Abstract
We used event-related functional MRI to investigate the neural bases of two categories of mental processes believed to contribute to performance of an alphabetization working memory task: memory storage and memory manipulation. Our delayed-response tasks required memory for the identity and position-in-the-display of items in two- or five-letter memory sets (to identify load-sensitive regions) or memory for the identity and relative position-in-the-alphabet of items in five-letter memory sets (to identify manipulation-sensitive regions). Results revealed voxels in the left perisylvian cortex of five of five subjects showing load sensitivity (as contrasted with alphabetization-sensitive voxels in this region in only one subject) and voxels of dorsolateral prefrontal cortex in all subjects showing alphabetization sensitivity (as contrasted with load-sensitive voxels in this region in two subjects). This double dissociation was reliable at the group level. These data are consistent with the hypothesis that the nonmnemonic executive control processes that can contribute to working memory function are primarily prefrontal cortex-mediated whereas mnemonic processes necessary for working memory storage are primarily posteriorly mediated. More broadly, they support the view that working memory is a faculty that arises from the coordinated interaction of computationally and neuroanatomically dissociable processes.
Elucidation of the cognitive and neural architectures underlying human working memory has been an important focus of cognitive neuroscience for much of the past decade (1–3). Two conclusions that arise from this research are that working memory, a faculty that enables temporary storage and manipulation of information in the service of behavioral goals, can be viewed as neither a unitary nor a dedicated system. For example, behavioral and functional neuroanatomical dissociations of working memory for different kinds of stimulus information attest to its componential nature (2, 4–6), and working memory performance recruits neuroanatomical systems that are also responsible for perceptuomotor information processing (7, 8). In the current experiment, we tested the hypothesis that the processes supporting the strictly mnemonic demands of a working memory task can be dissociated anatomically from the processes supporting the executive control demands of the same task. We developed this hypothesis after concluding from a review of the neuropsychological literature that performance on tests of immediate serial recall for verbal and spatial stimuli (i.e., memory “span”), widely accepted as indices of working memory storage capacity, is not impaired by damage to the prefrontal cortex (PFC) (9). This fact stands in contradistinction to the overwhelming evidence from functional neuroimaging research that working memory function is associated with PFC activation. Setting aside limitations on the ability to infer the necessity of a brain region’s contributions to a cognitive process from physiological observations (10, 11), this apparent disparity can be reconciled if one posits that the PFC activity seen in neuroimaging studies of working memory tasks reflects primarily the operation of executive control processes recruited by these tasks (3, 12). Candidate executive control processes that can contribute to working memory function include shifting attention among items held in working memory (13, 14), inhibition of prepotent responses (15), mediation of proactive interference (16, 17), updating mnemonic representations (18–20), and coordination of multiple task performance (21). [There is also evidence that rehearsal processes critical to performance on some tests of delayed response (as contrasted with tests of span) may depend on PFC (9)].
The preponderance of neuroimaging studies revealing working memory-related PFC activation have featured blocked experimental designs and have thus not permitted direct assessment of the relative contributions of mnemonic and executive control processes to PFC activation (22, 23). The present functional MRI (fMRI) study, in contrast, featured an event-related experimental design and analysis that permitted temporal isolation of the neural correlates of processes contributing to different components of the working memory task (i.e., target presentation/encoding, instructions, delay, probe/response) (24). It also featured manipulation of two variables, load and alphabetization, that index, respectively, mnemonic processes and executive control processes contributing to performance on our working memory task. Previous functional neuroimaging studies of working memory that have revealed effects of manipulating “load” in PFC (25–27) have done so in the context of tasks that do not permit dissociation of the strictly mnemonic consequences of varying the number of memoranda from commensurate effects on the executive control processes listed above.
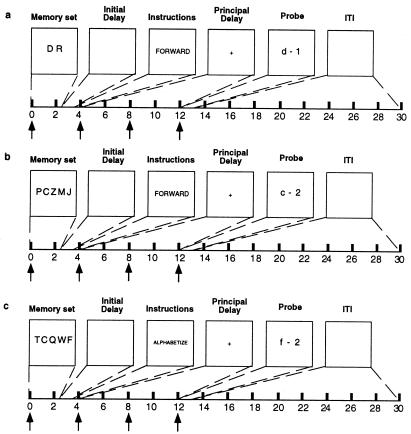
Previous research has identified a role for dorsolateral PFC in supporting the control processes required to manipulate information held in working memory (28, 29). A review of the neuropsychological (9, 30) and neuroimaging (31, 32) literatures, in contrast, suggests a role for left posterior perisylvian cortex in short-term storage of verbal information. In the present experiment, we operationalized the manipulation of information by requiring subjects to reorder into alphabetical order the five randomly ordered letters of a memory set (Alphabetize 5) and contrasting evoked fMRI signal from this condition with signal from trials in which no alphabetization of the five memoranda was required (Forward 5). We operationalized the storage of information by varying the number of items to be remembered during a delay (i.e., load: Forward 5 vs. Forward 2 conditions; Fig. 1). We predicted that regions of dorsolateral PFC would be sensitive to variations of alphabetization, but insensitive to variations of load, and that regions of the left posterior perisylvian cortex would be sensitive to variations of load, but insensitive to variations of alphabetization. Additionally, we predicted that, whereas maintenance of five letters across the delay period would engage dorsolateral and ventrolateral PFC (Forward 5), adding the requirement to alphabetize five letters during the delay (Alphabetize 5) would result in additional activity only in dorsolateral PFC, a result that would be consistent with processing models of the organization of working memory function in PFC (28, 29).
Figure 1.
The three conditions of the item-position recognition task. (a) Forward 2. (b) Forward 5. (c) Alphabetize 5. Arrows along the time lines represent the placement of independent variables positioned to detect variance in the fMRI signal associated with memory set, instruction, principal delay, and probe portions of the trial, respectively. ITI, intertrial interval
Methods
Subjects.
The five healthy, unmedicated subjects [four male; mean age = 24.2 (SD = 2.95)] were recruited from the undergraduate and medical campuses of the University of Pennsylvania, and all gave informed consent.
Behavioral Task.
Each trial in the item-position recognition task lasted 14 sec, presenting sequentially the memory set (2.5 sec), initial delay (1 sec), instructions (“forward” or “alphabetize”; 500 msec), principal delay (8 sec), and a letter-digit probe (1 sec; Fig. 1). The intertrial interval was 16 sec. The memory set for each trial type consisted of either two (Forward 2) or five (Forward 5 and Alphabetize 5) randomly selected and randomly ordered consonant letters. The probe always contained a letter from the memory set, and the digit represented with P = 0.5 the correct position of that letter. The Forward 2 and Forward 5 conditions assessed memory for the position of each letter as it appeared in the display of the memory set. The probe in the Alphabetize 5 condition assessed memory for the alphabetical position of each item in the memory set, relative to the other four items.
MRI Technique.
Imaging was carried out on a 1.5T SIGNA scanner (GE Medical Systems, Milwaukee, WI) equipped with a prototype fast gradient system for echo-planar imaging. A standard radiofrequency head coil was used with foam padding to restrict comfortably head motion. High resolution sagittal and axial T1-weighted images were obtained in every subject. A gradient echo, echoplanar sequence (TR = 2000 msec, TE = 50 msec) was used to acquire data sensitive to the blood oxygen level-dependent signal. Scans of the behavioral task were preceded by a scan in which we derived the impulse response function (IRF) for each subject. The IRF, which characterizes the fMRI response resulting from a brief impulse of neural activity (33), was used to convolve independent variables entered into the modified general linear model (see “Data Analysis” section below) (34) that we used to analyze the results of the scans of our behavioral task. This IRF derivation scan lasted 7 min (200 images/slice). Each fMRI scan of the working memory task lasted 6 min 20 sec (180 images/slice). fMRI data collection during all scans was preceded by 20 sec of dummy gradient and radiofrequency pulses to achieve a steady state of tissue magnetization.
Data Analysis.
Our method for deriving the IRF is described in detail elsewhere (35). In brief, we derived the IRF from primary sensorimotor cortex in each subject by scanning while the subject performed a simple button-press reaction-time task. The fMRI data from this scan were analyzed within the modified general linear model by using a Fourier basis set of four sines and four cosines. A partial F test was used to evaluate significance of activity in sensorimotor cortical voxels, and an IRF estimate was extracted from the suprathreshold voxels by averaging their time series, filtering the resultant spatially averaged fMRI time series to remove high (>0.244 Hz) frequencies, adjusting it to remove the effects of nuisance covariates (36), and trial-averaging it.
fMRI data from the item-position recognition task also were analyzed with the modified general linear model (34). The principle of the fMRI time series analysis was to model the fMRI signal changes occurring during particular temporal periods of the behavioral trials with covariates comprised of shifted, blood oxygen level-dependent IRFs (24, 37). Differences in delay-period fMRI signal were tested with contrasts of covariates that modeled the expected blood oxygen level-dependent signal response in the event of an increase in neural activity (relative to the intertrial interval) occurring during the delay period of each trial. For example, a contrast assessing a load effect would be effected as the difference of the parameter estimates associated with the covariates modeling delay-period activity in Forward 5 trials and Forward 2 trials: (DelayForward 5 − DelayForward 2). Importantly, we used this method to obtain measures of delay-period activity that were not contaminated by variance in the fMRI time series attributable to stimulus presentation or response activity. Smoothness of the fMRI response to neural activity allows fMRI responses to be resolved on the order of 4 sec (24). False positive rates for all contrasts were controlled at α = 0.05 by Bonferroni correction for the number of voxels per region of interest (ROI) (38). This correction for multiple comparisons, combined with the fact that we did not spatially smooth our data, meant that individual suprathreshold voxels identified by a particular contrast could be interpreted as demonstrating significant activity in relation to that contrast.
Defining ROIs.
ROIs were created for each subject by first creating a standard set of ROIs on a representation of a brain corresponding to a standardized coordinate frame (39). These ROIs defined dorsolateral PFC (areas 9 and 46), ventrolateral PFC (areas 44, 45, and 47), left posterior perisylvian cortex (areas 22, 39, and 40), and several right posterior cortical regions (selected with the procedure described in the next section, “Hypothesis Testing”). These standardized ROIs then were transformed into coordinates corresponding to each subject’s fMRI scan (effectively, a reverse normalization). Thus, the fMRI data for this experiment were analyzed in the coordinates in which they were acquired.
Hypothesis Testing.
Alphabetization sensitivity vs. load sensitivity in dorsolateral PFC.
The principal hypothesis tests were performed in dorsolateral PFC on single-subject data and proceeded in two steps. First, we searched for alphabetization-sensitive voxels with the contrast (DelayAlphabetize 5 − DelayForward 5), and, in parallel, we searched for load-sensitive voxels with the contrast (DelayForward 5 − DelayForward 2). Second, an averaged time series was extracted from the alphabetization-sensitive voxels and was assessed for load sensitivity with the contrast (DelayForward 5 − DelayForward 2), and an averaged time series was extracted from the load-sensitive voxels and was assessed for alphabetization sensitivity with the contrast (DelayAlphabetize 5 − DelayForward 5). Significance for these and all subsequent hypothesis testing contrasts performed on spatially averaged time series data was set at t = 1.96.
Alphabetization sensitivity vs. load sensitivity in left posterior perisylvian cortex.
The principle hypothesis tests were performed in the left posterior perisylvian cortex in a similar manner, except that our a priori predictions about the critical locus of memory storage activity were not as precise as were our predictions about the critical locus of alphabetization activity. Thus, our first step was to define the critical left posterior perisylvian ROI for each subject by identifying load-sensitive voxels (with the contrast [DelayForward 5 − DelayForward 2]) within the functionally and cytoarchitectonically defined ROIs in left posterior perisylvian cortex that have been implicated in previous neuropsychological studies: left inferior parietal lobule (areas 39 and 40), left posterior insular and opercular cortex within the Sylvian fissure, and left superior temporal gyrus (area 22) (30). When load-sensitive voxels had been identified and localized to a particular ROI, we next searched for alphabetization-sensitive voxels within that ROI (with the contrast [DelayAlphabetize 5 − DelayForward 5]). Finally, assessment of alphabetization sensitivity within load-sensitive posterior perisylvian voxels and of load sensitivity within alphabetization-sensitive posterior perisylvian voxels was accomplished by using the same method used in dorsolateral PFC.
Confirmatory group analyses.
To confirm that we were not biasing our results by only testing our hypothesis in voxels that showed suprathreshold alphabetization- or load-sensitive activity, we performed two additional analyses at the group level. The goal of these tests was to assess whether the activity of the suprathreshold voxels identified by the principal hypothesis-testing contrasts was representative of activity pooled across the ROI in which they were located, or whether these critical voxels displayed anomalous activity that did not characterize the behavior of that ROI as a whole. First, within each subject, we extracted region-wide time series by collapsing across all voxels in dorsolateral PFC and by collapsing across all voxels in the critical posterior perisylvian ROI. Next, we assessed alphabetization sensitivity and load sensitivity by applying to each of these two spatially averaged time series the contrasts (DelayAlphabetize 5 − DelayForward 5) and (DelayForward 5 − DelayForward 2). The resultant t values would index, for each subject, the extent to which these two ROIs exhibited, on average, alphabetization sensitivity and load sensitivity. Last, we performed two random effects group analyses, one for each ROI, to assess whether there was a reliable trend toward a greater alphabetization effect or a greater load effect in either ROI. These group analyses were realized as paired t tests (with 4 degrees of freedom), with each subject contributing an alphabetization-effect score and a load-effect score for each ROI (Table 1).
Table 1.
ROI-wide data (t values) by subject (and ROI), and associated group analyses
| Subject | Dorsolateral PFC
|
Left posterior
|
Right posterior
|
|||
|---|---|---|---|---|---|---|
| 5A-5F | 5F-2F | 5A-5F | 5F-2F | 5A-5F | 5F-2F | |
| 1 | 2.70 | 2.23 | −0.78 (BA 40) | 0.88 (BA 40) | −0.63 (BA 22) | 1.99 (BA 22) |
| 2 | 1.77 | 0.17 | 0.00 (BA 40) | 2.33 (BA 40) | 1.28 (BA 40) | 0.30 (BA 40) |
| 3 | 3.01 | 0.01 | 0.50 (BA 22) | 2.87 (BA 22) | 4.12 (BA 18) | 1.90 (BA 18) |
| 4 | 5.19 | 0.00 | −3.02 (insula) | 2.47 (insula) | 0.40 (BA 39) | 1.76 (BA 39) |
| 5 | 2.87 | 2.08 | 2.81 (BA 22 and BA 40) | 4.02 (BA 22 and BA 40) | 1.18 (BA 22) | 1.82 (BA 22) |
| Group results† | 2.6* | −3.5* | 0.3 | |||
A, Alphabetize; F, Forward; BA, Brodmann area.
Denotes a significant effect at the group level.
t-values with 4 degrees of freedom; positive values indicate a greater alphabetization than load effect, negative values the converse.
Right hemisphere group analysis.
We also planned to test the specificity of the predicted load-sensitive but alphabetization-insensitive nature of the left posterior perisylvian ROIs by applying the same hypothesis testing and group analysis procedures in the right hemisphere: first, identify load-sensitive voxels in right hemisphere posterior perisylvian regions; next, collapse across all voxels in these right hemisphere ROIs, extract spatially averaged time series, and assess the load sensitivity and alphabetization sensitivity in each ROI; finally, perform a random effects group analysis (paired t test) to assess, at the group level, whether there was a significant alphabetization effect or load effect. We predicted that these right posterior ROIs would not exhibit a reliable trend in either direction.
Maintenance vs. manipulation in dorso- and ventrolateral PFC.
Our experimental design also permitted us to effect a replication of a previous result from our group, that, whereas delay-period activity associated with Forward 5 trials was identified in both dorsolateral and ventrolateral PFC, voxels evincing significantly greater working memory manipulation (DelayAlphabetize 5) than working memory maintenance (DelayForward 5) activity were only found in dorsolateral PFC (29). This result was interpreted as evidence for a processing model of the functional organization of working memory in PFC (28). We performed the maintenance vs. manipulation analysis by applying the two-tailed contrast (DelayAlphabetize 5 − DelayForward 5) to dorso- and ventrolateral PFC ROIs in each subject.
Results
Maintenance vs. Manipulation in Dorso- and Ventrolateral PFC.
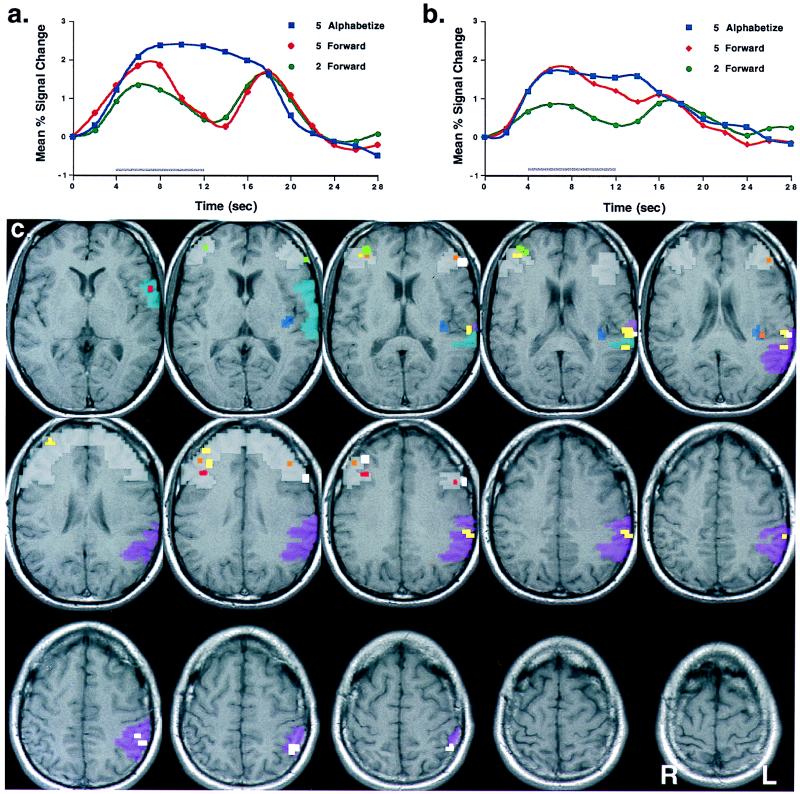
Performance on the two five-item conditions was equivalent [Forward 5 = 82.5% (SD = 13.0); Alphabetize 5 = 84.4% (SD = 11.0)] and slightly lower than Forward 2 performance [91.3% (SD = 8.4)]. Delay-period activity in the Forward 5 condition was observed (versus baseline) in dorsolateral PFC, bilaterally, in all five subjects and in four of five subjects in ventrolateral PFC: bilaterally in three subjects; in left hemisphere of subject 5; and in no ventrolateral PFC voxels of subject 2. Direct contrasts of blood oxygen level-dependent time series data in the Alphabetize 5 vs. Forward 5 conditions revealed significantly greater DelayAlphabetize 5 than DelayForward 5 activity in dorsolateral PFC, bilaterally, in each subject (Fig. 2 a and c). Such voxels were identified in ventrolateral PFC, however, in only one subject: in left hemisphere of subject 5.
Figure 2.
Manipulation vs. storage in working memory (a) Trial-averaged time series from dorsolateral PFC voxels of subject 5 demonstrating significant alphabetization sensitivity (DelayAlphabetize 5 − DelayForward 5) but no load sensitivity (DelayForward 5 − DelayForward 2). The gray bar along the horizontal axis in this panel, and in b, represents the duration of the principal delay period. (b) Trial-averaged time series from superior temporal gyrus and inferior parietal lobule (areas 22 and 40) voxels of subject 5 demonstrating significant load sensitivity but no alphabetization sensitivity. (c) Composite map displaying voxels from all five subjects, showing alphabetization sensitivity but load insensitivity in dorsolateral PFC and load sensitivity but alphabetization insensitivity in left posterior perisylvian cortex. T1-weighted anatomical slices are from a single subject and were normalized to the template brain in Talairach space that is provided in the spm96b package (http://www.fil.ion.ucl.ac.uk/spm). Four ROIs (also defined on the same normalized brain) are overlaid on the anatomical slices and depicted in four translucent colors: white, dorsolateral PFC; light blue, left area 22; dark blue, left posterior, superior insular cortex; purple, left area 40. Individual data from each subject, in the form of suprathreshold voxels identified by the alphabetization sensitivity and load sensitivity hypothesis tests, are displayed in five colors: subject 1, light green; subject 3, red; subject 4, orange; subject 5, yellow; white, locations with overlapping voxels from two or more subjects. (Subject 2 had no nonoverlapping voxels; subject identifying numbers correspond to the numbers in Table 1.) Loci of suprathreshold voxels (or clusters of contiguous voxels) for each subject, represented in normalized coordinates representing distances in mm of centers of voxels/voxel clusters in the x, y, and z directions from the origin, were: Subject 1, 10 PFC voxels (37.5, 41, 15), (−56, 30, 10), (37.5, 19, 50); 2 left area 40 voxels (−45, −60, 55). Subject 2: 2 PFC voxels (−56, 0, 45), (41, 7.5, 45); 1 left area 40 voxel (−64, −49, 17.5). Subject 3: 2 PFC voxels (32, 20, 35), (−50, 0, 40); 1 left area 22 voxel (−64, 15, 0). Subject 4: 10 PFC voxels (30, 45, 20), (35, 25, 35), (−37, 28, 35); 1 left posterodorsal insula voxel (−41.5, −26, 13). Subject 5: 14 PFC voxels (30, 37.5, 30), (37.5, 37.5, 15), (−60, 11.5, 35); 8 left areas 22 and 40 voxels (−71.5, −30, 5), (−67.5, −41, 10), (−71.5, −30, 30), (−52.5, −49, 50).
Alphabetization Sensitivity vs. Load Sensitivity in Dorsolateral PFC.
Within each subject, we found that the alphabetization-sensitive voxels in dorsolateral PFC were insensitive to variations of load (Fig. 2a). We also identified load-sensitive/alphabetization-insensitive voxels in dorsolateral PFC in two subjects: two voxels in right hemisphere in subject 5; and four voxels in left hemisphere in subject 1. The ROI-wide group analysis revealed significantly greater alphabetization than load sensitivity in dorsolateral PFC (Table 1).
Alphabetization Sensitivity vs. Load Sensitivity in Left Posterior Perisylvian Cortex.
Load sensitive voxels were identified in each subject: in left area 40 (inferior parietal lobule) in three subjects; in left area 22 (superior temporal gyrus) in two subjects; in both areas 40 and 22 in one subject; and in left posterior, superior insular cortex (proximal to the parietal operculum and the supramarginal gyrus) in one subject† (Fig. 2c.; Table 1). These suprathreshold voxels did not demonstrate alphabetization sensitivity in any subject. Within these ROIs, we identified alphabetization-sensitive voxels only in subject 5. These nine alphabetization-sensitive voxels were clustered along the border of areas 40 and 7 [centered at Talairach coordinates [−41, −56, 50)], a location markedly more caudal and superior than the clusters of load-sensitive voxels found in areas 40 and 22 in this subject (Fig. 2c). This cluster of nine alphabetization-sensitive voxels also demonstrated significant load-sensitivity [t(1236) = 2.8]. The ROI-wide group analysis revealed reliably greater load sensitivity than alphabetization sensitivity in left posterior perisylvian cortex (Table 1).
Alphabetization Sensitivity vs. Load Sensitivity in Right Posterior Cortex.
We identified suprathreshold load-sensitive voxels in right posterior hemisphere in area 22 in two subjects, area 40 in one subject, area 39 in one subject, and area 18 in one subject for whom there were no suprathreshold load-sensitive voxels in a right posterior perisylvian region. Group analyses performed on the spatially averaged time series from these ROIs revealed no evidence of a reliable trend toward greater load than alphabetization sensitivity (Table 1).
Discussion
The data presented in this report provide evidence for a functional neuroanatomical double dissociation of mnemonic processes and executive control processes that contribute to the storage and manipulation, respectively, of information in an item-position recognition working memory task. They are thus consistent with our view that working memory function arises from the coordinated recruitment of several independent cognitive processes that can be independently, differentially engaged depending on environmental demands. They are also consistent with the hypothesis that the nonmnemonic executive control processes that can contribute to working memory function are primarily PFC-mediated whereas mnemonic processes necessary for working memory storage are primarily posteriorly mediated (12). This hypothesis is orthogonal to, and compatible with, evidence of a role for PFC in maintenance and rehearsal processes (9) and the likelihood that control processes important for working memory storage (43) are supportd by posterior brain areas.
The absence of a reliable load effect in dorsolateral PFC in the present dataset is consistent with other results from our laboratory obtained with an item-recognition task. These results revealed load sensitivity in dorsolateral PFC during the target presentation/encoding portion of the task only and, as in the present dataset, a delay-period memory load effect in left posterior perisylvian cortex (44). Taken together, these two studies suggest that previous evidence of load-sensitivity in PFC of subjects performing the n-back working memory task (25–27) might be ascribed to the operation of encoding-mediated processes, or perhaps of other executive control processes, but not to differential memory storage demands per se.
Our finding of a reliable load-sensitivity effect in left posterior perisylvian cortex is broadly consistent with reports of patients with dramatically circumscribed short-term memory spans who have lesions in this area (30), as well as with the results of many previous neuroimaging studies (reviewed in refs. 45 and 46). The considerable variability in the precise localization of load-sensitive/manipulation-insensitive voxels across the five subjects in our study, however, raises questions about the nature of the mental processes underlying this load effect. This variability may reflect the fact that several discrete mental processes were sensitive to the variation of load in our experiment. The assembled evidence from patients with impairments of auditory-verbal short term memory (30), however, is inconclusive with respect to the expected spatial variability in the neural circuitry supporting phonologically based short-term storage of information (46). There is also a considerable amount of variability, both methodological and empirical, in functional neuroimaging data that is germane to this question (45, 46). The present study uses neuroimaging methods featuring sufficiently high power and spatial resolution to permit investigation of this question in individual subjects. Further refinement of theoretical and computational models of verbal working memory will be required to conclude whether the intersubject spatial variability seen in the present dataset is characteristic of the anatomical substrates of the mechanisms supporting phonological storage or whether our results reflect the participation of many different processes in our task.
The data reported here also provide a replication and an extension of previous demonstrations of the differential recruitment of dorsolateral PFC by working memory tasks requiring the manipulation of memoranda (29, 47). The results of these earlier studies had been open to the alternative interpretation that increases in the difficulty or complexity of working memory tasks might engender increased activity of the same process or system of processes that supported performance on the easier, or simpler tasks. Thus, according to this view, increased dorsolateral PFC activation associated with manipulation of letter (29) or spatial (47) stimuli may merely have reflected broader recruitment of the same processes involved in storage and maintenance of memoranda in working memory. The present results permit the rejection of this alternative hypothesis on two grounds. First, the double dissociation that they represent provides evidence that the neurophysiological factors underlying manipulation and storage differ qualitatively, and not merely quantitatively (48). Second, the equivalent levels of performance on Alphabetize 5 and Forward 5 trials, two conditions matched very closely for perceptual and procedural content, obviate the possibility that differences in difficulty underlay the differential patterns of PFC activation associated with manipulation and maintenance of information in working memory. One recent study of maintenance versus manipulation of spatial stimuli has marshaled similar evidence against the difficulty interpretation of spatial manipulation-related activity in dorsolateral PFC (49), and a second has replicated these results in an event-related fMRI study of maintenance versus manipulation in spatial working memory that featured closely matched behavioral conditions.‡
The model of the neural organization of working memory function in PFC that arises from the present study is one in which PFC can be recruited broadly by tasks requiring simple maintenance of information across a delay period—dorsolateral regions as well as ventrolateral regions. Dorsolateral regions alone, however, are recruited to an additional extent by the requirement to manipulate information held in working memory. This model is broadly consistent with the results of several neuropsychological studies in suggesting the attribution to dorsolateral PFC the support of the operation of executive control processes that can contribute to working memory function (12, 50–56). Our model differs in some details, however, from other processing models that suggest a strict segregation of maintenance- and manipulation-related function in PFC, with only ventrolateral regions supporting maintenance processes and only dorsolateral regions supporting manipulation processes (3, 48, 57). Our data are also at variance with the prediction arising from several previous neuroimaging studies (reviewed in ref. 57) of a left lateralized focus of working memory maintenance-related activity for verbal stimuli. Additional studies are required to determine the extent to which the instances of empirical variation that are reviewed in this paragraph are attributable to differences in behavioral methods, in neuroimaging technique, and/or in data analysis methods.
Acknowledgments
We thank Jessica Lease for assistance with programming and data collection and Geoff Aguirre, Saul Sternberg, and Eric Zarahn for helpful discussions of this work. This work was supported the American Federation for Aging Research and National Institutes of Health Grants NS01762 and AG13483. B.R.P. also received support from National Institutes of Health Grant AG 00255 (to Virginia M.-Y. Lee, University of Pennsylvania Medical Center).
Abbreviations
- PFC
prefrontal cortex
- fMRI
functional MRI
- IRF
impulse response function
- ROI
region of interest
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
The left posterior perisylvian ROI for this subject, subject 4, was defined on that subject’s axial T1 anatomical slices. The function of this area is poorly defined in humans, although the homologous region in the monkey is connected monosynaptically with polymodal areas of inferior parietal and superior temporal cortex and with ventral portions of frontal areas 8 and 46, as well as with somatosensory regions of the postcentral gyrus (40, 41). We limited this ROI to insular cortex in the posterior 10 mm of the Sylvian Fissure because more anterior regions of insular cortex are considered a part of the accessory motor system (42) and therefore are likely to be functionally distinct.
Postle, B. R., Berger, J. S., Taich, A. M. & D’Esposito, M., Cognitive Neuroscience Society Annual Meeting, April 11–13, 1999, Washington, DC, 1999, p. 25 (abstr.).
References
- 1.Ungerleider L G. Science. 1995;270:769–775. doi: 10.1126/science.270.5237.769. [DOI] [PubMed] [Google Scholar]
- 2.D’Esposito, Aguirre G K, Zarahn E, Ballard D. Cognit Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 3.Smith E E, Jonides J. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 4.Tresch M C, Sinnamon H M, Seamon J G. Neuropsychologia. 1993;31:211–219. doi: 10.1016/0028-3932(93)90085-e. [DOI] [PubMed] [Google Scholar]
- 5.Postle B R, Jonides J, Smith E, Corkin S, Growdon J H. Neuropsychology. 1997;11:1–9. doi: 10.1037//0894-4105.11.2.171. [DOI] [PubMed] [Google Scholar]
- 6.Halbig T, Mecklinger A, Schriefers H, Friederici A. Neuropsychologia. 1998;36:305–311. doi: 10.1016/s0028-3932(97)00127-9. [DOI] [PubMed] [Google Scholar]
- 7.Courtney S M, Ungerleider L G, Keil K, Haxby J V. Nature (London) 1997;386:608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- 8.Zarahn E, Aguirre G K, D’Esposito M. Cognit Brain Res. 1999;7:255–268. doi: 10.1016/s0926-6410(98)00029-9. [DOI] [PubMed] [Google Scholar]
- 9.D’Esposito M, Postle B R. Neuropsychologia. 1999;37:89–101. doi: 10.1016/s0028-3932(99)00021-4. [DOI] [PubMed] [Google Scholar]
- 10.D’Esposito M, Ballard D, Aguirre G K, Zarahn E. NeuroImage. 1998;8:274–282. doi: 10.1006/nimg.1998.0364. [DOI] [PubMed] [Google Scholar]
- 11.Sarter M, Berntson G G, Cacioppo J T. Am Psychol. 1996;51:13–21. doi: 10.1037//0003-066x.51.1.13. [DOI] [PubMed] [Google Scholar]
- 12.D’Esposito M, Postle B R. In: Attention and Performance XVIII. Monsell S, Driver J, editors. Cambridge, MA: MIT Press; 1999. , in press. [Google Scholar]
- 13.McElree B. J Mem Lang. 1998;38:225–252. [Google Scholar]
- 14.Garavan H. Mem Cognit. 1998;26:263–276. doi: 10.3758/bf03201138. [DOI] [PubMed] [Google Scholar]
- 15.Diamond A. In: The Development and Neural Bases of Higher Cognitive Functions. Diamond A, editor. New York: NY Acad. Sci.; 1990. pp. 267–317. [DOI] [PubMed] [Google Scholar]
- 16.Jonides J, Smith E E, Marshuetz C, Koeppe R A, Reuter-Lorenz P A. Proc Natl Acad Sci USA. 1998;95:8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Esposito M, Postle B R, Jonides J, Smith E E. Proc Natl Acad Sci USA. 1999;96:7514–7519. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salmon E, Van der Linden M, Collette F, Delfiore G, Maquet P, Degueldre C, Luxen A, Franck G. Brain. 1996;119:1617–1625. doi: 10.1093/brain/119.5.1617. [DOI] [PubMed] [Google Scholar]
- 19.Morris N, Jones D M. Br J Psychol. 1990;81:111–121. [Google Scholar]
- 20.Kiss I, Pisio C, Francois A, Schopflocher D. Cognit Brain Res. 1998;6:235–247. doi: 10.1016/s0926-6410(97)00035-9. [DOI] [PubMed] [Google Scholar]
- 21.D’Esposito M, Detre J A, Alsop D C, Shin R K, Atlas S, Grossman M. Nature (London) 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- 22.Aguirre G K, D’Esposito M. In: Functional MRI. Moonen C T W, Bandettini P A, editors. Berlin: Springer; 1999. pp. 369–380. [Google Scholar]
- 23.D’Esposito M, Zarahn E, Aguirre G K. Psychol Bull. 1999;125:155–164. doi: 10.1037/0033-2909.125.1.155. [DOI] [PubMed] [Google Scholar]
- 24.Zarahn E, Aguirre G K, D’Esposito M. NeuroImage. 1997;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]
- 25.Braver T, Cohen J D, Nystrom L E, Jonides J, Smith E E, Noll D C. NeuroImage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 26.Cohen J D, Perlstein W M, Braver T S, Nystrom L E, Noll D C, Jonides J, Smith E E. Nature (London) 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 27.Jonides J, Schumacher E H, Smith E E, Lauber E J, Awh E, Minoshima S, Koeppe R A. J Cognit Neurosci. 1997;9:462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- 28.Petrides M. In: Handbook of Neuropsychology. Boller F, Grafman J, editors. Vol. 3. New York: Elsevier; 1989. pp. 75–90. [Google Scholar]
- 29.D’Esposito, M., Postle, B. R., Ballard, D. & Lease, J. (1999) Brain Cognit., in press. [DOI] [PubMed]
- 30.Shallice T, Vallar G. In: Neuropsychological Impairments of Short-Term Memory. Vallar G, Shallice T, editors. New York: Cambridge Univ. Press; 1990. pp. 11–53. [Google Scholar]
- 31.Paulesu E, Frith C D, Frackowiak R S J. Nature (London) 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- 32.Awh E, Jonides J, Smith E E, Schumacher E H, Koeppe R A, Katz S. Psychol Sci. 1996;7:25–31. [Google Scholar]
- 33.Boynton G M, Engel S A, Glover G H, Heeger D J. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Worsley K J, Friston K J. NeuroImage. 1995;2:173–182. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 35.Aguirre G K, Zarahn E, D’Esposito M. NeuroImage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- 36.Friston K J, Holmes A P, Poline J-B, Heather J D, Frackowiak R S J. NeuroImage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- 37.Postle, B. R., Zarahn, E. & D’Esposito, M. (1999) Brain Res. Protocols, in press. [DOI] [PubMed]
- 38.Zarahn E, Aguirre G K, D’Esposito M. NeuroImage. 1997;5:179–197. doi: 10.1006/nimg.1997.0263. [DOI] [PubMed] [Google Scholar]
- 39.Talairach J, Tournoux P. Co-Planer Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 40.Mesulam M-M, Mufson E J. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 41.Cipollini P B, Pandya D N. J Comp Neurol. 1999;403:431–458. [PubMed] [Google Scholar]
- 42.Ceballos-Baumann A O, Passingham R E, Marsden C D, Brooks D J. Ann Neurol. 1995;37:746–757. doi: 10.1002/ana.410370608. [DOI] [PubMed] [Google Scholar]
- 43.Kieras D E, Meyer D E, Mueller S, Seymour T. In: Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. Miyake A, Shah P, editors. Cambridge, U.K.: Cambridge Univ. Press; 1999. pp. 183–223. [Google Scholar]
- 44.Rypma B, D’Esposito M. Proc Natl Acad Sci USA. 1999;96:6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jonides J, Schumacher E H, Smith E E, Koeppe R A, Awh E, Reuter-Lorenz P A, Marschuetz C, Willis C R. J Neurosci. 1998;18:5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Becker, J. T., MacAndrew, D. K. & Fiez, J. A. (1999) Brain Cognit., in press. [DOI] [PubMed]
- 47.Owen A M, Evans A C, Petrides M. Cereb Cortex. 1996;6:31–38. doi: 10.1093/cercor/6.1.31. [DOI] [PubMed] [Google Scholar]
- 48.Teuber H-L. Annu Rev Psychol. 1955;6:267–296. doi: 10.1146/annurev.ps.06.020155.001411. [DOI] [PubMed] [Google Scholar]
- 49.Owen A M, Herrod N J, Menon D K, Clark J C, Downey S P M J, Carpenter T A, Minhas P S, Turkheimer F E, Williams E J, Robbins T W, et al. Eur J Neurosci. 1999;11:567–574. doi: 10.1046/j.1460-9568.1999.00449.x. [DOI] [PubMed] [Google Scholar]
- 50.Petrides M, Milner B. Neuropsychologia. 1982;20:249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- 51.Shallice T. From Neuropsychology to Mental Structure. Cambridge, U.K.: Cambridge Univ. Press; 1988. [Google Scholar]
- 52.Petrides M. Proc R Soc London Ser B. 1991;246:293–298. doi: 10.1098/rspb.1991.0157. [DOI] [PubMed] [Google Scholar]
- 53.Petrides M, Alivisatos B, Evans A C, Meyer E. Proc Natl Acad Sci USA. 1993;90:873–877. doi: 10.1073/pnas.90.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrides M. J Neurosci. 1995;15:359–375. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chao L L, Knight R T. NeuroReport. 1995;6:1605–1610. doi: 10.1097/00001756-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Ptito A, Crane J, Leonard G, Amsel R, Caramanos Z. NeuroReport. 1995;6:1781–1784. doi: 10.1097/00001756-199509000-00018. [DOI] [PubMed] [Google Scholar]
- 57.Smith E E, Jonides J, Marshuetz C, Koeppe R A. Proc Natl Acad Sci USA. 1998;95:876–882. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]