Abstract
Correlates of virus load and characteristics of virus-producing cells in tonsillar tissue were investigated. Our results suggest that when less than 1:100 tonsillar CD4+ T cells from individuals infected with HIV type-1 (HIV-1) contain replication competent provirus, the level of CD4+ T cells in tonsils is comparable to that observed in uninfected individuals. Virus load at or above this level was associated with low CD4 cell numbers in tonsillar tissue. Only a few percent of all infected T cells in tonsillar tissue were active virus producers, with minor differences observed between individuals. Plasma viremia was found to correlate with infectious virus load in tonsillar tissue. With less than 1:1,000 of CD4 cells in lymphoid tissues being involved in active virus production, direct cytopathic effect by HIV-1 on infected CD4 cells is unlikely to fully explain the immunodeficiency seen in AIDS.
HIV type-1 (HIV-1) predominantly infects T cells that express CD4 (1). However, through years of clinically latent HIV-1 infection the proportion of CD4+ T cells in blood carrying replication competent provirus is typically less than 1:1,000 (1, 2). More recent studies have suggested higher viral loads in lymphoid tissues (3). Still, the proportion of HIV-1-producing cells has been considered by many to be insufficient to fully explain the overall loss of CD4+ T cells suggested by the low levels of CD4+ T cells in blood (4).
New data estimating a production of up to 1010 HIV-1 particles and a continuously high turnover of CD4+ T cells has profoundly affected current thinking on the immunopathogenesis of AIDS (5–8). These estimations were made by measurement of the concentration of CD4 cells in peripheral blood and virions in plasma after in vivo treatment with potent inhibitors of HIV-1 replication (5–7). However, only approximately 2% of all lymphocytes are found in peripheral blood; the majority of lymphocytes are located in lymphoid tissues. Thus, studies of cellular changes and virus load in lymphoid tissues are required to fully understand the forces driving the development of AIDS. The palatine tonsils, containing lymphoid tissue with characteristics similar to both lymph node tissue and gut-associated lymphoid tissue, may yield biopsy material suitable for such studies. Examining biopsies of tonsillar tissue from HIV-1-infected individuals at different clinical stages of infection, we have recently shown that in tonsillar tissue from asymptomatic HIV-1-positive individuals, CD4+ T cells are maintained at levels similar to those observed in uninfected controls, despite reduced CD4+ T cell counts in blood (9).
The present study focuses on correlates of virus load in tonsillar tissue. We show evidence suggesting that as long as the proportion of provirus-containing CD4 cells is below 1:100, the level of CD4 cells in lymphoid tissues remains relatively unaffected. Virus load at or above this threshold of infection was associated with low levels of tonsillar tissue CD4+ T cells. Moreover, we show that only a small fraction, approximately 3% of the HIV-1-infected CD4+ T cells in tonsils, were actually producing virus. We also demonstrate that measurements of HIV-1 RNA in plasma correlate with the tonsillar fraction of CD4+ T cells carrying infectious HIV-1 provirus.
MATERIALS AND METHODS
Study Participants and Collection of Material.
Thirteen HIV-1 seropositive individuals (median age 34 years) participated in the study. Tonsillar tissue was obtained by a partial biopsy from the palatine tonsil in local anesthesia. Written consent was obtained from the participants, and approval was given by the regional committee for medical ethics in research. The HIV-1-positive individuals were clinically classified according to Communicable Disease Center criteria (10). Clinical and laboratory data on all of the study participants are presented in Table 1.
Table 1.
Clinical and laboratory data on the 13 study participants
| Patient no. | CDC class* | Antiviral treatment | Blood
|
Tonsils
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 mm−3 | CD8 mm−3 | Plasma viremia† | Virus phenotype | CD4, % | CD8, % | B cells, % | Histology | Virus phenotype | |||
| 1 | A1 | No | 588 | 1,013 | 2.6 | s/l | 37.0 | 18.8 | 40.4 | ND | s/l |
| 2 | A2 | No | 415 | 1,006 | 3.8 | s/l | 40.0 | 12.0 | 48.0 | FH/FL | s/l |
| 3 | A2 | No | 371 | 371 | 3.3 | s/l | 32.1 | 27.2 | 34.0 | FH/FL | s/l |
| 4 | A1 | No | 677 | 450 | 5.4 | s/l | 41.8 | 10.4 | 45.0 | FL/FH | s/l |
| 5 | A1 | No | 742 | 723 | 5.4 | r/h | 35.4 | 21.5 | 40.0 | FI/FL | r/h |
| 6 | A3‡ | ZDV | 110 | 629 | 4.3 | s/l | 21.3 | 43.7 | 35.0 | FI/FL | s/l |
| 7 | B2 | No | 488 | 654 | 4.2 | r/h | 19.0 | 19.0 | 56.0 | FI/FL | s/l |
| 8 | B2 | ZDV | 260 | 852 | 4.1 | s/l | 21.2 | 19.3 | 53.0 | FH/FL | s/l |
| 9 | C3 | No | 84 | 1,500 | 4.4 | r/h | 7.2 | 56.6 | 31.5 | FI/DC | r/h |
| 10 | C3 | No | 132 | 384 | 5.9 | r/h | 14.8 | 13.6 | 70.0 | FI/FL | r/h |
| 11 | B3 | No | 174 | 1,245 | 5.0 | r/h | 3.1 | 44.3 | 43.0 | FI/DC | r/h |
| 12 | B3 | No | 97 | 516 | 4.1 | r/h | 10.7 | 19.6 | 67.8 | FI/FL | r/h |
| 13 | C3 | No | 2 | 114 | 3.4 | ND | 0.1 | 16.3 | 80.8 | DC | nd |
ZDV, zidovudine; s/l, slow/low; r/h, rapid/high (11); FH, follicular hyperplasia; FL, follicular lysis; FI, follicular involution; DC, diffuse changes (12); ND, not done.
Clinical classification according to 1993 revised classification system for HIV infection (10).
Plasma viremia is given as log10 HIV RNA copies/ml.
Patient 6 changed to C3 (pneumocystis carinii pneumonia) approximately 6 months after inclusion into the study.
Cell Separation.
The tonsillar specimens were minced and incubated for 45 min in MEM (Joklik modification, GIBCO/BRL) containing 1.5 mg/ml collagenase II (Sigma) at 37°C in 5% CO2 before the cells were filtered through a 40-μm nylon mesh. Mononuclear cell fractions were isolated from citrate anticoagulated peripheral blood samples and tonsillar cell suspensions by density gradient-separation (Lymphoprep–Nycomed Pharma, Oslo). CD4 cells were positively selected from mononuclear cell fractions by rosetting with immunomagnetic beads (Dynabeads, Dynal, Oslo) for 30 min at 4°C after which the rosetted cells were detached by incubation with a Fab-specific goat anti-mouse mAb (Detachabead, Dynal) according to instructions provided by the manufacturer. The detached cells were washed twice in PBS containing 1% fetal calf serum (GIBCO, Paisley, Scotland). The selected cells were >98% CD4+ and >99% CD3+ by flow cytometry.
Quantitation of the Proportion of CD4+ T Cells Containing Infectious Provirus in Blood and Tonsils.
The proportion of CD4+ cells infected by HIV-1 was quantified by a limiting dilution assay based on principles described earlier (2). Briefly, purified CD4 cells were cocultured in decreasing numbers (5-fold dilutions) with uninfected allogeneic CD4 cells from an HIV-1 seronegative donor and stimulated with a mAb to the T cell receptor (T10B9, kindly provided by J. S. Thompson, University of Kentucky Medical Center, Lexington, KY) coated onto Dynabeads. Cells were cultured in RPMI medium 1640 (GIBCO) containing 10% fetal calf serum and 5 units/ml interleukin 2 (Amersham), which was replaced twice weekly. Cell supernatants were collected on day 14 and analyzed for the presence of HIV-1 p24 antigen by an in-house p24 antigen ELISA (13). The proportion of infected CD4 cells was calculated according to the Poisson distribution formula based on the inverse fraction of the number of cells required to give 63% p24 positive culture supernatants (2).
Measurements of Absolute Levels of CD4 Cells Containing Infectious Provirus in Lymphoid Tissue.
CD4 cell levels in tonsils were calculated according to a recently described method (9). In that study the fraction of B cells among tonsillar mononuclear cells was found to remain relatively unaltered in asymptomatic HIV-1 infection. The ratio between the percentage of CD4+ T cells and the percentage of B cells in tonsils can thus be used as a measure of the absolute levels of CD4 cells in lymphoid tissue (9). Based on the same principle, the provirus burden in tonsils were calculated as the product of the CD4 cell levels and provirus frequency according to the formula:
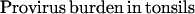 |
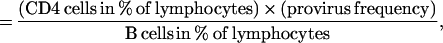 |
where provirus frequency is given as the number of CD4+ T cells containing replication competent HIV-1 per 106 CD4+ T cells.
Virus Isolation and Characterization.
HIV-1 was isolated from peripheral blood mononuclear cells or positively selected CD4 cells from blood and tonsils of the HIV-1-positive individual by coculture with phytohemagglutinin blasts obtained from HIV-negative blood donors. When virus replication was detected, infected cells were cocultured with cells of established lines of T cell (Jurkat-tat, MT-2) and monocytoid (U937–2) origin, and the virus isolates were classified as being of either rapid/high, syncytia-inducing, or slow/low nonsyncytia-inducing phenotype (11).
Quantitation of Plasma Viremia.
HIV-1-RNA levels in plasma were determined by a commercially available semiquantitative reverse transcriptase–PCR assay (Amplicore HIV-1 Monitor, Roche Diagnostics) according to the instructions provided by the manufacturer.
Detection of Cells Producing HIV-1 in Vivo.
Cytospin preparations of 1.5 × 105 purified CD4 cells per slide from tonsillar tissue of five HIV-1-positive individuals in different clinical stages of disease were fixed in methanol and stored at −20°C until analyzed. After washing and blocking with 0.5% BSA, the cells were incubated with mAb specific for HIV-1 p24 antigen (IgG1, clone EF7, generously provided by Jorma Hinkula, Stockholm, Sweden) at +4°C overnight in a moisture chamber. The next day, the slides were washed in PBS and incubated for 30 min at room temperature with an fluorescein isothiocyanate-labeled isotype specific secondary antibody (Southern Biotechnology Associates). After a final wash in PBS and rinsing in distilled water a coverslip was mounted in Slowfade-solution (Molecular Probes). All the cells on two slides (approximately 3 × 105 CD4 cells) were examined by UV microscopy.
To determine spontaneous HIV-1 production, 105 positively selected CD4 cells from tonsils and blood were cultured alone in RPMI medium 1640 with 10% fetal calf serum in the absence of mitogens and allogeneic recipient cells. Supernatant was harvested on day 5 and assayed for the presence of p24 antigen.
Statistics.
Differences between paired observations were determined by Wilcoxon rank sum test, differences between groups were calculated by the Mann–Whitney method for nonparametric measurements, and correlations were compared by linear regression analysis. Results were regarded as statistically significant when P = 0.05.
RESULTS
High Frequency of Infected Cells Correlates with Low CD4 Cell Level in Tonsillar Tissue: Evidence for a Threshold of Infection.
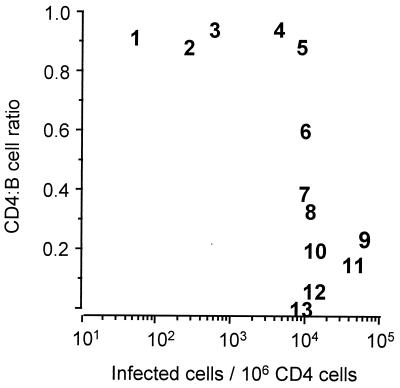
We previously have found the CD4:B cell ratio to be a useful indicator for the CD4 cell content in tonsillar lymphoid tissue in HIV-1 infection (9). Fig. 1 shows results of experiments correlating the proportion of CD4+ tonsillar T cells carrying replication-competent provirus with the tonsillar CD4:B cell ratio. We found that the CD4:B cell ratio in tonsillar tissue was stable for all study participants with less than 1:100 infected cells. This CD4:B cell ratio is similar to that observed in uninfected controls (9). Low levels of tonsillar CD4 cells were seen only in tonsils of individuals with infectious provirus levels ≥ 1:100. This level of infection thus seems to represent an infectious threshold at which a loss of tonsillar CD4+ T cells is observed. The biological phenotype of HIV-1 isolates from blood and tonsils was characterized, and the results are presented in Table 1. All the patients with low provirus levels (patients 1–4) had slow/low-type virus in the tonsillar cells, whereas 5 of the 8 patients at or above the infectious level had rapid/high-type virus in the tonsils (difference not significant). In patient 7 rapid/high-type HIV-1 isolates had been persistently cultured from blood-derived cells for the past year, but virus isolation from tonsillar tissue yielded a slow/low-type HIV-1.
Figure 1.
Correlation between the proportion of CD4+ T cells carrying replication competent HIV-1 provirus and the tonsillar CD4:B cell ratio. Values are represented by numbers that correspond with patient identification numbers in Table 1.
Plasma Viremia and Provirus Levels in Blood Reflect the Levels of HIV-Infected Cells in Lymphoid Tissue.
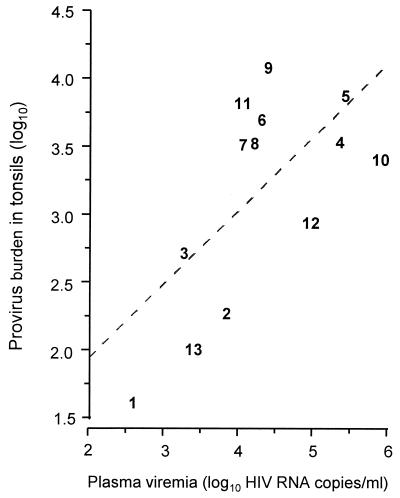
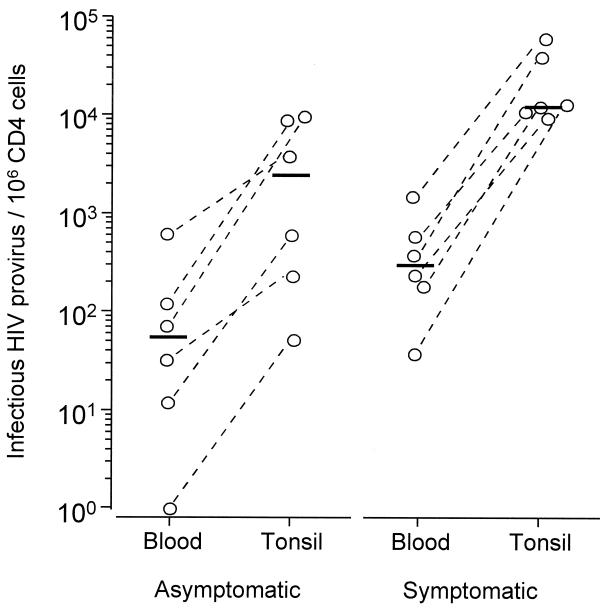
In previous studies, quantification of HIV-1 provirus-containing CD4+ T cells (14) and, subsequently, HIV-1 RNA concentration in plasma (15, 16) have been found to be of prognastic value. It is not yet known, however, if these indices of viral load in the peripheral circulation reflect viral load in lymphoid tissues. The product of the fraction of tonsillar CD4 cells carrying replication competent provirus and the CD4:B cell ratio gives an indication of the tonsillar provirus burden. As seen in Fig. 2, we found a significant correlation (r = 0.65, P = 0.016) between the tonsillar provirus burden and the number of HIV-1 RNA copies per ml of plasma. There was also a correlation between the proportion of CD4+ T cells in tonsils and peripheral blood carrying replication competent provirus (r = 0.82, P < 0.002). However, the percentage of provirus-containing cells was always much higher (median 44-fold) in tonsils than in peripheral blood (Fig. 3, P = 0.022). The fraction of HIV-1-infected CD4 cells in both blood and tonsils was significantly higher in persons with HIV-related symptoms compared with asymptomatic HIV-1-positive individuals (median 304 vs. 53 HIV-infected cells/106 CD4 cells, P < 0.05 for blood and median 12,000 vs. 2,343, P < 0.05 for tonsils, respectively), but there was no difference in the tonsil/blood ratio of provirus-containing CD4 cells between the clinical groups.
Figure 2.
Correlation between plasma viremia and the tonsillar provirus load (see Materials and Methods). Values are represented by patient identification numbers. A linear regression curve is also indicated (dotted line: r = 0.65, P = 0.016).
Figure 3.
Proportion of CD4+ T cells carrying replication competent HIV-1 in blood and tonsils of asymptomatic (Left, patients 1–6) and symptomatic HIV-1-infected individuals (Right, patients 7–12). Bars represent median values.
Quantification of Tonsillar CD4+ T Cells Producing HIV-1 in Vivo.
As a correlate for plasma HIV-1 viremia, the number of cells involved in active virus production in lymphoid tissues at any one time should be of even greater significance than the estimate of tissue provirus burden. By relating the proportion of tonsillar CD4 cells expressing HIV-1 p24 to the fraction of the CD4 cells calculated to contain replication competent HIV-1 provirus, an estimate of the percentage HIV-1-infected CD4 cells involved in active virus production was obtained. The results for five patients at different clinical stages and with phenotypically different virus isolates is shown in Table 2. The results differ little between individuals and show that only approximately 3% of the HIV-1-infected tonsillar CD4 cells in these individuals were producing viral proteins. HIV-1 p24 antigen positive cells were readily distinguishable from antigen negative cells in cytospin preparations. Many of the HIV-1 p24+ cells showed signs of nuclear condensation and fragmentation, while some were small and pyknotic (data not shown). When placed in unstimulated culture in the absence of cytokines, tonsillar CD4+ T cells yielded HIV-1 p24-positive supernatants within a few days (data not shown).
Table 2.
Estimation of the fraction of HIV-1-infected CD4+ T cells involved in active virus production in tonsillar tissue
| Patient no. | Cells containing infectious provirus* | p24+ cells† | Percent p24+ infected cells‡ |
|---|---|---|---|
| 1 | 50 | <3 | <6 |
| 2 | 250 | 7 | 2.8 |
| 4 | 4,125 | 113 | 2.7 |
| 10 | 12,000 | 240 | 2.0 |
| 12 | 12,000 | 347 | 2.9 |
Number of cells containing infectious provirus copies/106 CD4 cells.
Number of p24+ cells/106 CD4 cells. Values represent mean of two counts from independent slides.
Estimation of the fraction (in percent) of provirus-containing CD4 cells in tonsils staining positive for p24 antigen.
DISCUSSION
It becomes increasingly clear that to formulate theories on the immunopathogenesis of AIDS data on the virological and immunological changes occurring within the lymphoid tissues must be taken into account. Thus, we have recently shown that in asymptomatic HIV-1-infected individuals the CD4+ T cells in tonsillar tissue is maintained at normal levels despite reduced CD4+ T cell counts in peripheral blood (9). In the present study we show that assays measuring HIV-1 load in peripheral blood lymphocytes and plasma quite accurately reflect infectious provirus in tonsillar tissue. A gradient still exists, however: the proportion of CD4+ T cells carrying replication competent HIV-1 is approximately 40 times higher in tonsillar tissue than in peripheral blood. These results on viral load are comparable to results obtained by ID50 methodology (17) and quantitative PCR (18) using cells from lymph nodes. Also, early studies suggested a very low prevalence of actively HIV-1-producing cells in lymph nodes (19, 20). However, using PCR in situ double-label methods, Embretson et al. (21) detected HIV-1 DNA in 20–30% of CD4+ cells both in lymph node germinal centers and in paracortical regions. Some of the difference may be explained by the ability of sensitive hybridization techniques to identify replication incompetent provirus. Interestingly, only 1:100–1:400 cells with HIV-1 DNA in that study were found to express HIV-1 RNA, suggestive of active HIV-1 production. Thus, by all methods used so far only 1:1,000 or less lymphoid tissue CD4+ T cells are found to be involved in active HIV-1 production.
Our present results suggest that, while the provirus load gradually increases in tonsillar tissue, the CD4 cell level remains normal until a “threshold of infection” is reached. Few of the individuals in our study had tonsillar provirus load much greater than the threshold of infection. However, most of the individuals with this degree of provirus load showed signs of HIV-1-related disease and/or loss of CD4+ T cells from tonsillar tissue. There was a tendency for individuals with high levels of provirus load to carry rapid/high-type HIV-1, although the difference between individuals with high and low levels of provirus load did not reach significance in this small patient population. Thus, the immunopathogenic importance of this observation remains to be established. Among the tonsillar CD4+ T cells, less than 3:10,000 were found to be expressing HIV-1 p24. We have previously shown that programmed cell death of CD4+ tonsillar T cells correlates with the infectious HIV-1 provirus burden in tonsils (unpublished work). In the present study we observed that some of the virus antigen positive cells did indeed show morphological features consistent with programmed cell death. Still, death of all or most of the virus-producing CD4+ tonsillar T cells seems insufficient to account for the degree of apoptosis observed among tonsillar T cells (unpublished work), and insufficient as an explanation for the eventual depletion of CD4+ T cells in lymphoid tissues as long as the repopulation system for lymphocytes is functioning normally (22). The exact mechanisms leading to the loss of CD4 cells in lymphoid tissue thus remain elusive.
Our present results, combined with previously published observations of HIV-1 turnover rates, can be used in simple calculations of viral load parameters for the virologically, immunologically, and clinically stable stage of HIV-1 infection. The adult human being has been calculated to possess 1012 lymphocytes (22), of which perhaps 2–3 × 1011 are CD4+ T cells. We suggest that approximately 3:104 CD4+ T cells are involved in active virus production at a given time, which amounts to 108 CD4 cells overall. Assuming a production of 109–10 HIV-1 particles every day (5–8), every virus-producing cell must yield approximately 102 virions. This is in line with in situ hybridization data measuring intracellular HIV-1 RNA in lymph nodes (20, 21) and tonsils (24) and in in vitro infected cells (25). Using in situ hybridization techniques, one can detect a virus-producing cell after transcription of proviral DNA, whereas the detection of intracellular p24 antigen requires translation of mRNA. Our results may thus be an underestimate of the total number of cells involved in HIV-1 production in tonsillar tissue. On the other hand, tonsillar tissue, by virtue of its proximity to the external environment, contains a large proportion of activated CD4+ T cells (unpublished work). Activation is required for latently infected cells to produce virus particles (26). The proportion of infected CD4+ T cells involved in active virus production may thus be higher in tonsillar tissue than in other lymphoid tissues. These potential sources of error pull in different directions, which makes us believe that our estimate of the number of virions produced per CD4+ T cell in lymphoid tissues may be quite correct.
Taken together, our results suggest that a very small proportion of tonsillar CD4+ T cells carry replication competent HIV-1, and only a small proportion of these cells are involved in active virus production. Still, at a threshold level when only about 1:100 of tonsillar CD4+ T cells are infected, and only approximately 3:10,000 tonsillar CD4+ T cells are active virus producers, the balance between tonsillar CD4 cells lost and replaced is disturbed, and the level of tonsillar CD4+ T cells starts to fall. A direct cytopathic effect by HIV-1 on individual infected cells alone seems insufficient to explain this loss of CD4+ T cells.
Acknowledgments
We thank Dr. Robert Bjerknes, Dr. Rolf Reiertsen, and Dr. Håkon Sjursen for practical help and recruitment of patients to the study. Financial support was provided by the Norwegian Research Council.
ABBREVIATION
- HIV-1
HIV type 1
References
- 1.Schnittman S M, Psallidopoulos M C, Lane H C, Thompson L, Baseler M, Massari F, Fox C H, Salzman N P, Fauci A S. Science. 1989;245:305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- 2.Brinchmann J E, Albert J, Vartdal F. J Virol. 1991;65:2019–2023. doi: 10.1128/jvi.65.4.2019-2023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pantaleo G, Graziosi C, Demarest J F, Butini L, Montroni M, Fox C H, Orenstein J M, Kotler D P, Fauci A S. Nature (London) 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 4.Finkel T H, Banda N K. Curr Opin Immunol. 1994;6:605–615. doi: 10.1016/0952-7915(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 5.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, Saag M S, Shaw G M. Nature (London) 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 6.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Nature (London) 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 7.Perelson A S, Neumann A U, Markowitz M, Leonard J M, Ho D D. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 8.Wain-Hobson S. Science. 1995;373:102. doi: 10.1038/373102a0. [DOI] [PubMed] [Google Scholar]
- 9.Røsok B I, Bostad L, Voltersvik P, Bjerknes R, Olofsson J, Åsjö B, Brinchmann J E. AIDS. 1996;10:F35–38. [PubMed] [Google Scholar]
- 10.Anonymous Morbid Mortal Weekly Rep. 1992;41:1–19. [Google Scholar]
- 11.Fenyö E M, Morfeldt-Månson L, Chiodi F, Lind B, von Gegerfelt A, Albert J, Olausson E, Åsjö B. J Virol. 1988;62:4414–4419. doi: 10.1128/jvi.62.11.4414-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Öst Å, Baroni C D, Biberfeld P, Diebold J, Moragas A, Noèl H, Pallesen G, Rácz P, Schipper M, Tenner-Rácz K, van der Tweel J G. Acta Pathol Microbiol Immunol Scand Suppl. 1989;8:7–15. [PubMed] [Google Scholar]
- 13.Sundqvist V A, Albert J, Ohlsson E, Hinkula J, Fenyö E M, Wahren B. J Med Virol. 1989;29:170–175. doi: 10.1002/jmv.1890290305. [DOI] [PubMed] [Google Scholar]
- 14.Schnittman S M, Greenhouse J J, Lane H C, Pierce P F, Fauci A S. AIDS Res Hum Retrovirol. 1991;7:361–367. doi: 10.1089/aid.1991.7.361. [DOI] [PubMed] [Google Scholar]
- 15.Mellors J W, Rinaldo C R, Jr, Gupta P, White R M, Todd J A, Kinsley L A. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 16.Saag M S, Holodniy M, Kuritzkes D R, O’Brien W A, Coombs R, Poscher M E, Jacobsen D M, Shaw G M, Richmann D D, Volberding P A. Nature Med. 1996;2:625–629. doi: 10.1038/nm0696-625. [DOI] [PubMed] [Google Scholar]
- 17.Lafeuillade A, Tamalet C, Pellegrino P, Tourres C, Yahi N, Vignoli C, Quilichini R, de Micco P. AIDS. 1993;7:1527–1528. doi: 10.1097/00002030-199311000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Pantaleo G, Graziosi C, Butini L, Pizzo P A, Schnittman S M, Kotler D P, Fauci A S. Proc Natl Acad Sci USA. 1991;88:9838–9842. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harper M E, Marselle L M, Chayt K J, Josephs S F, Biberfeld P, Epstein L G, Oleske J M, O’Hara C J, Groopman J E, Gallo R C, Wong-Staal F. In: Biochemical and Molecular Epidemiology of Cancer. Harris C C, editor. New York: Liss; 1986. pp. 449–457. [Google Scholar]
- 20.Biberfeld P, Chayt K J, Marselle L M, Biberfeld G, Gallo R C, Harper M E. Am J Pathol. 1986;125:436–442. [PMC free article] [PubMed] [Google Scholar]
- 21.Embretson J, Zupancic M, Ribas J L, Burke A, Racz P, Tenner-Racz K, Haase A T. Nature (London) 1993;362:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 22.Brinchmann J E. The Immunologist. 1993;1:167–171. [Google Scholar]
- 23.Klein J. Immunology. Oxford: Blackwell; 1990. p. 8. [Google Scholar]
- 24.Haase A T, Henry T, Zupancic M, Sedgewick G, Faust R A, Melroe H, Cavert W, Gebhart K, Staskus K, Zhang Z-Q, Daily P J, Balfour H H, Jr, Erice A, Perelson A S. Science. 1996;274:985–989. doi: 10.1126/science.274.5289.985. [DOI] [PubMed] [Google Scholar]
- 25.Åsjö B, Sharma U K, Morfeldt-Månson L, Magnusson A, Barkhem T, Albert J, Olausson E, von Gegerfelt A, Lind B, Biberfeld P, Fenyö E M. AIDS Res Human Retrovir. 1990;6:1177–1182. doi: 10.1089/aid.1990.6.1177. [DOI] [PubMed] [Google Scholar]
- 26.Hannibal M C, Markovitz D M, Clark N, Nabel G J. J Virol. 1993;67:5035–5040. doi: 10.1128/jvi.67.8.5035-5040.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]