Abstract
Many primates, including humans, live in complex hierarchical societies where social context and status affect daily life. Nevertheless, primate learning studies typically test single animals in limited laboratory settings where the important effects of social interactions and relationships cannot be studied. To investigate the impact of sociality on associative learning, we compared the individual performances of group-tested rhesus monkeys (Macaca mulatta) across various social contexts. We used a traditional discrimination paradigm that measures an animal’s ability to form associations between cues and the obtaining of food in choice situations; but we adapted the task for group testing. After training a 55-member colony to separate on command into two subgroups, composed of either high- or low-status families, we exposed animals to two color discrimination problems, one with all monkeys present (combined condition), the other in their “dominant” and “subordinate” cohorts (split condition). Next, we manipulated learning history by testing animals on the same problems, but with the social contexts reversed. Monkeys from dominant families excelled in all conditions, but subordinates performed well in the split condition only, regardless of learning history. Subordinate animals had learned the associations, but expressed their knowledge only when segregated from higher-ranking animals. Because aggressive behavior was rare, performance deficits probably reflected voluntary inhibition. This experimental evidence of rank-related, social modulation of performance calls for greater consideration of social factors when assessing learning and may also have relevance for the evaluation of human scholastic achievement.
A distinction between learning, the internal cognitive process, and performance, the external behavioral expression of knowledge, has been long recognized (1, 2), but whether the social environment influences the magnitude of the separation remains unclear. For example, under certain conditions and for reasons that are incompletely understood, humans inhibit their performance, feign incompetence, or “play dumb” (3, 4). One anecdotal account describes skilled bowlers of low social status performing below their ability in the presence of higher-status, presumably intimidating bowlers (5). This observation suggests that seeming differences in ability can arise from social influences on behavior. High rank confers substantial social advantages in many primates (6), including humans, but is this competitive edge also reflected in cognitive performance?
We began exploring the possibility that social context influences learning and performance in nonhuman primates. Rhesus monkeys (Macaca mulatta) have been favored models for comparative investigations of learning (7); moreover, they compete fiercely within a social order based on dominance relationships (8). We selected the discrimination learning paradigm (9) because the performance of single animals in limited laboratory settings has been well documented (10). We modified the traditional task to allow group testing, and incorporated components of natural foraging behavior to enhance ecological relevance (11). As reported previously, in a social context only the dominant members of a 74-member colony showed evidence of discrimination learning (12). It remained unclear exactly why subordinate monkeys performed poorly. Here we investigated three hypotheses that offer contrasting explanations for performance deficits in group-tested subordinate animals.
First, the “cognitive disadvantage hypothesis” suggests that dominant and subordinate monkeys differ in cognitive ability, with poorer performance reflecting inferior skill. This hypothesis predicts lower performance by subordinates under any social circumstance, whether dominant animals are present or not. Second, the “failure to learn hypothesis” requires no rank-related difference in cognitive ability, but posits that the presence of dominant animals prevents subordinates from attending to salient features of the task, thereby disrupting learning. Accordingly, if subordinates flunk in mixed social contexts, their performance deficits should persist (at least initially) upon separation from dominant animals. Moreover, even after being segregated, they should do worse than if they had always been segregated. Thus, performance by subordinate animals should vary depending on their prior experiences or social learning history. Third, the “failure to perform hypothesis” rejects any differences in comprehension and instead proposes that subordinates fail to express their learning, either voluntarily or because of social suppression by dominant animals. According to this hypothesis, subordinates learn in all social contexts, but express their knowledge only when dominant animals are absent, regardless of learning history. Thus, subordinates who became proficient in seclusion would show a decline in performance upon the introduction of higher-ranking animals.
We addressed these hypotheses by presenting two comparable color discrimination problems to different hierarchical groupings of a rhesus monkey colony and comparing individual responses across problems and across social contexts. Based on matrilineal affiliation, we defined two social classes within the colony, a “dominant” class, comprising the higher-ranking families, and a “subordinate” class, comprising the lower-ranking families. During an initial learning or acquisition phase, we presented one problem to the whole colony (combined condition) and the other to the dominant and subordinate subgroups separately (split condition). During a subsequent testing phase, we used the same two discrimination problems but reversed the social contexts. Thus, the problem seen by all colony members simultaneously during acquisition was now presented independently to the two subgroups. Conversely, the problem seen separately was now presented to the whole troop. By comparing the performances of the two social classes in these different conditions it was possible to test critically the three hypotheses for explaining why subordinates perform poorly in mixed social groups.
Methods
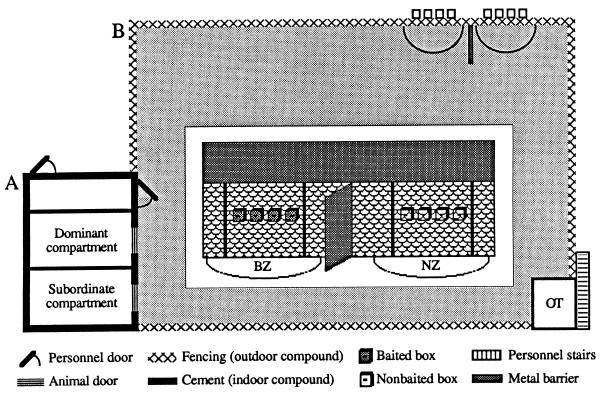
The subjects (n = 55) included all members of a long-established rhesus monkey colony, comprising 38 females and 17 males from 5 months to 18 years old. All animals were born and reared at the Yerkes Regional Primate Research Center, field station of Emory University in Atlanta, Georgia. The animals were housed in a large outdoor enclosure, with two attached indoor compartments (Fig. 1). The daily diet of Purina monkey chow and oranges was held until after behavioral trials, which were conducted from 8:00 a.m. to noon during the nonbreeding season. Animals were individually dye-marked with an aniline dye (no. 1 Nyanzol D. Flakes; N. Andover, MA). Yerkes is fully accredited by the American Association for the Accreditation of Laboratory Animal Care, all procedures were approved, and animal care met with institutional guidelines.
Figure 1.
Housing and color discrimination problem for a captive monkey colony. Two indoor quarters (A), each with access to the outdoor enclosure (1450 m2) and apparatus (top right corner) (B). Two sets of boxes, containing peanuts hidden in a sand-rock matrix, were attached to the outside of the fenced enclosure, 1 m off the ground, in two experimental zones (semicircles separated by metal barrier). (Inset) Monkey’s view of the apparatus under discrimination conditions in which sets were colored differently to signal food availability (baited ▩ vs. nonbaited □). Animals had to select between sets (visual discrimination), enter the appropriate zone, i.e., baited zone (BZ) or nonbaited zone (NZ), and search box contents (manual discrimination). Behavior was recorded and filmed from an observation tower (OT) that provided an unobstructed view of both zones. B is reproduced with permission from ref. 11 (copyright 1995, The Psychonomic Society).
The colony comprised members of 6 matrilines and 3 adult, nonnatal males that were introduced into the troop 4 yr before the study. The outcomes of dominance-submission encounters were used to assign rank to individuals. A hierarchy that produced the fewest reversals was then constructed, with the constraint that all members of a matriline be grouped together (12, 13). Based on the resulting matrix, we defined two social classes, a “dominant” class (n = 27), including the 3 highest-ranking matrilines and 1 nonnatal male, and a “subordinate” class (n = 28), including the 3 lowest-ranking matrilines and 2 nonnatal males. To manipulate the social context, the colony was trained to separate in the middle of their hierarchy, by sorting subgroup members into their appropriate indoor compartments (Fig. 1A). After 5 wk of training, the colony divided, on command, in under 2 min (13). Once the animals were trained on the separation technique and accustomed to the handling procedures, they were habituated to the apparatus.
The apparatus was a set of closed metal boxes (31 cm wide × 37 cm high × 21 cm deep), each with a central opening (8 × 8 cm) through which monkeys could insert an arm to search its contents. On habituation trials, all boxes were gray and each was baited with 15 peanuts and 5 stones hidden in gradually increasing depths of sand (to a maximum of 10 cm). With subgroups sequestered indoors (Fig. 1A), boxes were prepared and installed in two experimental zones that defined proximity (Fig. 1B). Depending on the social context of trials, each zone contained either two or four boxes to maintain equal subject-to-peanut ratios. All monkeys were released simultaneously for the combined condition, but each subgroup was released separately and in turn for the split condition. Thus, three 15-min trials were conducted daily, one per testing condition (one combined, one dominant subgroup, one subordinate subgroup). This testing schedule required two colony separations per day.
The monkey’s task was to approach the initially novel boxes (by entering an experimental zone and climbing the fence) and learn to dig for hidden peanuts (by manually discriminating between peanuts and similarly sized rocks). After each trial, animals were re-sequestered, boxes removed, and contents tallied. Split and combined trials were randomized and separated by 1 hr, during which time animals were reunited outdoors. Within the split condition, subgroup trials occurred sequentially, in random order, and separated by 10 min. Both subgroups were equally habituated to the apparatus and task after 27 days, i.e., 27 trials per condition (13).
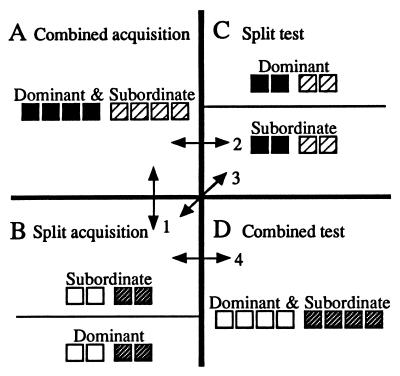
During subsequent discrimination trials, boxes were painted and the colors signaled whether or not food was available in the boxes (Fig. 1 Inset). Baited boxes contained 15 peanuts and 5 stones whereas nonbaited boxes contained 20 stones. During an initial acquisition phase, we presented two comparable discrimination problems daily. The first problem involved a choice between baited blue and nonbaited red boxes, and was presented in the combined context (Fig. 2A). The second problem involved a choice between baited yellow and nonbaited green boxes and was presented in the split context (Fig. 2B). In both cases, the location of baited boxes varied randomly between the left and right zones. Now the task involved visually discriminating between sets (i.e., learning to rely on the color cues). The duration of this phase was determined by an estimate of the monkey’s performance that could be obtained without detailed analysis of behavioral records. Accordingly, success was operationally defined, a priori, as significantly more subjects visiting baited boxes than nonbaited boxes in the first 30 s of five consecutive trials. Visits during this time period were tested against chance by using a two-tailed goodness of fit, G test (14). Subjects, regardless of rank, met criterion from days 18 to 28 in the combined context and, slightly later, from days 24 to 28 in the split context (P < 0.05). Therefore, the acquisition phase ran for 28 days.
Figure 2.
Experimental design. (A) Combined acquisition (the context in which each monkey was among all colony members) presented one discrimination problem, involving a choice between baited blue (■) vs. nonbaited red (▨) boxes. (B) Split acquisition (the context in which each monkey was among only the members of the same social class) presented the other discrimination problem, involving a choice between baited yellow (□) vs. nonbaited green ( ) boxes. In split testing (C) and combined testing (D), the discrimination problems remained the same, but the social contexts were reversed. Arrows 1–4 show the specific comparisons made to test the predictions regarding performance by subordinates.
Once acquisition criterion had been met on both problems, we began a testing phase in which the social context of each problem was reversed. Thus, the first problem was presented in the split context (Fig. 2C) and the second problem was presented in the combined context (Fig. 2D). This phase ran for six trials as only initial responses were necessary for resolution of the hypotheses. Each hypothesis generates unique predictions relating to the performance of subordinate animals in different social settings (combined vs. split context) or following different learning histories (acquisition vs. testing phase), and these predictions were assessed in four behavioral comparisons (arrows 1–4, Fig. 2).
Videotaped trials were scored for individual duration measures (time spent in zones and at boxes) and individual frequency measures (number of visits to zones and to boxes, manual searches, and peanut retrievals) (11, 12). We present two representative measures of discrimination performance, physical proximity (time spent in zones) and foraging success (peanut retrievals), for the last 6 acquisition trials and the 6 test trials. Each measure was handled differently, but both were analyzed in two stages. The first stage assessed individual performance differences by subgroup within experimental conditions (Fig. 2) and the second stage compared the performance of subordinates across experimental conditions (comparisons 1–4). Differences in proximity were assessed for each condition by using a two-factor analysis of variance (ANOVA, two subgroups × two zones) and planned comparisons between zones were made by using F tests for simple effects. For analyses of comparisons 1–4, we derived single mean difference scores (baited time − nonbaited time) per individual and compared values across all four conditions by using three-factor ANOVAs (two subgroups × two social contexts × two study phases). Planned comparisons between difference scores for members of the subordinate subgroup were made by using F tests for simple effects.
Total daily peanut retrievals by members of each subgroup in each condition were tested against chance with a two-tailed goodness of fit, G test (14). Comparisons 1–4 were analyzed as before, by using mean success per subgroup corrected for the number of peanuts available per social context. By using a finer grain definition of relative dominance status, each subgroup could be independently considered as a mixed social context, composed of members of a high-, middle-, and low-ranking matriline. To see whether rank-related patterns held within subgroups, retrieval frequencies were broken down by matriline for each social context of the acquisition phase. For the dominant subgroup, the relationship between matriline and foraging success was assessed by using a one-factor ANOVA and Kramer’s extension of multiple-range tests to group means with unequal numbers of replications (15); however, because of the disparity in family sizes within the subordinate subgroup, the zeta matriline (n = 2) was dropped and a t test was conducted for the remaining two matrilines.
Last, aggression occurring in the experimental zones was also monitored, and, because it involved dyadic interactions, we corrected aggression rates for the number of potential partners in any given social context (n − 1). Because there was no change in aggression across the study, the acquisition and testing phases were lumped, and corrected aggression rates were compared across the entire study by using a three-factor ANOVA (two subgroups × two social contexts × two zones).
Results
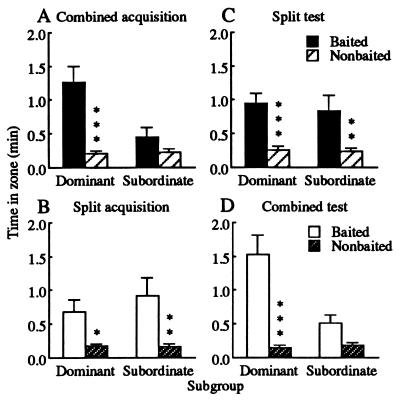
The mean time spent in proximity to baited boxes across acquisition trials 23–28 and test trials 1–6 is illustrated in Fig. 3. Subgroup members divided their time differently in the combined conditions (F > 10.0, df = 1/53, P < 0.005), with dominant animals spending more time in the baited zone (F > 32.1, df = 1/53, P < 0.001), but subordinates showing no preference (F < 2.3, df = 1/53, not significant, Fig. 3 A and D). By contrast, subgroups showed similar patterns of time partitioning in the split conditions (F < 0.7, df = 1/53, not significant), both preferring the baited zone (F > 5.0, df = 1/53, P < 0.05, Fig. 3 B and C).
Figure 3.
Mean ± SEM time spent in zones by subgroups across acquisition trials 23–28 and test trials 1–6: (A) combined acquisition; (B) split acquisition; (C) split testing; (D) combined testing. Both subgroups preferred the baited zone in split contexts, but only the dominant subgroup showed this preference in combined contexts (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Proximity difference scores were positive, reflecting a general preference for the baited zone, so comparisons 1–4 addressed differences in magnitude rather than direction. The subordinates’ preference for the baited zone was greater during split acquisition than during combined acquisition (comparison 1, F = 10.7, df = 1/53, P < 0.01), increased from combined acquisition to split testing (comparison 2, F = 5.3, df = 1/53, P < 0.025), was as strong during split acquisition as split testing (comparison 3, F = 0.9, df = 1/53, not significant), and decreased from split acquisition to combined testing (comparison 4, F = 7.2, df = 1/53, P < 0.01).
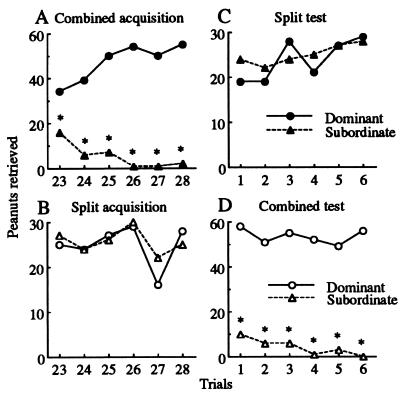
Foraging success on individual acquisition trials 23–28 and test trials 1–6 is illustrated in Fig. 4. Dominant animals foraged more successfully than subordinates on every trial in the combined conditions (G test, P < 0.05, Fig. 4 A and D), but subgroups were equally successful at retrieving peanuts in the split conditions (G test, not significant, Fig. 4 B and C).
Figure 4.
Total frequency of peanut retrievals by dominant (circles) and subordinate (triangles) subgroups per acquisition trials 23–28 and test trials 1–6: (A) combined acquisition; (B) split acquisition; (C) split testing; (D) combined testing. Dominant animals were more successful than subordinates in every combined trial (*, P < 0.05).
Using the corrected mean frequencies of peanut retrievals, we found that subordinates were more successful during split acquisition than combined acquisition (comparison 1, F = 17.2, df = 1/53, P < 0.001), more successful during split testing than combined acquisition (comparison 2, F = 15.9, df = 1/53, P < 0.001), equally successful during split acquisition and split testing (comparison 3, F = 0.03, df = 1/53, not significant), and less successful during combined testing than split acquisition (comparison 4, F = 19.8, df = 1/53, P < 0.001, Fig. 4).
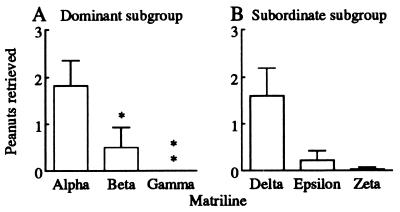
Within the dominant subgroup, there was a significant effect of matriline on foraging success in both acquisition conditions (F > 4.0, df = 2/24, P < 0.05). For instance, during split acquisition, the alpha matriline retrieved more peanuts than either the beta or gamma matrilines (Fig. 5A). This pattern was also apparent within the subordinate subgroup; however, foraging success by the delta matriline was not reliably greater than that of the epsilon matriline (t = 1.9, df = 24, P = 0.069, not significant, Fig. 5B). These results showed that rank-related differences in success between subgroups also were evident within subgroups.
Figure 5.
Mean ± SEM frequency of peanut retrievals by individual matrilines during split acquisition: (A) dominant subgroup; (B) subordinate subgroup. The alpha matriline was more successful than the beta and gamma matrilines (*, P < 0.05; **, P < 0.01). The same rank-related trend was evident for the subordinate subgroup, but was not statistically reliable.
The proximity (Fig. 3) and foraging success (Fig. 4) measures both led to the same conclusions for hypothesis testing. During combined acquisition, dominant animals demonstrated discrimination, whereas subordinates did not (Fig. 3A); dominant animals also retrieved more peanuts than did subordinates (Fig. 4A). By contrast, during split acquisition, both subgroups showed comparable discrimination (Fig. 3B) and equal foraging success (Fig. 4B). Because subordinates performed better in the split than the combined condition, we rejected the “cognitive disadvantage hypothesis.” Moreover, once dominant animals were removed during split testing, performance by subordinates immediately improved (Figs. 3C and 4C) and was indistinguishable from that shown during split acquisition (Figs. 3B and 4B). Because improvement was evident on the very first test day (Fig. 4C), inconsistent with any disruption in learning, we also rejected the “failure to learn hypothesis.”
Last, despite demonstrated excellence in segregated conditions (Figs. 3B and 4B), performance by subordinates decreased precipitously after reintroduction of dominant animals (Figs. 3D and 4D). All of these results were consistent with the “failure to perform hypothesis.”
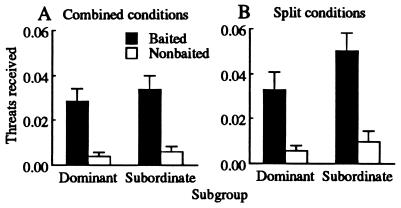
Given that poor performance reflected a failure to express learning, our final aim was to assess whether dominant monkeys suppressed subordinates through overt aggression. Threats occurred infrequently and performance (Figs. 3 and 4) was unrelated to the small amount of aggression received (Fig. 6). Overall, subordinates were threatened as often as were dominant animals (F = 4.0, df = 1/33, not significant). There were no differences in threat frequencies across social contexts (F = 2.3, df = 1/33, not significant) and no interaction between rank and social context (F = 1.0, df = 1/33, not significant). The only significant finding was that threats occurred more often in the baited zone (F = 55.1, df = 1/33, P < 0.001), reflecting the greater number of animals present. We therefore found that subordinates were not threatened more in the combined condition (Fig. 6A) in which their performance was the poorest (Fig. 3 A and D and Fig. 4 A and D). If anything, they tended to receive more threats in the split condition when all aggressors were other subordinates (Fig. 6B).
Figure 6.
Mean ± SEM frequency of threats received per trial for each subgroup: (A) combined conditions; (B) split conditions. Values are corrected for the number of potential partners in each social context (n − 1).
Discussion
Subordinate monkeys performed poorly on a simple discrimination task in the presence of higher-ranking animals, replicating previous findings for group-tested monkeys (12). Nevertheless, under segregated social conditions, subordinates were as capable of discrimination learning as were dominant animals. Moreover, the presence of dominant animals had not prevented subordinates from learning. Instead, our results confirm that meager performance by group-tested subordinates reflected a failure to express knowledge in the presence of dominant animals. Similar to early demonstrations of “latent” learning (1, 2), poor performance in low-ranking animals reflected factors other than cognitive ability, thereby underscoring the importance of distinguishing between performance and learning. Although our experimental procedure used concentrated food resources, a factor that could potentially increase competitive interactions (16), we found no evidence that aggression accounted for the deficits displayed by subordinates. We present a case of “playing dumb” in monkeys, similar to that in humans, in which a specific social setting promoted the voluntary inhibition of performance. Social context, therefore, can influence the extent to which performance underexpresses learning.
Social interactions often enhance or facilitate behavior (17), but they can also be inhibitory (18, 19). Social hierarchies are established through aggression or the threat of aggression (20, 21), but are as likely to be maintained through enforcement by high-ranking animals as through deference by low-ranking animals (22). Subtle cues emitted by dominant animals, and not easily detected by human observers, possibly affected the behavior of subordinates, but overt aggression was infrequent and subordinates were not obviously excluded. Most likely, subordinates adjusted their behavior, under changing social conditions, to minimize potential retaliation from dominant animals for transgressing social rules. Monkeys have representation of ordinal position (23), recognize individuals (24) or their associations with others (25, 26), and anticipate behavioral consequences (27). In this case, subordinates appeared to avoid socially difficult situations by inhibiting their behavior, although it meant missing out on desirable food items. Similar to the “subordinance hierarchy” proposed by Rowell (22) to explain the subordinates’ role in maintaining social hierarchies, our finding emphasizes the role individuals play in limiting their own behavior. Here, we find the subordinates’ comprehension of social position profoundly affected behavior apart from hierarchy maintenance.
Our study contributes to the growing literature on social learning (25, 28–30) by showing that social context modulates performance on simple learned tasks. Moreover, the influence exerted by the social milieu varies for different social classes. Within any grouping, individuals experience different social environments, some of which provide disincentives for expressing learning. Our results in monkeys could be analogous to circumstances in humans where competent individuals inhibit academic or athletic performance as the result of social status (5), gender (3, 4, 31), or racial influences (32). Eliminating social inhibition of performance has been cited as justification for sexually segregated instruction (33). Because performance is routinely used as the principal measure of learning, a situation that produces differential performance ultimately affects our perception of individual competence. Had we assessed the performance of our monkeys only in the whole colony, we might have concluded that subordinates were less intelligent than dominant animals. Varying the social context in which performance was measured led to a markedly different view of the subordinates’ cognitive ability. The capacity of social relations to inhibit performance shows that learning can be measured only in a context free of intimidation or social barriers.
Acknowledgments
We thank S. Brake, J. Eisler, M. Fox, K. Krebs, E. Nussbaum, P. Tannenbaum, and J. Sikes for technical assistance, and T. Gordon, H. Gouzoules, M. Tomasello, and M. Zeiler for helpful suggestions. This research was conducted as part of a doctoral degree program (C.M.D.) at Emory University. Research support was provided by a grant-in-aid from Σ Ξ and a dissertation fellowship from the Harry Frank Guggenheim Foundation for C.M.D., by a National Science Foundation grant (BNS86-07295) to K.W., and by a grant (RR-00165) from the National Institutes of Health, Division of Research and Resources to the Yerkes Regional Primate Research Center. Writing was completed under National Institutes of Health individual National Research Service Award (HD07684) to C.M.D.
References
- 1.Blodgett H C. Univ Calif Publ Psychol. 1929;4:113–134. [Google Scholar]
- 2.Tolman E C, Honzik C H. Univ Calif Publ Psychol. 1930;4:257–275. [Google Scholar]
- 3.Gove W R, Hughes M, Geerken M R. Soc Psychol Q. 1980;43:89–102. [PubMed] [Google Scholar]
- 4.Soltz D F. Psychol Rep. 1978;43:111–114. [Google Scholar]
- 5.Whyte W F. Street Corner Society. Chicago: Univ. Chicago Press; 1943. [Google Scholar]
- 6.Smuts B B, Cheney D L, Seyfarth R M, Wrangham R W, Struhsaker T T. Primate Societies. Chicago: Univ. Chicago Press; 1987. [Google Scholar]
- 7.Fobes J L, King J E. In: Primate Behavior. Fobes J L, King J E, editors. New York: Academic; 1982. pp. 289–326. [Google Scholar]
- 8.Lindburg D G. Primate Behav. 1971;2:1–106. [Google Scholar]
- 9.Harlow H H. J Gen Psychol. 1944;30:3–12. [Google Scholar]
- 10.Mackintosh N J. The Psychology of Animal Learning. New York: Academic; 1974. pp. 543–619. [Google Scholar]
- 11.Drea C M, Wallen K. Anim Learning Behav. 1995;23:1–8. [Google Scholar]
- 12.Drea C M. J Comp Psychol. 1998;112:170–182. doi: 10.1037/0735-7036.112.2.170. [DOI] [PubMed] [Google Scholar]
- 13.Drea C M. Am J Primatol. 1998;44:205–214. doi: 10.1002/(SICI)1098-2345(1998)44:3<205::AID-AJP3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Sokal R R, Rohlf J F. Biometry. 2nd Ed. San Francisco: Freeman; 1981. pp. 691–778. [Google Scholar]
- 15.Kramer C Y. Biometrics. 1956;12:307–310. [Google Scholar]
- 16.Isbell L A. Behavioral Ecol. 1991;2:143–155. [Google Scholar]
- 17.Zajonc R B. Science. 1965;149:269–274. doi: 10.1126/science.149.3681.269. [DOI] [PubMed] [Google Scholar]
- 18.Beauchamp G, Kacelnik A. Anim Behav. 1991;41:247–253. [Google Scholar]
- 19.Lefebvre L, Helder R. Behav Proc. 1997;40:201–207. doi: 10.1016/s0376-6357(97)00783-3. [DOI] [PubMed] [Google Scholar]
- 20.Ehardt C L, Bernstein I S. In: Coalitions and Alliances in Humans and Other Animals. Harcourt A H, De Waal F B M, editors. Oxford: Oxford Univ. Press; 1992. pp. 83–111. [Google Scholar]
- 21.Maestripieri D. J Comp Psychol. 1996;110:402–405. doi: 10.1037/0735-7036.110.4.402. [DOI] [PubMed] [Google Scholar]
- 22.Rowell T E. Behav Biol. 1974;11:131–154. doi: 10.1016/s0091-6773(74)90289-2. [DOI] [PubMed] [Google Scholar]
- 23.D’Amato M R, Colombo M. J Comp Psychol. 1988;103:252–261. doi: 10.1037/0735-7036.103.3.252. [DOI] [PubMed] [Google Scholar]
- 24.Dittrich W. Behav Process. 1994;33:139–154. doi: 10.1016/0376-6357(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 25.Cheney D L, Seyfarth R M. How Monkeys See the World. Chicago: Univ. Chicago Press; 1990. [Google Scholar]
- 26.Dasser V. Anim Behav. 1988;36:225–230. [Google Scholar]
- 27.Humphrey N K. In: Growing Points in Ethology. Bateson P P G, Hinde R A, editors. Cambridge, UK: Cambridge Univ. Press; 1976. pp. 303–317. [Google Scholar]
- 28.Byrne R W, Whiten A. Machiavellian Intelligence. Oxford: Clarendon; 1988. [Google Scholar]
- 29.Heyes C M, Galef B G., Jr . Social Learning in Animals: The Roots of Culture. San Diego: Academic; 1996. [Google Scholar]
- 30.Zentall T R, Galef B G., Jr . Social Learning: Psychological and Biological Perspectives. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 31.LaNoue J B, Curtis R C. Psychol Women Q. 1985;9:337–356. [Google Scholar]
- 32.Steele C M. Am Psychol. 1997;52:613–629. doi: 10.1037//0003-066x.52.6.613. [DOI] [PubMed] [Google Scholar]
- 33.Sadker M, Sadker D. Failing at Fairness: How America’s Schools Cheat Girls. New York: Charles Scribner’s Sons; 1994. [Google Scholar]