Abstract
Estrogen deficiency caused by ovariectomy (OVX) results in a marked bone loss due to stimulated bone resorption by osteoclasts. During our investigations of the pathogenesis of bone loss in estrogen deficiency, we found that OVX selectively stimulates B-lymphopoiesis which results in marked accumulation of B220-positive pre-B cells in mouse bone marrow. To examine the possible correlation between stimulated B-lymphopoiesis and bone loss, 8-week-old female mice were treated with interleukin (IL) 7, which stimulates B-lymphopoiesis in bone marrow. We also examined bone mass in IL-7 receptor-knockout mice that exhibit marked suppression of B-lymphopoiesis in the bone marrow. The increased B-lymphopoiesis induced by IL-7 administration resulted in marked bone loss by stimulation of osteoclastic bone resorption in mice with intact ovarian function. The changes in both B-lymphopoiesis and bone mass in IL-7-treated female mice were similar to those in age-matched OVX mice. In contrast, the trabecular bone volume of the femur was greatly increased in both female and male IL-7 receptor-knockout mice when compared with the respective wild-type and heterozygous littermates. These results show that the perturbation of B-lymphopoiesis in the bone marrow is closely linked to the change in bone mass. We propose here that the increased B-lymphopoiesis due to estrogen deficiency is involved in the mechanism of stimulated bone resorption.
Estrogen deficiency results in a marked bone loss due to increased bone resorption by osteoclasts. The pathologic bone loss induced by menopause can be restored by estrogen replacement, but the mechanism underlying this phenomenon remains unknown. There is a close relation between bone remodeling and hemopoiesis in bone marrow. We have reported that an estrogen deficiency caused by ovariectomy (OVX) selectively stimulates B-lymphopoiesis, resulting in marked accumulation of pre-B cells in mouse bone marrow (1). In contrast, B-lymphopoiesis is significantly reduced in bone marrow of pregnant mice with high plasma levels of estrogen (2). Like estrogen deficiency, androgen deficiency also results in bone loss and enhanced B-lymphopoiesis in bone marrow in male mice (3). These findings suggest that sex steroids somehow regulate growth and differentiation of B cell precursors. However, whether the increased B-lymphopoiesis by sex steroid deficiency is related to stimulated bone resorption is not known.
There is a close relationship between bone remodeling and hemopoiesis in bone marrow (4, 5). Various cytokines such as interleukin (IL) 1, IL-4, IL-6, IL-7, IL-11, and IL-13 are involved in the growth and differentiation of hemopoietic cells. Most of the cytokines are also involved in bone remodeling (6–10). Recent studies indicated the possible involvement of bone-resorbing cytokines such as IL-1, IL-6, and tumor necrosis factor α (TNF-α) in bone loss due to estrogen deficiency. Pacifici and coworkers (6, 11, 12) reported that estrogen inhibited IL-1 secretion by monocytes, and that an estrogen deficiency allowed monocytes to secrete more IL-1 and tumor necrosis factor α. Manolagas and coworkers (13, 14), on the other hand, reported that IL-6 was involved in the stimulation of bone resorption induced by estrogen deficiency. We reported that the enhanced bone resorption in OVX mice was due to cooperative effects of several local factors including IL-1, IL-6, and prostaglandins (15). These results suggest that the bone-resorbing cytokines are involved in the stimulated bone resorption due to estrogen deficiency.
It is well established that stromal cells play a key role in hemopoiesis in bone marrow (16–18). Adhesion molecules such as vascular cell adhesion molecule 1 expressed on bone marrow stromal cells is involved in the development of B cells, in particular in an early stage of B cell differentiation (18). In addition, bone marrow stromal cells support the development of B lymphocytes by a mechanism involving various growth factors including IL-7, which is produced by stromal cells. In both IL-7- and IL-7 receptor-deficient mice generated by the respective gene inactivation, the number of B lymphocytes is markedly reduced (19, 20). This suggests that the impairment of B-lymphopoiesis can be induced by the depletion of IL-7 signals in animals with intact ovarian function.
Estrogen suppresses both B-lymphopoiesis and bone resorption, and its deficiency stimulates both of them. In the present study, female mice with intact ovarian function were given IL-7 twice a day for 20 days to examine the possible correlation between stimulated B-lymphopoiesis and bone loss. We also examined bone mass in IL-7 receptor-knockout mice that exhibit marked suppression of B-lymphopoiesis in bone marrow. The increased B-lymphopoiesis induced by IL-7 administration resulted in marked bone loss in mice with intact ovarian function. In contrast, the trabecular bone volume of the femur was greatly increased in IL-7 receptor-knockout mice when compared with the wild-type littermates. We propose here that the increased B-lymphopoiesis due to estrogen deficiency is involved in the mechanism of stimulated bone resorption.
MATERIALS AND METHODS
Animals.
Eight-week-old female mice of the ddy strain were obtained from Shizuoka Laboratory Animal Center (Shizuoka, Japan). IL-7 receptor-knockout mice were generated using an IL-7 receptor disruption vector by replacing exon 2 of the IL-7 receptor gene with a neomycin-resistant cassette (19). E14-1 embryonic stem cells targeted with the IL-7 receptor gene were injected into blastocysts of C57BL/6 mice (19). Chimeric animals were bred with C57BL/6 mice, and the male and female mice of the F3 generation were used for the present study. Mice were maintained under specific pathogen-free conditions.
Study Protocols.
Female mice of the ddy strain were given subcutaneously 500 ng of recombinant mouse IL-7 dissolved in PBS containing 0.1% BSA twice a day for 20 days. Control mice were treated with vehicle solution (PBS/0.1% BSA) according to the same protocol. In another series of experiments, mice received either sham-operation or OVX and were fed a laboratory chow containing 1.15% calcium and 0.88% phosphorous for 4 weeks after operation. Then the uterine weight was measured, and the right and left tibiae were removed to prepare bone marrow cells. Urine samples were collected, and the excretion of pyridinoline measured by an ELISA kit (Metra Biosystems, Mountain View, CA) was expressed as pyridinoline/creatinine (nM/mM). The remaining right and left femora were used for radiographic analysis. Finally, the left femur was used for histological analysis, and the right was used to measure bone mineral density (BMD) and scan bone architecture by micro-computed tomography (μCT).
Flow Cytometric Analysis.
Bone marrow cells were stained with fluorescein isothiocyanate (FITC)-conjugated B220 (RA3-6B2; PharMingen) or Gr-1 (RB6-8C5, PharMingen), and analyzed on a flow cytometer (FACScalibur, Becton Dickinson). For two-color analysis, cells were stained with FITC-B220 and phycoerythrin-labeled anti-IgM μ-chain antibody (Tago). As a negative control, FITC rat IgG was used.
Histological and Radiographic Analyses of Cancellous Bone.
Radiographic analysis of the femora was performed using a soft x-ray system. BMD of the femur was measured by dual x-ray absorptiometry (model DCS-600R; Aloka, Tokyo). The bone mineral content (BMC) of mouse femur was closely correlated with the ash weight (r = 0.978). BMD was calculated by BMC and the measured area. The scanned area was divided into three parts: proximal metaphysis, diaphysis, and distal metaphysis. For histomorphometry, the distal metaphysis of the left femur was fixed with 70% ethanol and embedded in glycol methacrylate, and 3-μm-thick sections were stained for tartrate-resistant acid phosphatase (TRAP) and examined under a light microscope. The mean number of osteoclasts in each millimeter of the trabecular bone surface was determined in the area (1.08 mm2) of the secondary spongiosa of the distal metaphysis. In the same area, bone resorption surface, expressed as percent erosion surface/bone surface, was also determined according to the method of Parfitt et al. (21).
Three-Dimensional Analysis by μCT.
The femoral cancellous bone of the distal metaphysis was analyzed three-dimensionally by the μCT system (μCT-20; Scanco Medical, Zurich), as reported by Ruegsegger et al. (22). The mean tissue volume of the scanned area was 0.44 mm3 in the trabecular bone of the femoral distal metaphysis, which did not include any cortical bone. Using two-dimensional data from 200 scanned slices, three-dimensional analysis was performed to calculate morphometric indices such as bone volume density [bone volume (BV)/tissue volume (TV)], trabecular thickness [Tb.Th = 2 × BV/bone surface (BS)], trabecular number [Tb.N = (BV/TV)/Tb.Th], and trabecular separation [Tb.Sp = (1/Tb.N) − Tb.Th]. The indices were calculated by the same algorithm as reported by Parfitt et al. (21).
RESULTS AND DISCUSSION
Comparison of Stimulation of B-Lymphopoiesis Between IL-7 Treatment and Estrogen Deficiency in Mice.
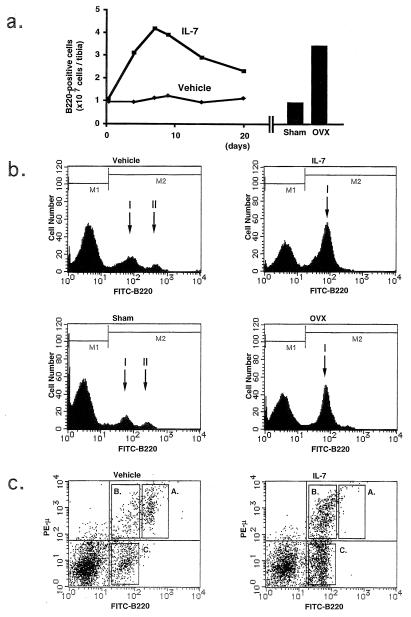
IL-7 is a growth factor responsible for early B cell differentiation, which selectively supports the growth of pre-B cells in vitro and in vivo (17, 23, 24). When female mice were treated with IL-7, the number of B220-positive B cells was selectively increased on days 4–20 (Fig. 1a Left). Other hemopoietic cells including Gr-1-positive granulocytes were not significantly affected by the treatment with IL-7 (data not shown). The number of B220-positive cells in the IL-7-treated mice reached 48% of the total nucleated cells in the bone marrow, which was similar to that in OVX mice 2 weeks after operation (Fig. 1a Right). In the vehicle-treated mice, B220-positive cells were separated into two subpopulations, peak I (B220low) and peak II (B220high) (Fig. 1b). Peak I was weakly stained with anti-B220 antibody, whereas peak II showed intense staining. B220high cells were mature B cells, and B220low cells consisted of immature B cells including pre-B cells in mouse bone marrow (25). In the IL-7-treated mice, most of the B220-positive cells appeared in peak I. The proportion of B220-positive cells in the IL-7-treated mice was similar to that in OVX mice (Fig. 1b). Fig. 1c shows a two-color analysis of bone marrow cells using FITC-labeled B220 and phycoerythrin-labeled μ-chain of IgM in vehicle- and IL-7-treated mice. In vehicle-treated normal mice, most of the B220high cells were μ-positive (B220high⋅μ+: quadrant A), whereas 70% of the B220low cells were negative for μ-chain (B220low⋅μ−: quadrant C) and the rest (30%) of the B220low cells were positive for μ-chain (B220low⋅μ+: quadrant B). The overall percentages of quadrants A, B, and C were 8.1%, 5.2%, and 13.7%, respectively. In the IL-7-treated mice, however, the population of quadrant A disappeared almost completely, whereas those of both quadrants B and C were markedly increased. The overall percentages of quadrants A, B, and C were 0.7%, 14.3%, and 29.1%, respectively. The ratio of quadrant B/C was 7:3 and was not changed by the IL-7 treatment. Therefore, it is concluded that the treatment with IL-7 and estrogen deficiency commonly induce B-lymphopoiesis, resulting in accumulation of pre-B cells in mouse bone marrow. The uterine weight was markedly reduced in OVX mice, whereas IL-7 treatment had no effect (data not shown), indicating that the IL-7-induced B-lymphopoiesis is not related to ovarian function.
Figure 1.
Comparison of the increased B-lymphopoiesis in bone marrow between the IL-7-treated female mice and OVX mice. (a) The number of B220-positive bone marrow cells was counted by flow cytometric analysis on days 4–20 in the vehicle- and IL-7-treated female mice (Left). The number of B220-positive cells in bone marrow was also counted in sham-operated and OVX mice 2 weeks after operation (Right). (b) Results of the flow cytometric analysis of B220-positive bone marrow cells were compared between the vehicle- and IL-7-treated female mice on day 14, and between the sham-operated and OVX mice 2 weeks after operation. Note that the proportion of B220-positive cells was markedly increased in the IL-7-treated mice compared with the vehicle-treated controls (% of positive cells shown in M2: vehicle, 27%; IL-7, 48%), and in the OVX mice mice compared with the sham-operated controls (sham, 28%; OVX, 49%). (c) Two-color immunofluorescence analysis was performed with bone marrow cells collected from the vehicle- and the IL-7-treated female mice on day 14 using FITC-B220 and phycoerythrin-anti-IgM μ-chain (PE-μ).
IL-7 Treatment Induces Bone Loss in Mice, as Estrogen Deficiency Does.
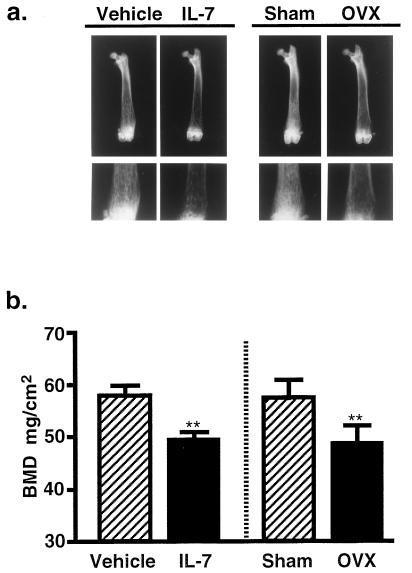
To examine the relationship between the perturbation of B-lymphopoiesis and the change in bone mass, the density of mineralized cancellous bone was analyzed using femora of IL-7-treated female mice. Radiographic analysis revealed that the mineralized cancellous bone was significantly decreased, especially in the distal metaphysis of the femur in the IL-7-treated mice (Fig. 2a). The BMD was significantly reduced in the femoral metaphysis by the treatment with IL-7, compared with that in the vehicle-treated mice (Fig. 2b). The degree of bone loss induced by IL-7 was quantitatively similar to that induced by estrogen deficiency (Fig. 2b). The BMD in the femoral diaphysis was not changed appreciably by either IL-7 treatment or estrogen deficiency (data not shown). The urinary excretion of pyridinoline was significantly elevated by the treatment with IL-7 on day 20 [vehicle, 281 ± 36 nM/mM (pyridinoline/creatinine); IL-7, 671 ± 150 nM/mM]. This suggests that the bone loss by the treatment with IL-7 is due to increased bone resorption.
Figure 2.
Increased B-lymphopoiesis by IL-7 treatment induces bone loss, as estrogen deficiency does. (a) X-ray analysis of femora collected from the vehicle- and IL-7-treated female mice on day 20, and from the sham-operated and OVX mice 4 weeks after operation. Note that marked bone loss occurred in the distal metaphysis of the femoral cancellous bone in the IL-7-treated mice compared with the vehicle-treated controls. The degree of bone loss due to IL-7 treatment was similar to that in the OVX mice. (Lower) High power views of the respective distal metaphyses (shown in the Upper panels). (b) BMD was measured in the femoral distal metaphysis of mice shown in a Lower. Data are expressed as means ± SEM of nine animals. ∗∗, P < 0.01.
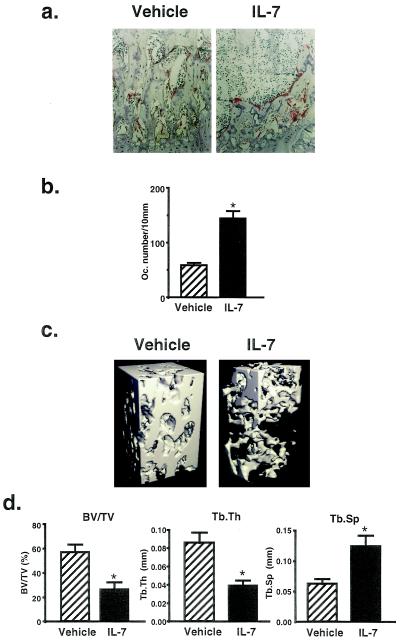
Histological analysis of femoral sections showed a marked loss of cancellous bone in the IL-7-treated mice, compared with the vehicle-treated controls (Fig. 3a). The number of TRAP-positive osteoclasts was markedly increased in the secondary spongiosa of the distal metaphysis in the IL-7 treated mice (Fig. 3b). In the same area, bone resorption surface expressed as percent erosion surface/bone surface was also increased in IL-7-treated mice (vehicle, 22.9 ± 1.3%; IL-7, 38.2 ± 1.9%). These results clearly show that the treatment with IL-7 stimulates osteoclastic bone resorption in vivo. To confirm the changes in cancellous bone evoked by IL-7 treatment, bone morphometric analysis was performed using μCT in the trabecular bone of the distal femoral metaphysis. Recent studies have shown that μCT scanning and its three-dimensional analysis are extremely useful for imaging and quantification of the volume of trabecular bone (22). The scan was focused on the area of trabecular bone and bone marrow in the metaphysis, and the morphometric indices were calculated. The distinct plate-like structure of bone could be easily seen in the vehicle-treated mice, and the connecting rods were well maintained (Fig. 3c Left). In the IL-7-treated mice, however, the plate-like structure was partially destroyed and many of the connecting rods were missing (Fig. 3c Right). Treatment with IL-7 markedly reduced the bone volume density (BV/TV) and trabecular thickness (Tb.Th), and enhanced the trabecular separation (Tb.Sp) as determined by μCT analysis (Fig. 3d). The increase in Tb.Sp indicated that the osteoclastic bone resorption was stimulated, which resulted in enhanced separation of trabecular rods. The IL-7-induced bone loss detected by μCT analysis (Fig. 3c) and the bone morphometry indices (Fig. 3d) were consistent with the histological findings shown in Fig. 3 a and b. In addition to the radiographic data shown in Fig. 2, μCT analysis revealed that increased bone resorption in the IL-7-treated mice occurred on day 20, similarly to the OVX mice (data not shown). We reported previously that IL-7 did not stimulate bone resorption in fetal mouse long bones in organ culture (9). Therefore, it is suggested that the change in bone marrow microenvironment accompanying the increased B-lymphopoiesis stimulates osteoclastic bone resorption, resulting in bone loss in vivo.
Figure 3.
Histological analysis and μCT scanning of trabecular bone collected from the vehicle- and IL-7-treated female mice on day 20. (a) Histological sections of femoral metaphysis stained for TRAP. Note that osteoclastic bone resorption was stimulated and loss of trabecular bone occurred in the IL-7-treated mice. (b) The number of osteoclasts (Oc. number) present in the trabecular bone surface was counted in the area (1.08 mm2) of the secondary spongiosa of femoral metaphysis using TRAP-stained sections from the vehicle- and IL-7-treated mice. The results are expressed as means ± SEM of nine animals. ∗, P < 0.01. (c) The three-dimensional trabecular bone architecture of femoral metaphysis collected from the vehicle- and IL-7-treated mice. Note that the plate-like structure was markedly reduced and the connecting rods of trabecular bone were lost in the IL-7-treated mice. (d) Bone morphometry by three-dimensional analysis using μCT shown in c. Morphometric indices were determined as described in Materials and Methods. BV/TV, bone volume density (bone volume/tissue volume); Tb.Sp, trabecular separation; Tb.Th, trabecular thickness. Data are expressed as means ± SEM of nine animals. ∗, P < 0.01.
Increased Cancellous Bone Volume in IL-7 Receptor-Knockout Mice.
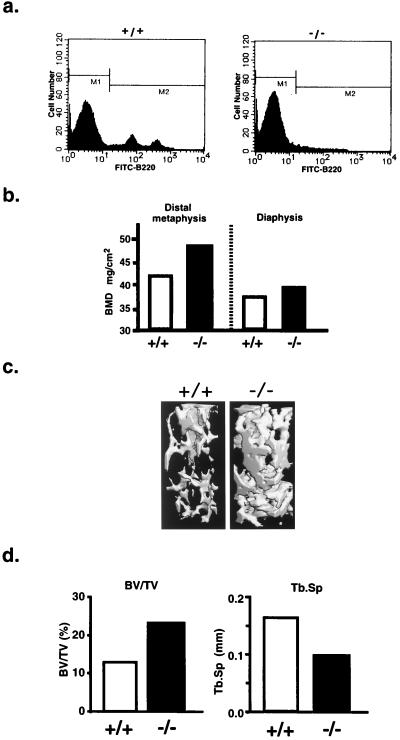
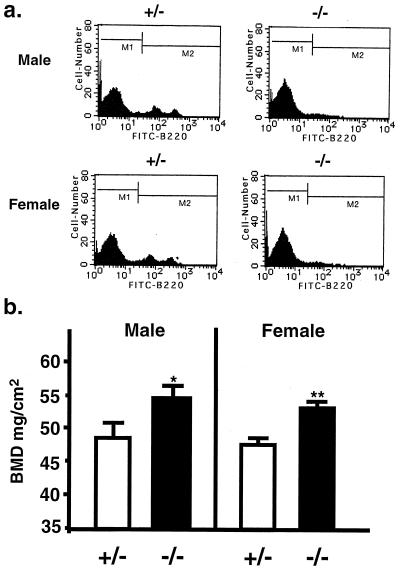
To examine further the effects of perturbation of B-lymphopoiesis on bone mass, we analyzed bone mineral density of 22-week-old female IL-7 receptor-knockout mice. The number of B-lymphocytes in bone marrow was markedly reduced in the IL-7 receptor-knockout mice (Fig. 4a), which was consistent with the previous reports by us (19) and other investigators (20). A higher BMD was detected in the distal metaphysis of the femora collected from the IL-7 receptor-knockout mice compared with the age-matched wild-type littermates (Fig. 4b). The BMD in the femoral diaphysis from the IL-7 receptor-knockout mice was similar to that from the wild-type controls (Fig. 4b). Three-dimensional analysis of the μCT scanning results clearly showed a marked increase in the volume of cancellous bone in the distal metaphysis of the femora collected from the IL-7 receptor-knockout mice (Fig. 4c). The plate-like structure and connecting rods of trabecular bone were more prominent in the IL-7 receptor-knockout mice than in the wild-type littermates. The trabecular bone volume density (BV/TV) was increased (2-fold), whereas the trabecular separation (Tb.Sp) was reduced in the knockout mice, compared with those in the wild-type controls (Fig. 4d). The value of BV/TV of the 22-week-old wild-type mice (Fig. 4d) was lower than that of 11-week-old vehicle-treated controls in the experiments shown in Fig. 3d. This may have been due to differences in the age and strain of mice used. Using μCT analysis, it has been reported that the BV/TV in the trabecular bone is markedly reduced by aging (22). The relationship between the age-related bone changes and the B-lymphopoiesis-induced bone changes should be examined in more detail in future.
Figure 4.
Increased bone volume and suppressed B-lymphopoiesis in IL-7 receptor-knockout female mice. B-lymphopoiesis and bone volume were compared between 22-week-old IL-7 receptor-knockout female mice (−/−) and the age-matched wild-type female littermates (+/+). (a) Flow cytometry of B220-positive cells in bone marrow. Note that B-lymphopoiesis was markedly suppressed in the IL-7 receptor-knockout female mice. (b) BMD in the distal metaphysis and diaphysis of femur. (c) Three-dimensional architecture of trabecular bone of femoral metaphysis. Note that the plate-like structure and the connecting rods of trabecular bone were markedly increased in the IL-7 receptor-knockout mice. (d) Bone histomorphometry in the IL-7 receptor-knockout mice. Morphometric indices were determined by three-dimensional analysis of μCT scanning results. Note that the cancellous bone volume was markedly increased, whereas the trabecular separation was reduced in the IL-7 receptor-knockout mice, compared with the age-matched wild-type littermates. Data are expressed as means of two littermates for IL-7 receptor-knockout mice and the age-matched wild-type controls, respectively. BV/TV, bone volume density (bone volume/tissue volume); Tb.Sp, trabecular separation.
To compare B-lymphopoiesis and bone volume between female and male IL-7 receptor-knockout mice, we analyzed the proportion of B220-positive cells in bone marrow and the femoral BMD using 14-week-old IL-7 receptor-knockout mice and their age-matched heterozygous mice. Both female and male heterozygous mice possessed a normal range of B-lymphocytes in bone marrow, the range of which was similar to that in the wild-type mice shown in Fig. 4a (Fig. 5a). Not only female but also male IL-7 receptor-knockout mice had a higher BMD than the heterozygous mice (Fig. 5b). It is therefore likely that the perturbation of B-lymphopoiesis is closely related with bone mass in both sexes. Treatment of normal female mice with a high dose of estrogen induced marked suppression of B-lymphopoiesis in bone marrow and an increase in bone mass by inhibiting osteoclastic bone resorption (1, 26). These results suggest that the increased B-lymphopoiesis due to estrogen deficiency is involved in the mechanism of stimulated bone resorption.
Figure 5.
Comparison of B-lymphopoiesis and bone volume between female and male IL-7 receptor-knockout mice. B-lymphopoiesis and bone volume were compared between 14-week-old female and male IL-7 receptor-knockout mice (−/−) and their respective age-matched heterozygous littermates (+/−). (a) Flow cytometry of B220-positive cells in bone marrow. In both sexes, the proportion of B220-positive B cells in the +/− mice was similar to that in the wild-type female mice shown in Fig. 4a. Note that B-lymphopoiesis was markedly suppressed in both male and female −/− mice. (b) Using male and female mice, BMD in the distal metaphysis of femur was compared. Data are expressed as the means ± SEM of five to seven animals. ∗, P < 0.05; ∗∗, P < 0.01.
Possible Mechanisms of the Relationship Between Increased B-Lymphopoiesis and Bone Loss.
Estrogen deficiency induces not only bone resorption but also B-lymphopoiesis in female mice (1, 27). Androgen deficiency also induces both bone loss and B-lymphopoiesis in male mice (3). The mechanism of the increased B-lymphopoiesis due to sex steroid deficiency is not known at present, but it is likely that the increased B-lymphopoiesis is involved in osteoclastic bone resorption, leading to bone loss due to sex steroid deficiency. To confirm this hypothesis, the effect of OVX on B-lymphopoiesis and bone mass has to be examined in IL-7 receptor-knockout mice. This experiment is under way in our laboratory.
Several lines of evidence have indicated that there is a possible relationship between hemopoiesis and bone remodeling (4, 5, 12, 14, 15). Vascular cell adhesion molecule 1, an adhesion molecule expressed on stromal cells, is essential for not only B cell differentiation but also osteoclastogenesis in bone marrow (18, 28). This suggests that there is a close relationship between B-lymphopoiesis and osteoclastogenesis. In addition, cell-to-cell interactions between hemopoietic cells and bone marrow stromal cells could trigger production of bone-resorbing cytokines by stromal cells. Adhesion of multiple myeloma cells to stromal cells triggers IL-6 secretion by stromal cells (29, 30), which may be involved in increased bone resorption in multiple myeloma. Adhesion of activated T cells induces marked production of bone-resorbing cytokines such as IL-1 and IL-6 by osteoblasts (31). Similarly, adhesion of pre-B lymphocytes to bone marrow stromal cells could induce the production of bone-resorbing cytokines including IL-6 (32), suggesting that the accumulation of pre-B cells in bone marrow could trigger the induction of osteoclast formation and bone resorption of trabecular bone in the metaphysis of long bones. We have reported that bone marrow supernatants collected from OVX mice stimulate bone resorption in vitro, and that the bone-resorbing activity was suppressed by neutralizing antibodies against IL-1, IL-6, and IL-6 receptors (15). Taken together, the changes of cytokines such as IL-1, IL-6, tumor necrosis factor α, and IL-7 have to be examined in more detail to elucidate the mechanism of bone resorption associated with increased B-lymphopoiesis.
The present study indicates that the enhanced B-lymphopoiesis by IL-7 administration markedly stimulates bone resorption. A similar phenomenon is recognized in bone loss due to estrogen deficiency. Further studies are needed to confirm that the increased B-lymphopoiesis triggers off bone loss in estrogen deficiency. Determination of the mechanism of increased bone resorption induced by accumulated pre-B cells in bone marrow could lead to understanding not only of the relationship between hemopoiesis and bone metabolism, but also of the pathogenesis of bone loss in postmenopausal osteoporosis. It is important to examine B-lymphopoiesis in postmenopausal women whether bone marrow microenvironment accompanying increased B-lymphopoiesis is involved in bone loss due to sex steroid deficiency in humans as well.
Acknowledgments
We thank Dr. T. Sato (Daiichi Pharmaceutical Co., Ltd.) for measuring the bone mineral density, and Ms. Y. Nagai (Kureha Chemical Co.) for her helpful assistance in histological analysis. We also thank Dr. T. Sudo (Toray Industries) for his helpful discussion. This work was supported by the Grants-in-Aids (08407060 for T.S., and 08457493 for C.M.) from the Ministry of Science, Education, and Culture of Japan.
ABBREVIATIONS
- IL
interleukin
- OVX
ovariectomy
- BMD
bone mineral density
- μCT
micro-computed tomography
- FITC
fluorescein isothiocyanate
- TRAP
tartrate-resistant acid phosphatase
References
- 1.Masuzawa T, Miyaura C, Onoe Y, Kusano K, Ohta H, Nozawa S, Suda T. J Clin Invest. 1994;94:1090–1097. doi: 10.1172/JCI117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medina K L, Smithson G, Kincade P W. J Exp Med. 1993;178:1507–1515. doi: 10.1084/jem.178.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson C A, Mrose S A, Thomas D W. Blood. 1995;85:1535–1539. [PubMed] [Google Scholar]
- 4.Mundy G R, Roodman G D. Bone Miner Res. 1987;5:209–281. [Google Scholar]
- 5.Suda T, Takahashi N, Martin T J. Endocr Rev. 1995;4:266–270. [Google Scholar]
- 6.Kitazawa R, Kimble R B, Vannice J L, Kung V T, Pacifici R. J Clin Invest. 1994;94:2397–2406. doi: 10.1172/JCI117606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishimi Y, Miyaura C, Jin C H, Akatsu T, Abe E, Nakamura Y, Yamaguchi A, Yoshiki S, Matsuda T, Hirano T, Kishimoto T, Suda T. J Immunol. 1990;145:3297–3303. [PubMed] [Google Scholar]
- 8.Girasole G, Passeri G, Jilka R L, Manolagas S C. J Clin Invest. 1994;93:1516–1524. doi: 10.1172/JCI117130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onoe Y, Miyaura C, Kaminakayashiki T, Nagai Y, Noguchi K, Chen Q R, Seo H, Ohta H, Nozawa S, Kudo I, Suda T. J Immunol. 1996;156:758–764. [PubMed] [Google Scholar]
- 10.Tamura T, Udagawa N, Takahashi N, Miyaura C, Tanaka S, Yamada Y, Koishihara Y, Ohsugi Y, Kumaki K, Taga T, Kishimoto T, Suda T. Proc Natl Acad Sci USA. 1993;90:11924–11928. doi: 10.1073/pnas.90.24.11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacifici R, Brown C, Puscheck E, Friedrick E, Slatopolsky E, Maggio D, McCracken R, Avioli L V. Proc Natl Acad Sci USA. 1991;88:5134–5138. doi: 10.1073/pnas.88.12.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimble R B, Vannice J L, Bloedow D C, Thompson R C, Hopfer W, Kung V T, Brownfield C, Pacifici R. J Clin Invest. 1994;93:1959–1967. doi: 10.1172/JCI117187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girasole G, Jilka R L, Passeri G, Boswell S, Boder G, Williams D C, Manolagas S C. J Clin Invest. 1992;89:883–891. doi: 10.1172/JCI115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jilka R L, Hangoc G, Girasole G, Passeri G, Williams D C, Abrams J S, Boyce B, Broxmeyer H, Manolagas S C. Science. 1992;257:88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- 15.Miyaura C, Kusano K, Masuzawa T, Chaki O, Onoe Y, Aoyagi M, Sasaki T, Tamura T, Koishihara Y, Ohsugi Y, Suda T. J Bone Miner Res. 1995;10:1365–1373. doi: 10.1002/jbmr.5650100914. [DOI] [PubMed] [Google Scholar]
- 16.Kincade P W. Adv Immunol. 1987;41:181–267. doi: 10.1016/s0065-2776(08)60032-2. [DOI] [PubMed] [Google Scholar]
- 17.Namen A E, Lupton S, Hjerrild K, Wignall J, Mochizuki D Y, Schmierer A, Mosely B, March C J, Urdal D, Gillis S, Cosman D, Goodwin R G. Nature (London) 1988;333:571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- 18.Miyake K, Medina K, Ishihara K, Kimoto M, Auerbach R, Kincade P W. J Cell Biol. 1991;114:557–565. doi: 10.1083/jcb.114.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maki K, Sunaga S, Komagata Y, Kodaira Y, Mabuchi A, Karasuyama H, Yokomuro K, Miyazaki J, Ikuta K. Proc Natl Acad Sci USA. 1996;93:7172–7177. doi: 10.1073/pnas.93.14.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peschon J J, Morrissey P J, Grabstein K H, Ramsdell F J, Maraskovsky E, Gliniak B C, Park L S, Ziegler S F, Williams D E, Ware C B, Meyer J D, Davison B L. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parfitt A M, Mathews C H E, Villanueva A R, Kleerekoper M, Frame B, Rao D S. J Clin Invest. 1983;72:1396–1409. doi: 10.1172/JCI111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruegsegger P, Koller B, Muller R. Calcif Tissue Int. 1996;58:24–29. doi: 10.1007/BF02509542. [DOI] [PubMed] [Google Scholar]
- 23.Lee G, Namen A E, Gillis S, Ellingsworth L R, Kincade P W. J Immunol. 1989;142:3875–3883. [PubMed] [Google Scholar]
- 24.Morrissey P J, Conlon P, Charrier K, Braddy S, Alpert A, Williams D, Namen A E, Mochizuki D. J Immunol. 1991;147:561–568. [PubMed] [Google Scholar]
- 25.Hardy R R, Carmack C E, Shinton S A, Kemp J D, Hayakawa K. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medina K L, Kincade P W. Proc Natl Acad Sci USA. 1994;91:5382–5386. doi: 10.1073/pnas.91.12.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smithson G, Beamer W G, Shultz K L, Christianson S W, Shultz L D, Kincade P W. J Exp Med. 1994;180:717–720. doi: 10.1084/jem.180.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feuerbach D, Feyen J H M. FEBS Lett. 1997;402:21–24. doi: 10.1016/s0014-5793(96)01495-0. [DOI] [PubMed] [Google Scholar]
- 29.Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann T A, Anderson K C. Blood. 1996;87:1104–1112. [PubMed] [Google Scholar]
- 30.Barille S, Collette M, Bataille R, Amiot M. Blood. 1995;86:3151–3159. [PubMed] [Google Scholar]
- 31.Tanaka Y, Morimoto I, Nakano Y, Okada Y, Hirota S, Nomura S, Nakamura T, Eto S. J Bone Miner Res. 1995;10:1462–1469. doi: 10.1002/jbmr.5650101006. [DOI] [PubMed] [Google Scholar]
- 32.Seo H, Miyaura C, Sato S, Sudo T, Suda T. J Bone Miner Res. 1995;10:S159. (abstr.). [Google Scholar]