Abstract
It is generally thought that an effective vaccine to prevent HIV-1 infection should elicit both strong neutralizing antibody and cytotoxic T lymphocyte responses. We recently demonstrated that potent, boostable, long-lived HIV-1 envelope (Env)-specific cytotoxic T lymphocyte responses can be elicited in rhesus monkeys using plasmid-encoded HIV-1 env DNA as the immunogen. In the present study, we show that the addition of HIV-1 Env protein to this regimen as a boosting immunogen generates a high titer neutralizing antibody response in this nonhuman primate species. Moreover, we demonstrate in a pilot study that immunization with HIV-1 env DNA (multiple doses) followed by a final immunization with HIV-1 env DNA plus HIV-1 Env protein (env gene from HXBc2 clone of HIV IIIB; Env protein from parental HIV IIIB) completely protects monkeys from infection after i.v. challenge with a chimeric virus expressing HIV-1 env (HXBc2) on a simian immmunodeficiency virusmac backbone (SHIV-HXBc2). The potent immunity and protection seen in these pilot experiments suggest that a DNA prime/DNA plus protein boost regimen warrants active investigation as a vaccine strategy to prevent HIV-1 infection.
Keywords: CTL, virus neutralization, vaccine, SHIV, T lymphocyte
Although the correlates for immune protection against HIV infection are currently unknown, it is probable that an effective HIV vaccine should elicit both strong neutralizing antibody and cytotoxic T lymphocyte (CTL) responses. Antibody responses have provided protection from HIV infection in chimpanzee challenge models (1). Potential protective roles for CTL also have been recognized recently. It has become increasingly clear that CTL may play a key role in clearing viremia during primary HIV-1 infection in humans before the development of virus-neutralizing antibodies (2, 3) and in maintaining disease-free infection (4, 5). Furthermore, it has been suggested that anti-HIV CTL responses, detected in some HIV-exposed but uninfected individuals in the absence of HIV-specific antibodies, may have prevented establishment of infection (6, 7).
Although a number of live vector, recombinant protein, and peptide vaccination strategies have been shown to elicit HIV-specific antibodies and CTL in nonhuman primate models and humans, it would be desirable if these responses were more potent and durable. The demonstration of the utility of plasmid DNA immunization for the induction of virus-specific CTL and neutralizing antibodies in a variety of animal disease models (8–11) suggests that this vaccine modality may prove useful for an AIDS vaccine. In fact, DNA vaccination has been shown to elicit HIV-specific CTL and antibody responses in rhesus monkeys (12–14). We recently demonstrated that immunization regimens with HIV-1 env DNA elicit potent, long-lived envelope (Env)-specific CTL responses in rhesus monkeys (ref. 15 and unpublished work). These experiments demonstrated that (i) antigen-specific CTL responses were elicited in all monkeys after one or two vaccinations; (ii) an additional immunization after several months of “rest” substantially boosted CTL responses; and, (iii) these responses persisted at least 36 weeks. Although these HIV env DNA vaccines also elicited antigen-specific antibodies in monkeys, only low titers of virus-neutralizing antibodies generally were obtained (13).
The full potential for the induction of HIV-specific immune responses using DNA immunization combined with other vaccine modalities has not been determined. In the present studies, we have immunized rhesus monkeys with HIV env DNA vaccines in combination with recombinant HIV-1 envelope glycoprotein, characterized the resulting immune responses, and assessed their protective efficacy against a SHIV-HXBc2 challenge.
MATERIALS AND METHODS
Vaccination Vectors and Proteins.
The preparation and purification of HIV gp120 and gp160t DNA vaccines, which express secreted and membrane-anchored forms of HXBc2-derived Env, respectively, have been described (12, 13, 15, 17). In brief, the expression vector, V1Jns, used for construction of the vaccine plasmids, used the promoter, enhancer, and intron A from the human cytomegalovirus and the bovine growth hormone termination and polyadenylation sequences contained within a pUC plasmid backbone from which the entire lac operon had been removed and the ampicillin resistance gene replaced by one conferring resistance to kanamycin. The HIV gp120 and gp160t vaccine constructs were made by preparing chimeric genes in which the signal peptide from the human tissue-specific plasminogen activator gene replaced the native signal peptide from env. The gp120 construct expresses secreted gp120 protein, and the gp160t construct encodes a truncated gp160 gene that expresses a membrane-anchored form of gp160 protein with most of the intracellular domain of gp41 deleted. Rhesus (Macaca mulatta) monkeys were obtained from and maintained within a closed breeding colony. Vaccinations were performed by i.m. administration of 1–2 mg of DNA in normal saline solution or 100 μg of o-gp160 IIIB (Advanced BioScience Laboratories) mixed with 100 μg of saponin-derived adjuvant. Control vaccines consisted of plasmid DNA without an inserted gene and ovalbumin.
Anti-HIV Antibody Assays.
Recombinant gp120 (IIIB) protein (Intracel, Cambridge, MA) was used at a concentration of 2 μg/ml to coat 96-well microtiter plates to measure serum anti-Env antibody responses by ELISA as described (12, 13). An anti-human IgG secondary antibody-conjugated horseradish peroxidase enzyme (Bethyl Laboratories, Montgomery, TX), which is cross-reactive with rhesus IgG, was used to detect antibodies bound to gp120 for rhesus and human sera. End point titers were calculated using softmax (Molecular Devices) using cutoff values of twice the OD of control sera. Virus neutralization assays using SHIV-HXBc2 infection of MT-2 cells were performed as described (18, 19).
T Lymphocyte Assays.
Preparation of blood-derived peripheral blood mononuclear cells (PBMCs) and assay methods for in vitro CTL and proliferation experiments have been described (12, 13, 20). In brief, rhesus PBMCs were tested for cytotoxicity after restimulation in vitro for 7 days with autologous B cell lines infected with vaccinia-env and tested for lysis in a 4-h 51Cr release assay at 37°C using similar antigen-sensitized cells for targets. Precursor CTL frequencies were determined by testing between 2.5 × 103 and 2.5 × 105 lymphocytes as sets of 24 replicates in a final volume of 0.2 ml. After 10 days of culture, each sample was tested for cytotoxic activity with 51Cr-labeled autologous B cell lines in a 5-h incubation at 37°C. Samples were scored as positives if specific lysis was observed that exceeded the mean of control wells containing target cells alone by at least 3 SD. A single-hit Poisson distribution was used to model the generation of a positive response in the limiting-dilution assay. To simplify comparisons, effector cell frequencies were normalized to the number of effector cells per 106 lymphocytes.
For lymphocyte proliferation assays, stimulation indices were calculated for each sample by determining the ratio of incorporated [3H]-thymidine by PBMCs in the presence of antigen to that in media alone. Cytokine determinations were performed using commercially available ELISA kits according to the manufacturer’s instructions: human IL-2 (Immunotech, Luminy, France); human IL-4, IL-10, and rhesus monkey γ-interferon (BioSource International, Camarillo, CA); and human tumor necrosis factor α (Genzyme). The human ELISA kits were selected on the basis of cross-reactivity with cytokines present in the supernatants of activated rhesus monkey PBMCs (21) and may not represent the exact concentrations of rhesus cytokines.
SHIV Challenge Experiments.
The chimeric HIV/simian immunodeficiency virus (SIV) SHIV-HXBc2, comprised of HIV IIIB tat, vpu, rev, and env contained within an SIVmac239 backbone, has been described (22). For virus challenge experiments, rhesus monkeys were injected i.v. with 24 tissue culture 50% infective dose of a titrated virus stock predetermined to give >99% probability of successful infection. SHIV infection of rhesus monkeys was determined by three assays: ELISA detection (Coulter) of SIV p27 Gag antigen after 14 days of in vitro culture of PBMCs recovered from challenged animals; Western blot detection of anti-HIV-2 Gag antibodies using a kit (Cambridge Biotech); and PCR detection of SIV gag/pol from 200-ng samples of DNA from PBMCs. The PCR amplification was performed for 30 cycles, and reaction products were electrophoresed on agarose gels, transferred to nitrocellulose paper, and visualized by hybridization using gene-specific [32P]-labeled synthetic DNA oligomers.
RESULTS
DNA/Protein Dual Modality Vaccinations.
Our previous experiments using HIV env DNA to immunize rhesus monkeys demonstrated that these vaccines were capable of eliciting potent, long-lived, antigen-specific CTL activity and that maximal responses were obtained by using a regimen consisting of a priming series of vaccinations followed by several months of rest before boosting (unpublished results). As part of our ongoing experiments evaluating different types of HIV env DNA vaccines and immunization regimens, a dual modality vaccine comprised of HIV env DNA and recombinant protein arms also was tested. During an exploratory series of experiments intended to evaluate the immunogenicity of different HIV env DNA constructs, three rhesus monkeys were immunized with 2 mg (dose arbitrarily chosen) of a gp120 DNA vaccine a total of five times at 4-week intervals (12). Maximal antibody titers were obtained after the third vaccination (anti-gp120 ELISA endpoint titers ≈103), but no virus neutralizing antibodies were detected. The subsequent injections administered at that time had no effect on these responses. These animals, after an interval of 1 year, received additional immunizations with 1 mg of a gp160t DNA vaccine four times at 4-week intervals. A significant boost in ELISA antibody responses was elicited by the initial injection (end point titers ≈104), and low levels of virus neutralizing antibodies were detected (titers < 100). Neither the ELISA nor the neutralization antibody responses were enhanced by the three subsequent injections.
After an additional 4-month rest, two of these monkeys were vaccinated a single time at two separate sites with HIV gp160t DNA (2 mg) and o-gp160 protein (100 μg). The third monkey was excluded from further study for reasons unrelated to the experimental protocol. Two sets of control monkeys (six total) were added to the experiment at this time: one group of three control animals was injected with 2 mg of vector DNA (not coding for any protein but otherwise identical to the env DNA) and with 100 μg ovalbumin/saponin adjuvant, and a second group of three control animals received only 100 μg of o-gp160 (IIIB)/saponin adjuvant.
Helper T Lymphocyte Responses.
The HIV-1 Env-specific cellular immune responses of these eight monkeys were assessed after the boosting immunization. HIV-1 Env-specific CD4+ T cell responses were evaluated by measuring rgp120-elicited proliferative and cytokine responses in PBMCs obtained 3 weeks after antigen boosting (Table 1). The PBMCs of the six control monkeys did not exhibit rgp120-specific helper T cell responses with stimulation indices of <3 and no measurable production of IL-2, IL-4, γ-interferon, or tumor necrosis factor α. PBMCs of the two experimental monkeys had stimulation indices of 9 (monkey 0003) and 57 (monkey 0092), produced no measurable IL-2 or IL-4, but made 356 (0003) and 47 (0092) pg/ml γ-interferon, and 25 (0003) and 11 (0092) pg/ml tumor necrosis factor α. The quantity of tumor necrosis factor α detected for monkey 0092 is only 2-fold greater than the detection limit we have determined for this assay using rhesus PBMC culture supernatants and may represent only a weak positive for this cytokine. Thus, the DNA env/o-gp160 vaccinees exhibited antigen-specific T lymphocyte memory responses.
Table 1.
HIV-1 gp120-induced PBMC in vitro proliferation and cytokine secretion after HIV env DNA and o-gp160 vaccinations
| Prime/boost regimen | Monkey | SI* | IL-2† | IL-4† | IL-10† | IFNγ† | TNFα† |
|---|---|---|---|---|---|---|---|
| envDNA/envDNA plus o-gp120 | 0092 | 56.8 | <5 | <5 | 7 | 47 | 11 |
| 0003 | 8.6 | <5 | <5 | 7 | 356 | 25 | |
| naive/o-gp160 | 0094 | 2.9 | <5 | <5 | 7 | <5 | <5 |
| 0076 | 2.00 | <5 | <5 | 6 | <5 | <5 | |
| 0342 | 2.2 | <5 | <5 | 7 | <5 | <5 | |
| vectorDNA/OVA | 0053 | 1.8 | <5 | <5 | 8 | <5 | <5 |
| 0065 | 1.7 | <5 | <5 | 6 | <5 | <5 | |
| 0059 | 0.9 | <5 | <5 | 7 | <5 | 6 |
SI, stimulation index: determined by dividing the mean gp120-induced thymidine incorporation that observed with media alone.
Cytokine production measured by ELISA: IL-2 from Immunotech; IL-4, IL-10, and rhesus monkey γ-interferon from Biosource; tumor necrosis factor from Genzyme. Results expressed as pg/mL. Cell culture supernatant harvested from 2 × 105 peripheral blood lymphocytes cultured in the presence of 2 μg/mL rgp120 for 72 h.
CTL Responses.
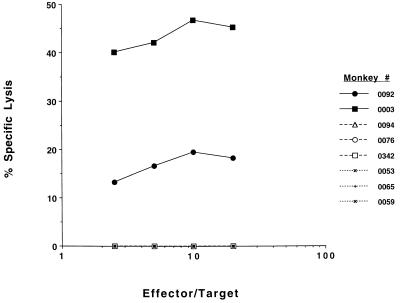
HIV-1 Env-specific bulk and precursor CTLs were quantitated in PBMCs before and after final immunization. Fig. 1 shows that significant bulk CTL responses were obtained after the final HIV env DNA/protein vaccination whereas neither set of control monkeys exhibited any Env-specific CTL responses. Using a more quantitative CTL precursor frequency assay, PBMCs of the six control monkeys had no detectable HIV-1 Env-specific CTL precursors before or 12 days after immunization. PBMCs of the experimental monkey 0003 had 7.4 HIV-1 Env-specific effector cells/106 PBMCs before boosting; the frequency of these cells rose to 14.6/106 PBMCs 12 days after boosting. PBMCs of the experimental monkey 0092 had HIV-1 Env-specific effector cells in PBMCs that rose from 6.6/106 PBMCs before boosting to 10.0/106 PBMCs by 12 days after boosting.
Figure 1.
Anti-HIV Env CTL responses in rhesus monkeys after HIV env DNA and o-gp160 vaccinations. Rhesus PBMCs were tested for cytotoxicity after restimulation in vitro for 7 days with autologous B cell lines infected with vaccinia-env and tested for lysis in a 4-h 51Cr release assay using similar antigen-sensitized cells for targets. Cytoxicity curves are shown for individual monkeys for each vaccination group: HIV env DNA plus o-gp160 vaccinees (solid lines), naive/o-gp160 vaccinees (broken lines), and naive/vector DNA plus ovalbumin-vaccinated monkeys (dotted lines). No specific killing was found using wild-type vaccinia-treated targets.
Anti-gp120 ELISA and Virus Neutralizing Antibody Responses.
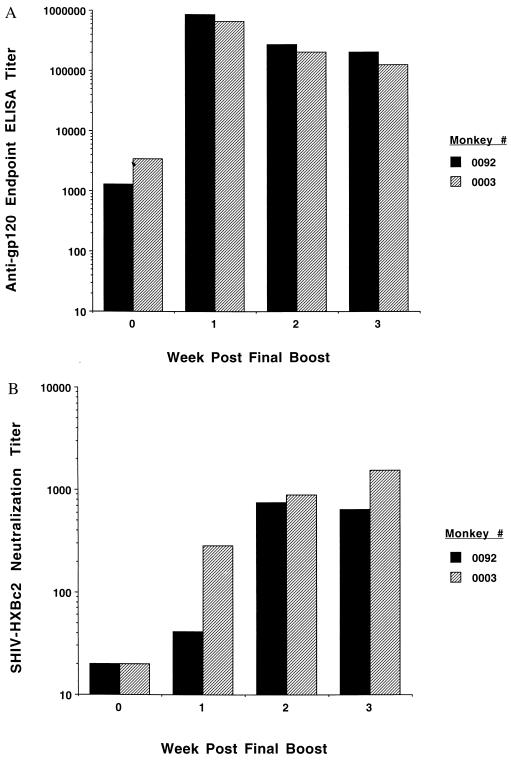
Fig. 2A shows that high ELISA antibody titers to rgp120 protein developed in both HIV Env vaccinees that were comparable to those observed in sera from an HIV-infected human (data not shown; see Fig. 5 for analogous data). Neutralization of SHIV-HXBc2 by sera from these vaccinated monkeys was assessed in an MT-2 cell-killing assay (18, 19). Although sera of the two experimental animals obtained before boosting did not neutralize this virus, by 3 weeks after boosting, serum of monkey 0003 had a neutralization titer of 1551 and serum of monkey 0092 had a neutralization titer of 637 (see Fig. 2B). These neutralization titers exceeded those observed with sera from rhesus monkeys chronically infected with SHIV-HXBc2 [geometric mean titer = 159 ± 38 for four animals infected from 147 to 225 days (19)]. These results demonstrate that the combination immunization with HIV env DNA and o-gp160 protein vaccines generated very high levels of neutralizing antibodies. Sera of the six control animals obtained before and after antigen boosting did not neutralize SHIV-HXBc2 at any timepoint tested (data not shown).
Figure 2.
Anti-HIV Env antibody responses in rhesus monkeys after HIV env DNA and o-gp160 vaccinations. Sera from vaccinated monkeys were tested at the indicated timepoints relative to the final vaccination for anti-gp120 ELISA antibody responses (shown as end point titers in A) and for SHIV-HXBc2-neutralizing antibody titers (B). Data for individual monkeys are depicted in each figure.
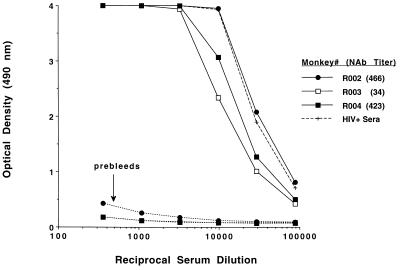
Figure 5.
Antibody responses of rhesus monkeys after HIV gp160t DNA (3X) and gp160t DNA/o-gp160 (1X) vaccinations. Serial dilutions of sera were tested for anti-gp120 ELISA antibody responses 2 weeks after the final vaccination (solid lines) compared with preimmune sera from the same monkeys (dotted lines). ELISA responses are also shown for a pool of sera obtained from HIV+ humans (dashed line). Anti-SHIV-HXBc2 neutralization titers for each serum sample are indicated in the legend.
SHIV-HXBc2 Challenge of Rhesus Monkeys.
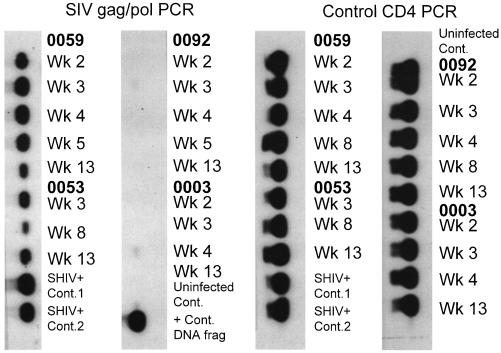
We next sought to determine whether the immunity generated in monkeys by this DNA priming and DNA plus protein boosting afforded them protection against an AIDS virus challenge. The eight rhesus monkeys were challenged by i.v. route with 24 tissue culture 50% infective doses of cell-free SHIV-HXBc2 4 weeks after the boosting immunization. Animals were then bled at 2, 3, 4, 6, 8, 11, 13, 17, and 21 weeks after challenge and assessed for SHIV-HXBc2 isolation, seroconversion to SIV Gag, and evidence of SIV gag DNA in PBMCs. Con A-stimulated, CD8+ cell-depleted PBMCs from every bleed yielded positive SHIV-HXBc2 isolations from all six control but not from the DNA primed/DNA and protein-boosted monkeys (Table 2). Consistent with these findings, sera from weeks 3, 6, and 13 after challenge were positive by Western blot analysis for antibodies cross-reactive with HIV-2 Gag in the control but not the DNA primed/DNA and protein-boosted animals (Fig. 3). Finally, PCR amplification using SIV gag/pol-specific primers and DNA from PBMCs of the HIV env DNA primed/HIV env DNA plus o-gp160-boosted monkeys did not detect any challenge virus-related genome (Fig. 4). Thus, in this pilot study, the monkeys primed with HIV-1 env DNA and boosted with HIV-1 env DNA plus o-gp160 were protected from infection with SHIV-HXBc2.
Table 2.
SHIV-HXBc2 was not isolated from PBMC of env DNA/env DNA plus o-gp160 vaccinated rhesus monkeys after virus challenge
| Prime/boost regimen | Monkey | SHIV isolation week after challenge
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 6 | 8 | 11 | 13 | 17 | 21 | ||
| env DNA/env | 0092 | − | − | − | − | − | − | − | − | − |
| DNA plus o-gp160 | 0003 | − | − | − | − | − | − | − | − | − |
| naive/o-gp160 | 0094 | + | + | + | + | + | + | + | + | + |
| 0076 | + | + | + | + | + | + | + | + | + | |
| 0342 | + | + | + | + | + | + | + | + | + | |
| naive/vector | 0053 | + | + | + | + | + | + | + | + | + |
| DNA plus OVA | 0065 | + | + | + | + | + | + | + | + | + |
| 0059 | + | + | + | + | + | + | + | + | + | |
PBMCs of the rhesus monkeys were obtained at the noted times after SHIV-HXBc2 challenge, stimulated with Con A for 18 h, then depleted of CD8+ cells using immunomagnetic beads and maintained in culture for 2 weeks in IL-2-containing medium. The medium was changed regularly, and supernatants were assessed for SIVp27 antigen as determined by enzyme immunoassay. A virus isolation is noted as + if the supernatants of that culture contained measurable SIVp27 antigen.
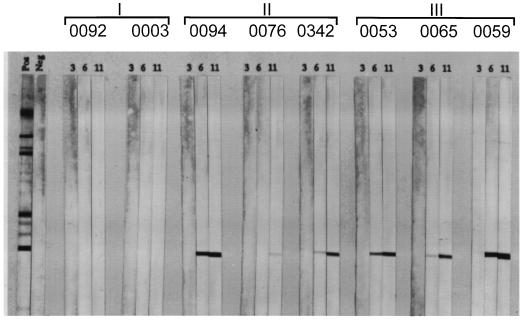
Figure 3.
Seroconversion to SIV Gag antigen reactivity was detected in plasma of control but not experimental vaccinated monkeys after SHIV-HXBc2 challenge. Plasma from the HIV env DNA plus o-gp160 (Group I), naive/o-gp160 (Group II), and naive/vector DNA plus ovalbumin (Group III) vaccinated monkeys were assessed for antibody reactivity to HIV-2 Western immunoblot strips at 3, 6, and 11 weeks after virus inoculation. The two panels at left show positive and negative plasma control sample Western reactivities.
Figure 4.
PBMCs from HIV env DNA plus o-gp160-immunized rhesus monkeys were negative for SIV gag/pol DNA by PCR analysis. DNA was isolated from PBMCs of the experimental vaccinated monkeys (0092 and 0003) and two of the control naive/vector DNA plus ovalbumin-vaccinated monkeys (0059 and 0053) at the times noted and subjected to PCR amplification using oligomers specific for either SIV gag/pol or, as a control, the cellular CD4 gene.
It was important to determine whether a smaller number of inoculations could elicit similar neutralizing antibody responses. Three rhesus monkeys were vaccinated three times at 4-week intervals with 1 mg of HIV gp160t DNA only, rested for 4 months, and boosted once with gp160t DNA and o-gp160 protein as before. Fig. 5 shows that all three monkeys developed high ELISA antibody titers that were comparable to sera obtained from HIV+ humans. Two of these monkeys also exhibited high levels of neutralizing antibodies (titers >400). Thus, comparable virus-neutralizing responses were elicited with a total of four immunizations as were obtained in the previous monkey group that had received 10 immunizations.
DISCUSSION
Several live recombinant vectors and peptide formulations have been shown to elicit SIV- or HIV-specific CTL in nonhuman primates (23). A boosting of such CTL responses by sequentially combining a live vector and a subunit immunogen also has been shown. However, repeated inoculations of the same live vector or subunit immunogen have not substantially increased effector precursor frequency or durability of these responses. Thus, our previous demonstration of the elicitation of a potent HIV-1 Env-specific CTL response using a plasmid DNA encoding a single immunogen makes naked DNA unique among vaccine modalities. Moreover, this DNA vaccine-elicited HIV-1 Env-specific CTL response in rhesus monkeys was substantially greater in magnitude and was maintained for a longer period of time than that seen with other modalities (unpublished results). The DNA plus protein-combined modality vaccinations described in the present study combine the advantages offered by the DNA arm alone (boostable, durable CTL responses) and extremely potent antibody responses obtained from using both DNA and protein.
Although the SIV-infected macaque has proven a powerful model for exploring approaches to AIDS vaccination, its utility has been somewhat limited in assessing viral envelope-based immunization strategies. The SIV and HIV-1 envelope glycoproteins are distinct enough antigenically that it is difficult to extrapolate from observations concerning SIV to those relevant for HIV. It has been shown that macaques can be infected by chimeric viruses comprised of SIVmac239 expressing HIV-1 Env and the associated HIV-1 auxiliary genes tat, vpu, and rev (22). This chimeric virus provides a model for assessing envelope-based vaccine approaches for the prevention of HIV-1 infection.
Although this pilot protection study used extremely small numbers of monkeys, the high levels of HIV-specific immune responses and evidence of complete protection from infectious challenge in the DNA primed/DNA plus protein-boosted monkeys is compelling. The ultimate potency of protective immunity generated by this immunization strategy is unclear. The only immunity that has reproducibly protected macaques from challenge with the highly pathogenic SIVmac is that generated by prior infection with a live, attenuated SIVmac (24). Protection of chimpanzees from infection by HIV-1 SF2, a virus isolate that does not replicate very efficiently in that species, has proven relatively easy to achieve with a variety of HIV-1 envelope subunit immunogens eliciting protective immunity in that model (1, 16, 25). The fact that SHIV-HXBc2 replicates to lower levels during primary infection in rhesus monkeys than SIVmac and is nonpathogenic in this species suggests that this challenge model may not be as rigorous as the SIVmac/macaque system. In addition, SHIV-HXBc2 uses an env gene derived from a neutralization-sensitive, T cell line-cultured laboratory strain of HIV, and the high viral neutralization titers obtained with the vaccine combination in this study may not be realized with vaccines directed against more neutralization-resistant, primary HIV strains. Nevertheless, the potent immunity and protection seen in these pilot studies suggest that the immunization strategy used in this work bears further active investigation.
We thank the veterinary associates Tamara Montgomery and Karen Wright, who assisted with monkey vaccinations, bleeds, and the SHIV challenge and Miroslawa Bilska for performing the virus neutralization experiments. This work was supported by funds from Merck Research Laboratories and by National Institute of Health Grants A1-20729 and CA-50139.
ABBREVIATIONS
- CTL
cytotoxic T lymphocyte
- Env
envelope
- PBMC
peripheral blood mononuclear cells
- SIV
simian immunodeficiency virus
- SHIV-HXBc2
chimeric HIV/SIV
References
- 1.Johnston M I. Int Arch Allergy Immunol. 1995;108:313–317. doi: 10.1159/000237173. [DOI] [PubMed] [Google Scholar]
- 2.Koup RA, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B A. J Virol. 1994;68:5103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rinaldo C, Huang X-L, Fan Z, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, Gupta P. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P M, Eeftinck-Schattenkerk J-K M, Osterhaus A D M E, Schuitemaker H, Miedema F. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinto L A, Sullivan J, Berzofsky J A, Clerici M, Kessler H A, Landay A L, Shearer G M. J Clin Invest. 1995;96:867–876. doi: 10.1172/JCI118133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.RowlandJones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, Takiguchi M, Schultz T, McMichael A, Whittle H. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 8.Ulmer J B, Donnelly J J, Parker SE, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, Hawe L A, Leander K R, Martinez D, Perry H C, Shiver J W, Montgomery D L, Liu M A. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly J J, Friedman A, Martinez D, Montgomery D L, Shiver J W, Motzel S L, Ulmer J B, Liu M A. Nat Med. 1995;1:583–587. doi: 10.1038/nm0695-583. [DOI] [PubMed] [Google Scholar]
- 10.Fynan E F, Webster R G, Fuller D H, Haynes J R, Santoro J C, Robinson H L. Proc Natl Acad Sci USA. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B, Ugen K E, Srikantan V, Sato A I, Boyer J, Williams W V, Weiner D B. Proc Natl Acad Sci USA. 1993;90:4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiver J W, Perry H C, Davies M-E, Freed D L, Liu M A. Ann NY Acad Sci. 1995;772:198–208. doi: 10.1111/j.1749-6632.1995.tb44745.x. [DOI] [PubMed] [Google Scholar]
- 13.Shiver J W, Davies M-E, Perry H C, Freed D C, Liu M A. J Pharm Sci. 1996;85:1317–1324. doi: 10.1021/js9600991. [DOI] [PubMed] [Google Scholar]
- 14.Wang B, Boyer J, Srikantan V, Ugen K, Gilbert L, Phan C, Dang K, Merva M, Agadjanyan M G, Newman M, Carrano R, McCallus D, Coney L, Williams W V, Weiner D B. Virology. 1995;211:102–112. doi: 10.1006/viro.1995.1383. [DOI] [PubMed] [Google Scholar]
- 15.Liu M A, Yasutomi Y, Davies M-E, Perry H C, Freed D C, Letvin N L, Shiver J W. Antibiot Chemother. 1996;48:100–104. doi: 10.1159/000425163. [DOI] [PubMed] [Google Scholar]
- 16.Berman P W, Murthy K K, Wrin T, Vennari J C, Cobb E K, Eastman D J, Champe M, Nakamura G R, Davison D, Powell M F, Bussiere J, Francis D P, Matthews T, Gregory T J, Obijeski J F. J Infect Dis. 1996;173:52–59. doi: 10.1093/infdis/173.1.52. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery D L, Shiver J W, Leander K R, Perry H C, Friedman A, Martinez D, Ulmer J B, Donnelly J J, Liu M A. DNA Cell Biol. 1993;12:777–783. doi: 10.1089/dna.1993.12.777. [DOI] [PubMed] [Google Scholar]
- 18.Montefiori D C, Robinson W E, Jr, Schuffman S S, Mitchell W M. J Clin Microbiol. 1988;26:231–237. doi: 10.1128/jcm.26.2.231-235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reimann K A, Li J T, Voss G, Lekutis C, Tenner-Racz K, Racz P, Lin W, Montefiori D C, Lee-Parritz D E, Lu Y, Collman R G, Sodroski J, Letvin N L. J Virol. 1996;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasutomi Y, Koenig S, Woods R M, Madsen J, Wassef N M, Alving C R, Klein H J, Nolan T E, Boosts L J, Kessler J A, Emini E A, Conley A J, Letvin N L. J Virol. 1995;69:2279–2284. doi: 10.1128/jvi.69.4.2279-2284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lekutis C, Shiver J W, Liu M A, Letvin N L. J Immunol. 1997;158:4471–4477. [PubMed] [Google Scholar]
- 22.Li J, Lord C I, Haseltine W, Letvin N L, Sodroski J. J Acquired Immune Defic Syndr. 1992;5:639–646. [PubMed] [Google Scholar]
- 23.Letvin N L. N Engl J Med. 1993;329:1400–1405. doi: 10.1056/NEJM199311043291908. [DOI] [PubMed] [Google Scholar]
- 24.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 25.El-Amad Z, Murthy K K, Higgins K, Cobb E K, Haigwood N L, Levy J A, Steimer K S. AIDS. 1995;9:1313–1322. doi: 10.1097/00002030-199512000-00003. [DOI] [PubMed] [Google Scholar]