Abstract
Expression of the γ-aminobutyric acid type A receptor α6 subunit gene is restricted to differentiated granule cells of the cerebellum and cochlear nucleus. The mechanisms underlying this limited expression are unknown. Here we have characterized the expression of a series of α6-based transgenes in adult mouse brain. A DNA fragment containing a 1-kb portion upstream of the start site(s), together with exons 1–8, can direct high-level cerebellar granule cell-specific reporter gene expression. Thus powerful granule cell-specific determinants reside within the 5′ half of the α6 subunit gene body. This intron-containing transgene appears to lack the cochlear nucleus regulatory elements. It therefore provides a cassette to deliver gene products solely to adult cerebellar granule cells.
γ-aminobutyric acid type A receptors are transmitter-gated chloride channels mediating fast neuronal inhibition. They are built as pentameric subunit assemblies selected from a large gene family (α1-α6, β1-β3, γ1-γ3, δ, and ɛ) (1–6). The subunit genes are transcribed in complex patterns throughout the brain (7, 8), but nothing is known about how these patterns are generated. Many of the subunit genes are clustered, e.g., the α1, β2, γ2, and α6 genes are on mouse chromosome 11/human chromosome 5q (9, 10), which might imply coordinated regulation. As an entry point for analysis, we chose to study the subunit gene (α6), which has the simplest expression pattern. When the whole brain is surveyed, the α6 gene is found to be transcribed in just two cell types: cerebellar granule cells and the lineage-related cochlear nucleus granule cells (11–15). Within this lineage, the α6 locus is active only in postmigratory and differentiated cells, i.e., expression first begins to appear at the beginning of the second postnatal week (5, 14, 16–19).
With one exception (20), it has not proved possible to isolate regulatory DNA fragments that direct expression to just one type of neuronal cell. Most brain-specific transgenes drive wide expression (e.g., refs. 21 and 22). Here, we have identified an α6-based transgene capable of directing high-level expression uniquely to adult cerebellar granule cells.
MATERIALS AND METHODS
Transgene Constructions.
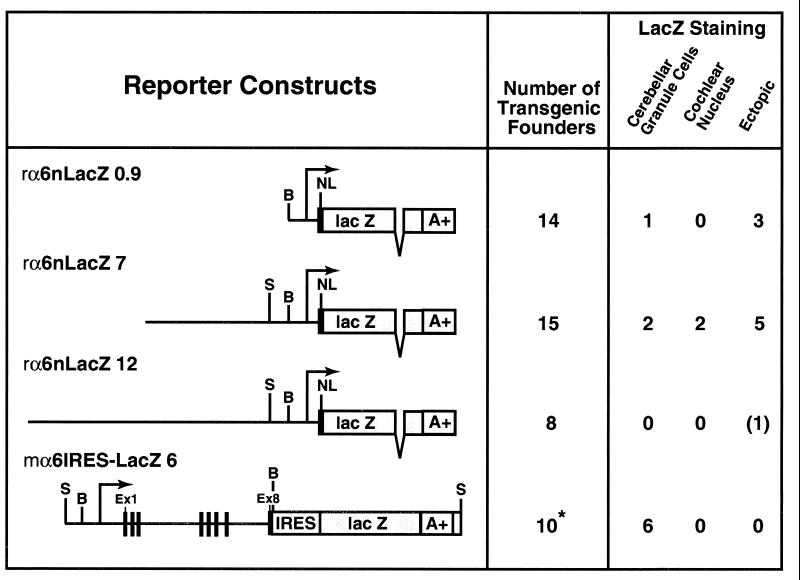
Plasmids prα6nLacZ7 and prα6nLacZ12 were built with the vector prα6nLacZ0.9 (23). This contains 500 bp of 5′ nontranscribed region, the proximal promoter including the transcription start site(s), and the complete 5′ untranslated segment (approx. 350 bp). The first methionine of the α6 gene is replaced with the lacZ coding region incorporating a simian virus 40-derived nuclear localization sequence (24). The prα6nLacZ0.9 plasmid was first modified to give prα6nLacZ0.9ΔNotXho, by inserting unique NotI and XhoI sites into the 5′ and 3′ polylinkers, respectively. Two α6 gene restriction fragments, spanning upstream portions of the α6 gene, were isolated from a λ Dash II Sprague–Dawley rat testis genomic library (Stratagene) (23). These 6.5-kb NotI/BamHI and 12-kb NotI/BamHI fragments were placed into the 5′ polylinker of prα6nLacZ0.9ΔNotXho to create prα6nLacZ7 and prα6nLacZ12, respectively (Fig. 1). In each case, the 5′ NotI site derives from the λ Dash II polylinker, with the 12-kb fragment originating from a phage with more 5′ α6 gene fragments. For pronuclear injections, the plasmid backbone of both constructs was removed by NotI and XhoI digestion.
Figure 1.
Transgenic data summary: Expression of lacZ-based α6 reporter genes in the brains of adult mice. A+, simian virus 40 polyadenylation signal; Ex, exon; LacZ, β-galactosidase; NL, simian virus 40 nuclear localization sequence; B, BamHI; S, SphI. Arrow indicates the transcriptional start site(s). The open box at the 3′ end of the mα6IRES-LacZ6 transgene marks the residual 5′ fragment of the neomycin (neo) resistance gene (see ref. 25). *, three independent lines were derived, all of which expressed the transgene.
The transgene pmα6IRES-lacZ6 was isolated from homozygous Δα6lacZ 129/Sv × C57BL/6 mouse liver genomic DNA (25). Δα6lacZ liver genomic DNA was partially Sau 3A-digested, ligated into a λ Fix II Xho Partial Fill-In vector (Stratagene), packaged, and amplified. The resulting library was screened with an α6 cDNA probe (12). A phage containing the promoter region through to the exon8-internal ribosome entry site (IRES)-lacZ and 5′ end of the neo gene was digested with SphI to give a lacZ-positive 12-kb fragment. This was subcloned into pUC BM20 (Boehringer Mannheim) to give pmα6IRES-lacZ6 (Fig. 1). The 5′ SphI site is 1 kb 5′ of the transcription start site(s); the 3′ end of the transgene contains a herpes simplex virus thymidine kinase promoter linked to the region encoding the first 180 amino acids of the neomycin phosphotransferase protein. For pronuclear injections, the insert was released using SphI. All constructs were verified by sequencing across fragment boundaries.
Transgenic Mouse Production and Analysis.
Transgenic mice (CBA/cba × C57BL/6) were produced by pronuclear microinjection (26). Founders were identified by blotting BamHI-digested tail-derived genomic DNA and hybridizing with a lacZ probe. Anesthetized adult mice were transcardially perfused with 4% paraformaldehyde. Sections from brains and selected organs (liver, kidney, and heart) then were incubated with 5-bromo-4-chloro-3-indoyl β-d-galactoside (X-Gal) (27). Low-power images were obtained by photographing wet, noncoverslipped sections using a Leica (Deerfield, IL) Wild Heerbrugg microscope. Selected sections were counterstained with neutral red (Sigma), coverslipped, and photographed with a Leica Orthomat E microscope.
RESULTS AND DISCUSSION
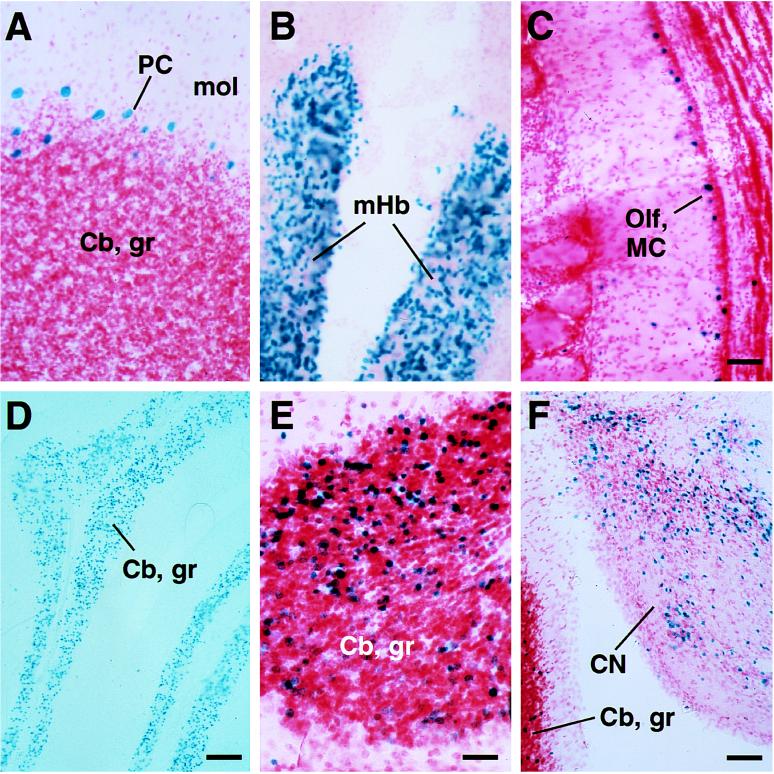
We searched for α6 gene regions that confer cerebellar granule cell-specific expression. Previous analysis showed that the proximal 500-bp promoter fragment (rα6lacz0.9) has neuronal specificity and occasionally can direct weak variegated expression to cerebellar granule cells in certain lobules (Figs. 1 and 2D) (23). More usually, integration events did not generate any expression (23). Here, we have extended this analysis using lacZ reporter transgenes containing more upstream regions of the rodent α6 subunit gene. The rα6nlacZ7 transgene contains 7 kb upstream of the start site(s) (Fig. 1). Seven of 15 independent transgene integrations gave expression (Fig. 1), all of which was neuron-specific, but ectopic. Differing genomic integration positions generated, for example, Purkinje cell (Fig. 2A), medial habenula (Fig. 2B), and mitral cell (Fig. 2C) expression. Two founders with rα6nlacZ7 integrations had cerebellar granule cell-specific expression (Fig. 2E). As for the rα6lacZ0.9 transgene (Fig. 2D), cerebellar granule cell expression obtained with rα6nlacZ7 was markedly mosaic; the majority of granule cells did not express the gene (Fig. 2E). However, the expressing cell minority had high lacZ activity (Fig. 2E). Additionally, the two rα6nlacZ7 founders with cerebellar granule cell expression had strong lacZ expression in the dorsal cochlear nucleus granule cells (Fig. 2F). Overall, granule cell-specific expression from the rα6nlacZ7 transgene did not significantly improve on that obtained with rα6lacZ0.9, except that the frequency of expression was roughly 2-fold higher. Expression was even less from a transgene incorporating a 12-kb upstream fragment (Fig. 1, and data not shown). With this construct, only weak ectopic expression was detected in dispersed midbrain cells. It seems that the 500-bp proximal promoter fragment is, to a certain extent, primed to give granule cell-specific expression, but that the chromosomal integration position usually dominates. Adding on more 5′ DNA does not significantly improve expression. Even when expressed, all these constructs showed strong position effect variegation (28).
Figure 2.
Ectopic (A–C), granule cell-specific mosaic (D–E), and cochlear nucleus (F) expression of the rα6LacZ7 transgene. (A) Cerebellar Purkinje cells (granule cells are nonexpressing). (B) Medial habenulae. (C) Olfactory bulb mitral cells. (D) Mosaic rα6LacZ0.9 expression in cerebellar granule cells. (E) Mosaic rα6LacZ7 expression in cerebellar granule cells. (F) rα6LacZ7 in dorsal cochlear nucleus granule cells. Sections are stained for β-galactosidase activity (blue) and counterstained with neutral red. Cb gr, cerebellar granule cells; CN, cochlear nucleus; mol, cerebellar molecular layer; Olf, MC, olfactory bulb mitral cells; mHb, medial habenulae. (Scale bars: A–C, 120 μm; D, 170 μm; E, 60 μm; and F, 120 μm.)
We tested, therefore, the role of sequences downstream of the promoter in regulating the α6 subunit gene expression. Previously, we generated a lacZ “knock-in” mouse line (Δα6lacZ) via homologous recombination at the α6 gene (25). In the Δα6lacZ line, an IRES-lacZ cassette insertion in exon 8 gave high levels of lacZ activity in cerebellar and cochlear nucleus granule cells (25). This mouse line contains “ready-made” transgenes embedded in the α6 gene locus. In particular, a 12-kb SphI genomic fragment (mα6IRES-lacZ6) contains 1 kb upstream of the transcription start site(s), 5 kb of exon and intronic regions through to exon 8, followed by an IRES-lacZ poly(A) sequence (ref. 25; and see Fig. 1). We therefore cloned this fragment out from a Δα6lacZ mouse liver genomic library (see Materials and Methods).
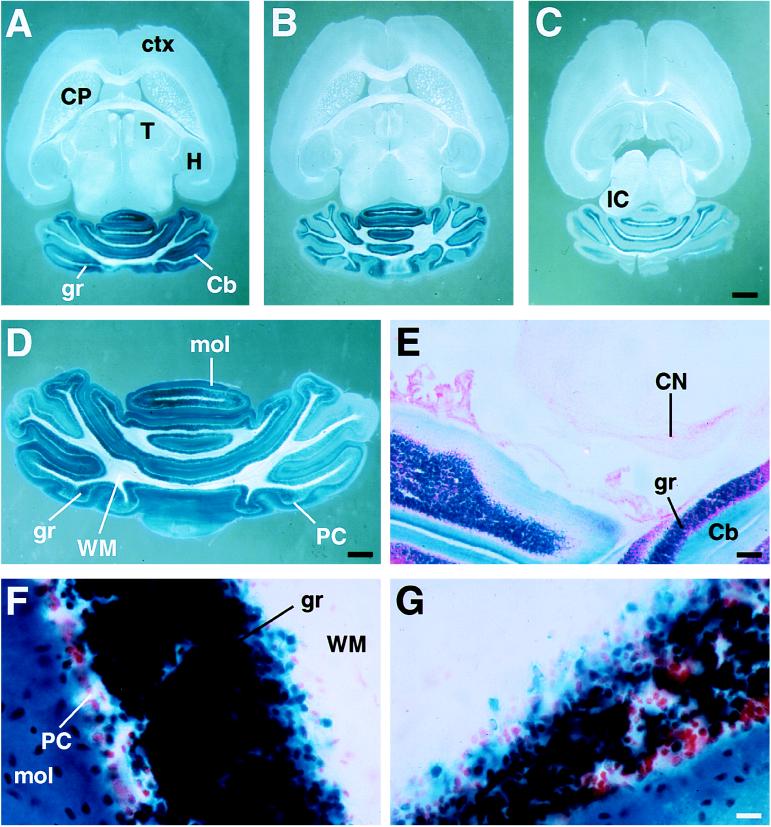
Expression from the microinjected mα6IRES-lacZ6 transgene closely resembles that of the native α6 gene (Figs. 1 and 3). Cerebellar granule cell-restricted lacZ expression was seen in every expressing transgenic founder, and the number of expressing transgenes was high (occurring in 6 of 10 independent integrations; see Fig. 1). In adult mice, depending on the genomic integration position, mα6IRES-lacZ6 transgene expression ranged from very strong (Fig. 3A) to strong (Fig. 3B) to moderate (Fig. 3C). No ectopic expression in other parts of the brain (Fig. 3 A–C), white matter (Fig. 3D) or in heart, liver, and kidney (data not shown) was found. In some cases, expression was so strong that lacZ activity could be detected in the molecular layer corresponding to enzyme transported into the granule cell axons—the parallel fibers (Fig. 3 F and G). In these cases, sections required only a short incubation in 5-bromo-4-chloro-3-indoyl β-d-galactoside (X-Gal) reagent to fully develop the blue reaction product. Some variegation in expression, depending on the exact cerebellar lobule was seen. This ranged from virtually all granule cells expressing (Fig. 3F) to a minority not expressing (Fig. 3G) the transgene. In the strongest expressing founders, variegation was at a minimum. Three of the founders were bred as heterozygotic lines. LacZ expression was stably inherited and correctly developmentally regulated, with transgene expression only appearing in postmigratory granule cells after the first postnatal week (data not shown).
Figure 3.
Expression of the mα6IRES-lacZ6 transgene is restricted to cerebellar granule cells. (A–C) Horizontal sections of three representative independent adult founder brains from the highest expressing (A) to the lowest expressing (C) mα6IRES-laZ6 integration. (D) Higher power view of a cerebellar section from founder shown in A. (E) No cochlear nucleus expression is observed even though very strong expression is found in the granule cell layer. (F–G) High-power views of granule cell layer of an expressing founder from different subregions of the cerebellum to illustrate mosaicism. In F, all cells are heavily stained. In G, some granule cells are not expressing. In both cases, the molecular layer is heavily stained with 5-bromo-4-chloro-3-indoyl β-d-galactoside (X-Gal) product because of β-galactosidase in the granule cell axons, the parallel fibers. A-D are stained only for β-galactosidase activity. E–G are counterstained with neutral red. Cb, cerebellum; CN, dorsal cochlear nucleus; CP, caudate-putamen; ctx, neocortex; gr, cerebellar granule cells; H, hippocampus; IC, inferior colliculus; mol, cerebellar molecular layer; PC, Purkinje cells; T, thalamus; WM, white matter tracts. (Scale bars: A–C, 1 mm; D, 0.5 mm; E, 170 μm; and F–G, 30 μm.)
So far, no cochlear nucleus granule cell lacZ expression has been observed in any mα6IRES-lacZ6 expressing brain (Fig. 3E, CN), even though cerebellar granule cell lacZ expression is prominent in the same sections (Fig. 3E, gr). This might imply the existence of discrete cochlear nucleus- and cerebellar granule cell-specific regulatory elements. Such a cochlear nucleus-specific element could reside between −7 and − 1 kb of the α6 gene, because the rα6nlacZ7 transgene can direct cochlear nucleus granule cell expression. We did not test the contribution of more 3′ elements (the 7-kb intron 8, and fragments 3′ to exon 9).
Clearly, information that directs gene expression specifically to adult cerebellar granule cells is contained in the 5′ half (6 kb) of the α6 subunit gene. Expression from mα6IRES-lacZ6 transgene is robust; no ectopic expression has so far been seen with this construct, and it gives a high-expression frequency, i.e., it is apparently relatively insensitive to genomic integration position. Therefore, α6 gene regulation can be uncoupled from the α1-β2-γ2 subunit gene cluster. This could be because of powerful granule cell-specific enhancer elements. The proximal 500-bp promoter contains information for neuronal specificity and seems primed to drive expression in granule cells, but with low efficiency (23). It might interact with a downstream element that enforces transcriptional specificity. Alternatively, an optimum gene architecture could account for the high fidelity of transgene expression in granule cells, e.g., the exon-intron structure may be particularly suited to granule cell expression.
Throughout the cerebellum’s evolutionary history, α6 subunit gene transcription has remained restricted to granule cells (29–30). Presumably, DNA regulatory elements also have been conserved. Comparative sequencing across the 5′ half of the gene might help to identify these putative regulatory regions (31). Eventually, by combining this approach with transgene deletions and point mutations, we will be able to identify the transcription factors that act on this gene. Similar factors may be involved in regulating other γ-aminobutyric acid type A receptor subunit genes (32), or more generally in defining the final stages of neuronal differentiation (33).
The cerebellum participates in the acquisition and deployment of motor skills (34–35), and in higher cognitive aspects of learning and anticipating patterns (36–37). The mα6IRES-lacZ6 transgene described here can be adapted to deliver other gene products uniquely to cerebellar granule cells. By coupling this with recent developments in transgenic technology (22, 38), the specific contributions of granule cell components to the physiology of cerebellar processes now can be investigated.
Acknowledgments
We thank R. Palmiter for the pnLacF vector, A. Lenton for help with figure preparation, and H. Bading, M. Goedert, A. L. Grant, S. P. Hunt, and S. Munro for discussion. G. King, T. Langford, I. Lavenir, and T. Rabbitts provided invaluable support with transgenic mice. S.B. holds a European Community Training and Mobility of Researchers Fellowship (category 20).
ABBREVIATION
- IRES
internal ribosome entry site
References
- 1.Seeburg P H, Wisden W, Verdoorn T A, Pritchett D B, Werner P, Herb A, Lüddens L, Sprengel R, Sakmann B. Cold Spring Harb Symp Quant Biol. 1990;55:29–40. doi: 10.1101/sqb.1990.055.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Tyndale R F, Olsen R W, Tobin A J. In: Handbook of Receptors and Channels: Ligand- and Voltage-Gated Ion Channels. North R A, editor. Boca Raton: CRC; 1995. pp. 265–290. [Google Scholar]
- 3.Costa E, Guidotti A. Trends Pharmacol Sci. 1996;17:192–200. doi: 10.1016/0165-6147(96)10015-8. [DOI] [PubMed] [Google Scholar]
- 4.McKernan R M, Whiting P J. Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 5.Wisden W, Korpi E R, Bahn S. Neuropharmacology. 1996;35:1139–1160. doi: 10.1016/s0028-3908(96)00076-7. [DOI] [PubMed] [Google Scholar]
- 6.Davies P A, Hanna M C, Hales T G, Kirkness E F. Nature (London) 1997;385:820–823. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- 7.Wisden W, Laurie D J, Monyer H, Seeburg P H. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritschy J-M, Möhler H. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 9.Garrett K M, Haque D, Berry D, Niekrasz I, Gan J, Rotter A, Seale T W. Mol Brain Res. 1997;45:133–137. doi: 10.1016/s0169-328x(96)00290-2. [DOI] [PubMed] [Google Scholar]
- 10.Hicks A A, Bailey M E S, Riley B P, Kamphuis W, Siciliano M J, Johnson K J, Darlison M G. Genomics. 1993;20:285–288. doi: 10.1006/geno.1994.1167. [DOI] [PubMed] [Google Scholar]
- 11.Kato K. J Mol Biol. 1990;214:619–624. doi: 10.1016/0022-2836(90)90276-r. [DOI] [PubMed] [Google Scholar]
- 12.Lüddens H, Pritchett D B, Köhler M, Killisch I, Keinänen K, Monyer H, Sprengel R, Seeburg P H. Nature (London) 1990;346:648–651. doi: 10.1038/346648a0. [DOI] [PubMed] [Google Scholar]
- 13.Laurie D J, Seeburg P H, Wisden W. J Neurosci. 1992;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varecka L, Wu C-H, Rotter A, Frostholm A. J Comp Neurol. 1994;339:341–352. doi: 10.1002/cne.903390304. [DOI] [PubMed] [Google Scholar]
- 15.Nusser Z, Sieghart W, Stephenson F A, Somogyi P. J Neurosci. 1996;16:103–114. doi: 10.1523/JNEUROSCI.16-01-00103.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korpi E R, Uusi-Oukari M, Kaivola J. Neuroscience. 1993;53:483–488. doi: 10.1016/0306-4522(93)90212-x. [DOI] [PubMed] [Google Scholar]
- 17.Kuhar S G, Feng L, Vidan S, Ross M E, Hatten M E, Heintz N. Development (Cambridge, UK) 1993;117:97–104. doi: 10.1242/dev.117.1.97. [DOI] [PubMed] [Google Scholar]
- 18.Laurie D J, Wisden W, Seeburg P H. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng T, Santi M-R, Bovolin P, Marlier L N J-L, Grayson D R. Dev Brain Res. 1993;75:91–103. doi: 10.1016/0165-3806(93)90068-l. [DOI] [PubMed] [Google Scholar]
- 20.Oberdick J, Smeyne R J, Mann J R, Zackson S, Morgan J I. Science. 1990;248:223–226. doi: 10.1126/science.2109351. [DOI] [PubMed] [Google Scholar]
- 21.Andra K, Abramowski D, Duke M, Probst A, Wiederhold K H, Bürki K, Goedert M, Sommer B, Staufenbiel M. Neurobiol Aging. 1996;17:183–190. doi: 10.1016/0197-4580(95)02066-7. [DOI] [PubMed] [Google Scholar]
- 22.Mayford M, Bach M E, Huang Y Y, Wang L, Hawkins R D, Kandel E R. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 23.Jones A, Bahn S, Grant A L, Köhler M, Wisden W. J Neurochem. 1996;67:907–916. doi: 10.1046/j.1471-4159.1996.67030907.x. [DOI] [PubMed] [Google Scholar]
- 24.Mercer E H, Hoyle G W, Kapur R P, Brinster R L, Palmiter R D. Neuron. 1991;7:703–716. doi: 10.1016/0896-6273(91)90274-4. [DOI] [PubMed] [Google Scholar]
- 25.Jones A, Korpi E R, McKernan R M, Pelz R, Nusser Z, Mäkelä R, Mellor J R, Pollard S, Bahn S, Stephenson F A, Randall A D, Sieghart W, Somogyi P, Smith A J H, Wisden W. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hogan B, Beddington R, Constantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. [Google Scholar]
- 27.Bonnerot C, Nicolas J-F. Methods Enzymol. 1993;225:451–469. doi: 10.1016/0076-6879(93)25031-v. [DOI] [PubMed] [Google Scholar]
- 28.Dobie K, Methali M, McLenaghan M, Lathe R. Trends Genet. 1997;13:127–130. doi: 10.1016/s0168-9525(97)01097-4. [DOI] [PubMed] [Google Scholar]
- 29.Bahn S, Harvey R J, Darlison M G, Wisden W. J Neurochem. 1996;66:1810–1818. doi: 10.1046/j.1471-4159.1996.66051810.x. [DOI] [PubMed] [Google Scholar]
- 30.Hadingham K L, Garrett E M, Wafford K A, Bain C, Heavens R P, Sirinathsinghji D J S, Whiting P J. Mol Pharmacol. 1996;49:253–259. [PubMed] [Google Scholar]
- 31.Aparicio S, Morrison A, Gould A, Gilthorpe J, Chaudhuri C, Rigby P, Krumlauf R, Brenner S. Proc Natl Acad Sci USA. 1995;92:1684–1688. doi: 10.1073/pnas.92.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motejlek K, Häuselmann R, Leitgeb S, Lüscher B. J Biol Chem. 1994;269:15265–15273. [PubMed] [Google Scholar]
- 33.Hatten M E, Alder J, Zimmerman K, Heintz N. Curr Opin Neurobiol. 1997;7:40–47. doi: 10.1016/s0959-4388(97)80118-3. [DOI] [PubMed] [Google Scholar]
- 34.Kim J J, Thompson R F. Trends Neurosci. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- 35.Raymond J L, Lisberger S G, Mauk M D. Science. 1996;272:1126–1131. doi: 10.1126/science.272.5265.1126. [DOI] [PubMed] [Google Scholar]
- 36.Allen G, Buxton R B, Wong E C, Courchesne E. Science. 1997;275:1940–1943. doi: 10.1126/science.275.5308.1940. [DOI] [PubMed] [Google Scholar]
- 37.Fiez J A. Neuron. 1996;16:13–15. doi: 10.1016/s0896-6273(00)80018-5. [DOI] [PubMed] [Google Scholar]
- 38.Tsien J Z, Chen D F, Gerber D, Tom C, Mercer E H, Anderson D J, Mayford M, Kandel E R, Tonegawa S. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]