Abstract
Immunologic research in nonhuman primates is occasionally limited by the availability of reagents that cross react in nonhuman primates. One major limitation has been the lack of a monoclonal antibody to CD45RO. Although the monoclonal antibody UCHL-1 is used to detect CD45RO isoforms in humans, it does not react with nonhuman primates, mandating the use of alternative strategies to define “memory” T cell responses in nonhuman primates. The current study examined the reactivity and specificity of another antibody against CD45RO, clone OPD4, in macaques. Here we demonstrate that OPD4 specifically labels memory CD4+ T cells in ~44% of rhesus macaques (Macaca mulatta) of Indian, but not Chinese origin. In contrast, tissues from pigtail macaques (Macaca nemestrina) react with this clone, indicating that OPD4 may be useful for examining memory CD4+ T cells in certain macaques, but its utility may be limited in other species or even among individual macaques.
Keywords: macaque, nonhuman primate, CD4+ T cell, naïve/memory, CD45RO, immunohistochemistry, OPD4, immunology
1. Introduction
Distinguishing naïve and memory T cell responses has greatly facilitated the investigation of T cell function and homeostasis in nonhuman primates, which are critical for understanding the pathogenesis and immunology of infectious diseases. The ability to distinguish naïve and memory subsets in macaques led to the discovery that simian immunodeficiency virus rapidly and selectively infects and eliminates “memory” CD4+ T cells, particularly in mucosal tissues [1–3], findings that were recently confirmed in HIV-infected patients [4, 5]. These findings have revolutionized our understanding of HIV pathogenesis by demonstrating that HIV eliminates memory CD4+CCR5+ T cells in primary infection, which are mostly found in mucosal lymphoid tissues. By rapidly and continuously eliminating CD4+ T cells that have previously responded to antigen (memory CD4+ T cells), it is now believed that the events leading to the significant immunological impairment that characterize acquired immune deficiency syndrome (AIDS) of HIV infection may begin much earlier than previously believed.
Unfortunately, accurately distinguishing naïve and memory T cell subsets in nonhuman primates is complicated by a limitation of reagents that cross-react in nonhuman primates. Originally, isoforms of the common leukocyte antigen CD45 were believed to reliably distinguish the conversion of naïve and memory T cell subsets in humans. Following antigenic stimulation, resting, naïve T cells undergo splicing of the CD45 molecule into isoforms, of which CD45RA and CD45RO were originally reported to distinguish naïve (CD45RA+) from memory (CD45RO+) T cell subsets [6–9]. Since this is a dynamic process, cells undergoing this conversion may at least transiently express both of these isoforms, so reliably distinguishing naïve and memory cells required simultaneous examination of both on the T cell subsets of interest. In addition, recent observations indicated that at least memory CD8+ T cells could revert to a CD45RAhigh phenotype [10], thus complicating the analysis of naïve and memory cell phenotypes based on phenotyping alone. Regardless, the CD45RO isoform can still be used to identify memory T cell subsets in humans, even if it does not label all such cells. In other words, even though not all memory CD8+ T cells may express CD45RO, it is still believed that all CD45RO+ cells are memory cells, making this a reliable marker of memory CD4+ and CD8+ T cells in humans.
Unfortunately, the most commonly used monoclonal antibody (UCHL-1) to CD45RO does not cross react in macaques, and thus, alternate methods of delineating naïve and memory T cells by immunophenotyping have been developed, including CD95/CD28 +/− CCR7 and other strategies such as staining T cell subsets with CD45RA and interpreting CD45RA negative cells as “memory”. Having a single monoclonal antibody (i.e., CD45RO) that cross reacts with memory cell subsets in tissues would facilitate research on immune responses in nonhuman primates.
Previous studies have shown that clone OPD4 recognizes an antigen with a molecular weight of 200 Kd, corresponding to that of leukocyte common antigen isoform CD45RO [11, 12]. Furthermore, OPD4 was reported to be reactive with CD45RO at the Fifth International Leukocyte Typing Workshop [13]. OPD4 is similar to UCHL1, and specifically labels memory CD4+ T cells, yet unlike UCHL-1, it does not cross react with monocytes, macrophages and granulocytes [11, 14]. In humans, OPD4 reacts with CD45 in formalin-fixed, paraffin- embedded tissue sections [11]. If this antibody were to reliably work in nonhuman primates and specifically label memory CD4+ T cells it would expand the number of analyses that could be made in tissues of nonhuman primates.
While OPD4 has been shown to cross-react in rhesus macaques [1, 15] its specificity has not been thoroughly examined in rhesus or other macaque species. In this study, we examined the reactivity, distribution, and specificity of OPD4 in rhesus macaques (Macaca mulatta) of both Chinese and Indian origin, as well as Pigtail macaques (Macaca nemestrina). Although they differ somewhat with respect to their response to SIV infection [16] both Indian and Chinese-origin rhesus macaques are currently considered the same genus and species (Macaca mulatta) and both are widely used in nonhuman primate studies of AIDS and other diseases. Similarly, pigtailed macaques are widely used as a model for AIDS. Thus, the current study was designed to determine the cross reactivity of the monoclonal antibody OPD4 in these three macaques. Reactivity and specificity of OPD4 was tested in blood and tissues of three different macaque species/subspecies by multi-color flow cytometry, and by immunohistochemistry on frozen and formalin-fixed, paraffin embedded tissues.
2. Materials and methods
2.1 Sample collection and criteria for reactivity
Cross reactivity of the OPD4 monoclonal antibody in macaques was confirmed by flow cytometry on peripheral blood mononuclear cells (PBMC) and/or by immunohistochemistry on snap frozen tissues collected immediately after necropsy. Cross-reactivity was also assessed by reactivity on formalin-fixed, paraffin embedded tissues. Peripheral blood for flow cytometry was collected in EDTA anti-coagulant from 28 Rhesus macaques of Chinese origin, 33 of Indian origin (7 infants and 26 adults) and 4 pigtail macaques. Formalin fixed or frozen tissues were examined in an additional 45 macaques of Indian origin (28 infant and 17 adults) and 6 pigtail macaques. In all, tissues were examined from 68 Indian origin macaques (28 infants and 40 adults), 28 Chinese origin macaques, and 10 pigtail macaques.
2.2 Multi-color Flow cytometry
For flow cytometry, peripheral blood mononuclear cells (PBMC) were stained with OPD4 using an indirect staining procedure. PBMC were prepared by Ficoll density gradient separation and 106 cells were incubated with 1 ul unconjugated OPD4 (Dako, Inc., Carpinteria, CA) for 20 min at room temperature (RT), washed with phosphate buffered saline (PBS), centrifuged at 400×g, the supernatent decanted, and then incubated with 1 ul 1:100 goat anti-mouse IgG1 labeled with Alexa 488 (Invitrogen, Carlsbad, CA) for 20 min at RT. Cells were washed again and surface stained with CD3-pacific blue, CD95-PE-Cy5, CD20-APC, CD28-APC, CD45RA-PE (Becton Dickinson, Carpinteria CA), CD4-AmCyan (NIH Nonhuman Primate Resource Reagent), and/or CD8 PE-Texas Red (Caltag Inc.), and incubated for 30 min at 4°C. Cells were then washed and fixed overnight in 1% paraformaldehyde. Samples were acquired the next day on a FACS Aria flow cytometer (Becton Dickinson). Data were analyzed with Flowjo software (Tree star, Inc). At least 10,000 lymphocytes were collected for analysis from each sample, and data were analyzed by gating through lymphocytes and then through cells of interest as described for individual analyses.
2.3 Immunochemistry
Paraffin embedded sections were de-paraffinized and “unmasked” using high temperature antigen retrieval which consisted of heating slides in a steam bath chamber (Black and Decker Flavor Center Steamer Plus) with 0.01 M citrate buffer, pH 6.0 for 20 min, cooled, and washed twice in PBS.
Slides were blocked using peroxidase blocking solution (Dako Inc.) for 10 min at RT, washed in PBS, then blocked again using serum-free protein block (Dako Inc.) for 30 min. Slides were washed again and incubated with purified OPD4 (1:20 dilution) for 1 hour at room temperature. Slides were washed and labeled with secondary antibodies using an ABC peroxidase detection kit (Vector labs, Burlingame, CA) according to manufacturer’s instructions. Slides were developed using the chromogen 3-amino-9-ethylcarbazole (AEC) or 3′, 3-diaminobenzidine tetrahydrochloride (DAB)(Dako Inc, Carpinteria, CA) for single labeling to assess the distribution of OPD4+ cells in sections, respectively.
For double-label immunohistochemistry slides developed with AEC were then labeled with anti-CD4 and the DakoCytomation EnVision doublestain system (Dako, Inc). Briefly, following AEC development, the sections were washed and blocked with Doublestain block and incubated with anti-CD4 mAb (1:50, Biocare Medical) for 1 hr. Envision alkaline-phosphatase anti-mouse/rabbit polymer (Dako, Inc) was used as the secondary antibody. Slides were developed with Vector blue (Vector labs). Specimens were mounted in aqueous based mounting medium and examined by light microscopy.
2.4 Three-color immunofluorescent staining and Confocal microscopy
For confocal microscopy, 3-color fluorescent immunostaining was performed on formalin-fixed tissues. Briefly, sections were incubated with OPD4 as above but incubated with Alexa Fluor 488 (green) labeled secondary antibody (goat anti-mouse IgG1, Invitrogen, Carlsbad, CA ) instead. Slides were then washed and incubated with appropriately diluted primary antibodies, CD45RA (mouse IgG2b, 1:50, Caltag Laboratories) and CD3 (rabbit polyclonal, 1:100, Dako Inc.), washed, and incubated with Alexa Fluor 568 labeled goat anti-mouse IgG2b (Invitrogen, Carlsbad, CA) and Alexa Fluor 633 labeled anti-rabbit IgG (H+L), (Invitrogen, Carlsbad, CA) secondary antibody to detect CD45RA (red) and CD3 (blue), respectively. Finally, slides were mounted with fluorescent mounting medium (Dako, Inc.) and visualized using a confocal microscope.
Confocal microscopy was performed using a Leica TCS SP2 confocal microscope equipped with three lasers (Leica Microsystems, Exton PA). Individual optical slices represent 0.2 mm and 32 to 62 optical slices were collected at 512 × 512 pixel resolution. NIH Image (version 1.62) and Adope Photoshop (version 7.0) were used to assign colors to the channels collected.
3. Results
3.1 Reactivity of OPD4 in macaques
To determine whether OPD4 cross-reacted in different species of macaques, and in rhesus macaques of Chinese and Indian origin, labeling was assessed by flow cytometry and/or immunohistochemistry. Interestingly, OPD4 did not cross react with any of the 28 Chinese origin rhesus macaques tested (Table 1).
Table 1.
Cross reactivity of OPD4 (anti-CD45RO) in macaques
| Species | Origin | Age (yrs) | Number tested | OPD4+ | %OPD4+ |
|---|---|---|---|---|---|
| Rhesus | Indian origin | <0.5 | 28 | 19 | 68 |
| 2–20 | 40 | 11 | 28 | ||
| Total | 68 | 30 | 44 | ||
| Chinese origin | 3.5–15.1 | 28 | 0 | 0 | |
| Pigtail | 1.2–13 | 10 | 8 | 80 |
In marked contrast, tissues from 8 of 10 Pigtail macaques cross-reacted with OPD4. Four of 4 pigtails were positive for OPD4 by flow cytometry and 4 of 6 were positive by immunohistochemistry on formalin-fixed, paraffin embedded tissues. However, fresh tissues from the two pigtails that did not cross-react by immunohistochemistry were not available, so it is not certain whether the latter was due to cross linking of the antigen by formalin fixation. Regardless, the majority (80%) of pigtailed macaques cross-reacted with this antibody, whereas none of the Chinese origin macaques cross reacted (Table 1).
The Indian origin macaques were more heterogenous in their reactivity to OPD4. In total, 30 of 68 Indian origin macaques reacted with OPD4 either by flow cytometry or immunohistochemistry. Ten macaques were tested by both immunohistochemistry and flow cytometry with consistent results, in that macaques that reacted by flow cytometry were always positive by immunohistochemistry. Furthermore, immunohistochemistry on both snap-frozen and formalin-fixed tissue sections was performed on 25 animals, and the results (negative or positive) always correlated. Combined, this suggests that formalin fixation of paraffin embedded tissues did not alter the antigenicity of the OPD4 epitope or cross-reactivity of the antibody. Furthermore, 13 of 21 Indian macaques were negative by flow cytometry, demonstrating a bona-fide lack of recognition of the native CD45RO antigen by the OPD4 mAb. This is based on the deduction that flow cytometry on fresh (unfixed) tissue samples is the “gold standard” for testing cross reactivity of a monoclonal antibody, since the target epitope is not manipulated or altered by fixation/high temperature, and/or antigen retrieval techniques.
Interestingly, there appeared to be a difference in reactivity between neonatal and adult macaques. Nineteen of the 28 neonatal macaques tested reacted with the monoclonal antibody (19 of which were positive by immunohistochemistry on formalin fixed tissues) whereas only 11 of the 40 adults tested cross-reacted with OPD4 (Table 1).
3.2 Specificity of OPD4 in Macaques
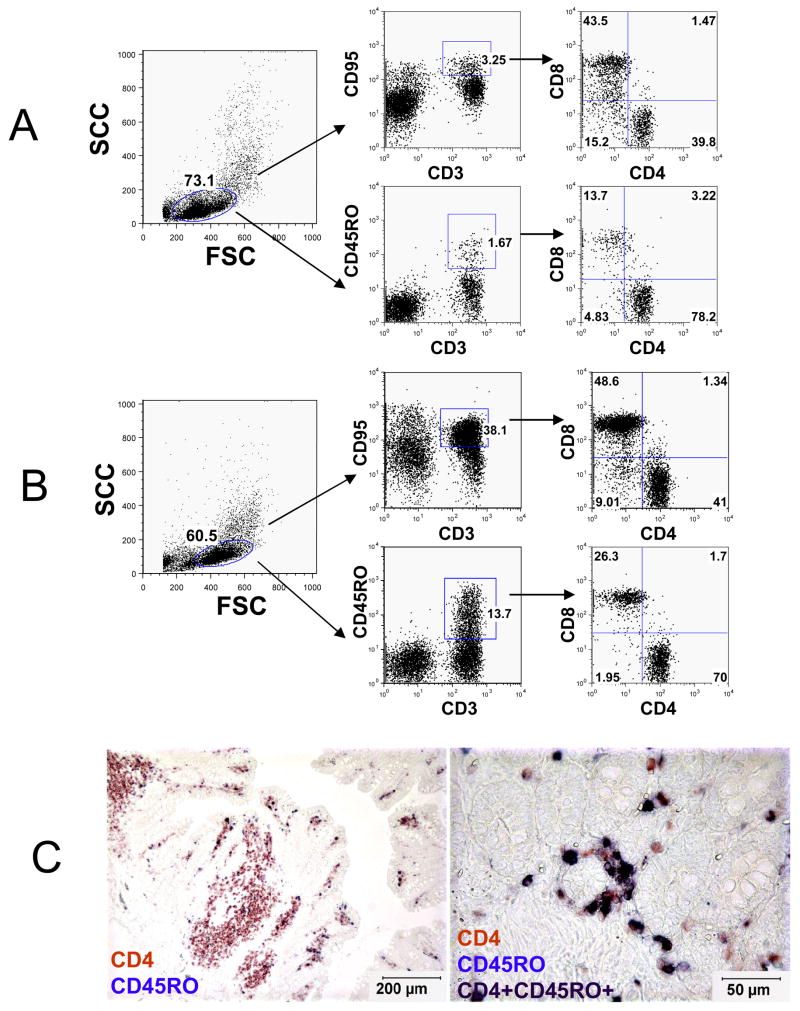
To identify the specificity of OPD4 in macaques for memory CD4+ T cells, we examined the expression of OPD4 using a series of flow cytometry and immunochemistry analyses. Flow cytometry revealed that, unlike CD95, essentially all OPD4+ cells co-expressed CD3 (Fig 1), and OPD4 did not cross-react with B cells or monocytes (data not shown). Of the CD3+CD45RO+ T cells, approximately 70–80% were CD4+, and 14–26% were CD8+ T cells (Fig 1). In contrast, of the CD3+CD95+ T cells, only about 40% were CD4+ while 45% were CD8+ (Fig 1). This indicates that the CD45RO preferentially recognizes memory CD4+ T cells.Furthermore, dual enzyme-linked immunohistochemistry for CD45RO and CD4 in tissue sections suggested that OPD4+ cells were mostly CD4+ (Fig 1C). Combined, these results demonstrated that, as in humans [11] the expression of OPD4 is restricted to memory T cells, and largely (but not exclusively) restricted to CD4+ T memory cells in macaques.
Figure 1.
Flow cytometry dot plots (A, B) and dual immunohistochemistry staining (C) demonstrating that CD45RO (OPD4) is predominantly expressed on CD4+ T cells in rhesus macaques (A is from a 14 day old neonatal macaque (GL06) and B is from an adult 12 year old macaque (R533). Note that essentially all OPD4+ lymphocytes from both neonates (A) and adults (B) co-express CD3 in contrast to CD95, which also labels a subpopulation of non-CD3+ lymphocytes. The left panel indicates the lymphocyte gate and the center plots demonstrate CD45RO expression on CD3+ cells. The right plots indicate that the majority of OPD4+ (CD45RO) T cells are also CD4+ in both adult and neonatal macaques. C) Dual immunohistochemistry for CD4 (red) and CD45RO (blue) on paraffin-embedded intestinal sections showing CD45RO (OPD4) is expressed mostly on CD4+ T cells (purple/black, black arrows). The image on the right is a higher magnification of the intestine showing CD4+CD45RO+ cells as dark purple (black arrows) and a few CD4+ CD45RO neg cells in red (white arrows).
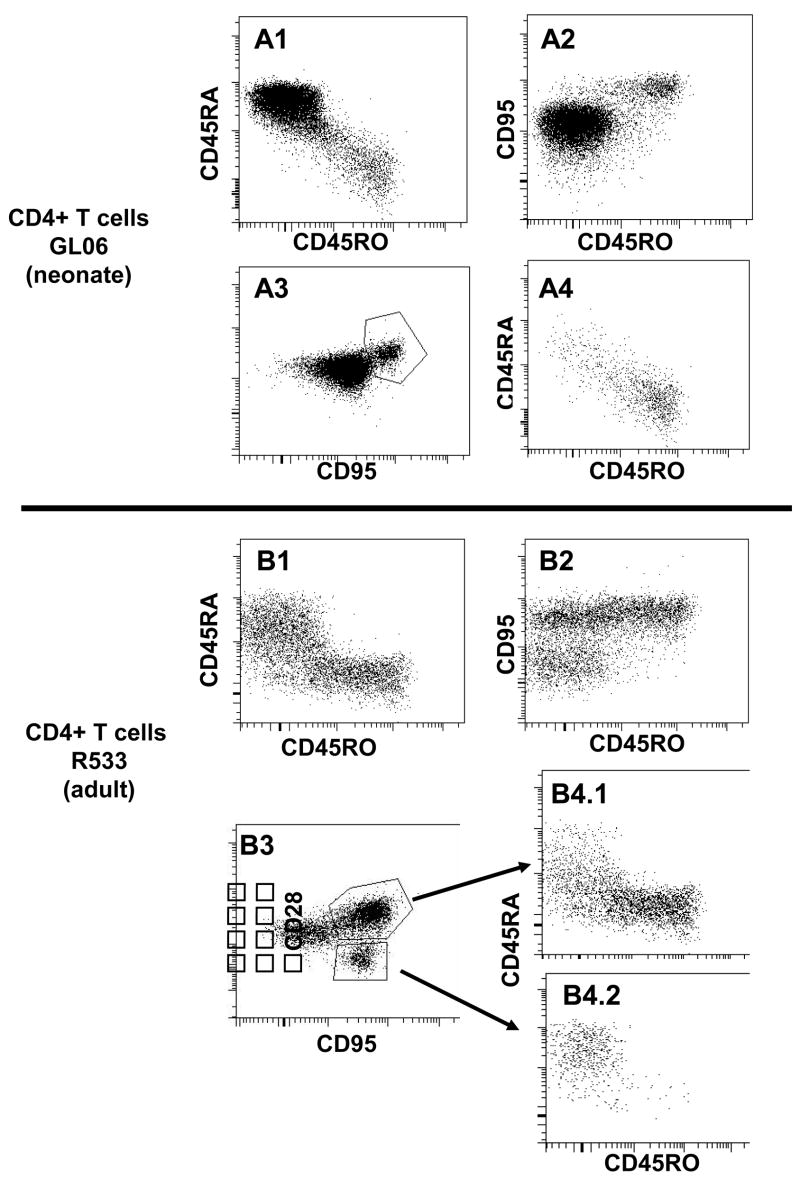
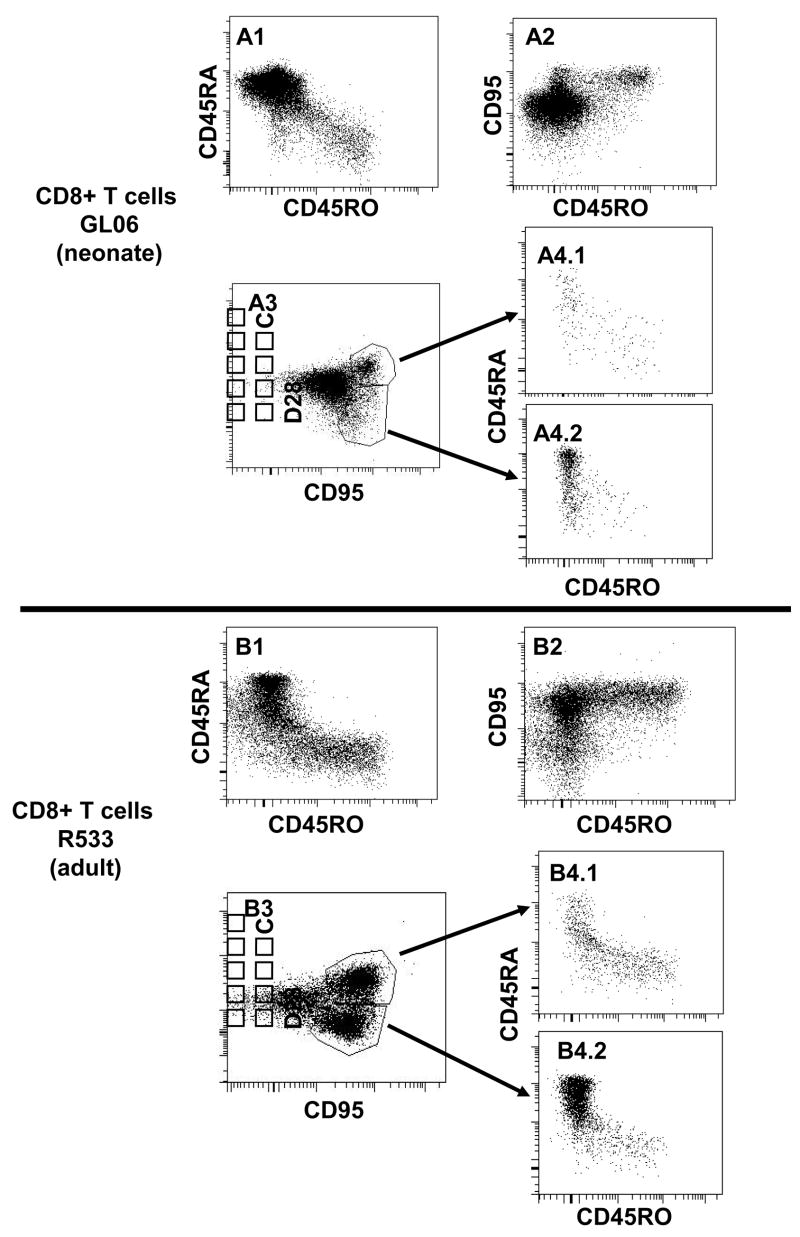
The co-expression of other naïve and memory markers (CD45RA and CD95) was also examined on OPD4+ cells (Fig 2). CD95 is considered to be a reliable marker for memory cells in macaques [17], and CD45RA is, in general, a marker for naïve cells [7, 18]. As shown in Fig 2, CD4+ T cells that co-expressed CD45RO (OPD4+) in both neonates and adults were essentially all CD95+, but did not co-express CD45RA. Furthermore, central memory cells (CD95+CD28+) were mainly CD45RO+CD45RA−, although a small population was CD45RAdimCD45ROdim in both neonatal and adult rhesus macaques (Fig 2). Not surprisingly, adult macaques had effector memory (CD95+CD28−) cells, which were absent in neonates. Interestingly however, these effector memory CD4+ T cells were CD45RA+ and CD45RO− (Fig 2). Although functional studies have not (to our knowledge) been performed with memory CD4+ T cells, these results are reminiscent of those previously reported for CD8+ T cells, which may revert to a CD45RA+ phenotype following antigen stimulation [10]. Consistent with this, similar expression patterns were detected in the CD8+ T cells that labeled with OPD4 (Fig. 3). In summary, these data indicate that OPD4 consistently identifies CD4+ memory cells in macaques, when it labels at all, and may be more useful for delineating naïve and memory T cell subsets than CD45RA alone.
Figure 2.
Multi-color flow cytometry demonstrating CD45RO staining is largely restricted to memory CD4 T cells in both neonate (A; GL06, 14 days old) and adult (B; R533, 12 years old) rhesus macaques. Plots were generated by first gating on CD3+ lymphocytes then through CD4 cells, so only CD4+ T cells are shown. A1, B1) Note that CD45RA bright cells generally do not co-localize with CD45RO+ cells. A2, B2) CD45RO+ cells all co-express CD95, and A3, B3) central memory cells (defined here as CD95+CD28+) are mostly CD45RO+, verifying that OPD4+ cells are “memory” cells. Finally, note that adult macaques have an effector memory (CD95+CD28−) population (B3) that is absent in neonates (A3). Interestingly, these cells are CD45RA+ and CD45RO−, suggesting that terminally differentiated memory CD4+ T cells may revert to a CD45RA+ state (see text).
Figure 3.
Multi-color flow cytometry demonstrating CD45RO expression on CD8+ T cells. Similar to CD4+ T cells, CD45RO expression is largely restricted to CD95+ memory CD8 T cells in both neonate (A; GL06, 14 days old) and adult (B; R533, 12 years old) rhesus macaques. Plots were generated by first gating on CD3+ lymphocytes then through CD8 cells, so only CD8+ T cells are shown. A1, B1) Note that CD45RA bright cells generally do not co-localize with CD45RO+ cells. A2, B2) CD45RO+ cells all co-express CD95, and A3, B3) central memory cells (defined here as CD95+CD28+) are mostly CD45RO+, verifying that OPD4+ cells are “memory” cells. Finally, note that adult macaques have a much higher proportion of effector memory (CD95+CD28−) CD8+ T cells (B3) compared to neonates (A3). Interestingly, these “effector memory” CD8+ T cells are mostly CD45RA bright and CD45RO negative, suggesting that terminally differentiated memory CD8+ T cells revert to a CD45RA+ state (see text).
3.3 Localization of CD45RO+ cells in rhesus macaque tissues
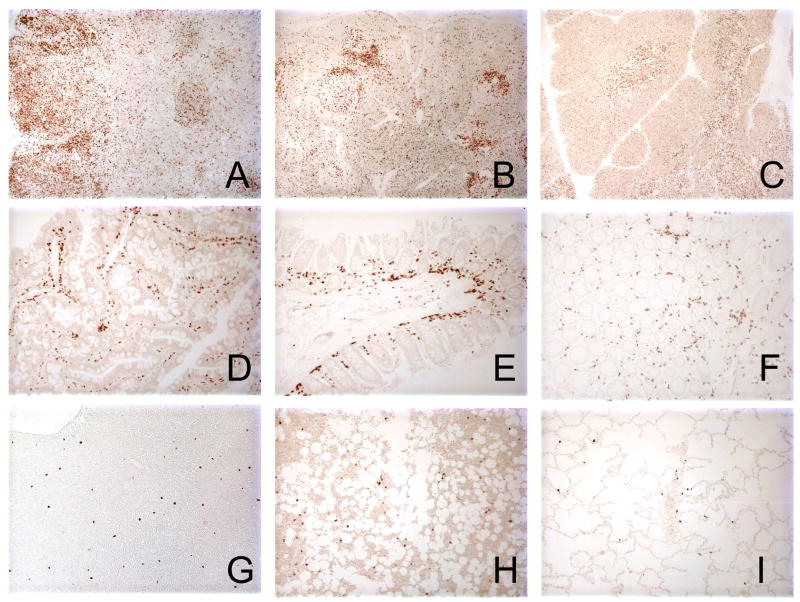
Similar to the staining pattern in human tissues [11], OPD4 reacted mainly with cells in the marginal T-cell zone of rhesus macaque organized lymphoid tissues such as lymph nodes and spleen (Fig. 4). A few scattered lymphocytes in the mantle and germinal centers were also positive for OPD4 (CD45RO), but morphologically, lymphocytes were the only cells that reacted with this antibody, as tingible-body macrophages, dendritic cells, sinus histiocytes and endothelial cells were all negative (Fig. 3A and B). We also found that OPD4 weakly reacted with cortical, and strongly with medullary thymocytes (Fig. 4C). In addition, abundant CD45RO+ cells could be detected throughout the intestine (Fig. 4D, E and F), particularly in the lamina propria. Finally, a few cells were detected in the liver, bone marrow and lung (Fig. 4G, H and I).
Figure 4.
Immunohistochemistry for OPD4 (CD45RO) in normal lymphoid tissues of an adult Indian-origin rhesus macaque (AJ79, 7.2 years old). A–B), Large numbers of CD45RO+ cells are present in the T cell zone of lymph node (A) and spleen (B). C) Section of thymus showing weakly positive cells in the cortical region but the intensely stained cells in the medulla. D–F) Intestinal sections including jejunum (D), ileum (E) and colon (F) demonstrating large numbers of OPD4+ T cells in the lamina propria (F). G–I) Rare OPD4+ T cells are present and scattered in the liver (G), bone marrow (H) and lung (I). All photomicrographs were taken at an original magnification of 200X.
3.4 Phenotype of OPD4 positive cells by three-color immunofluorescent staining in tissues
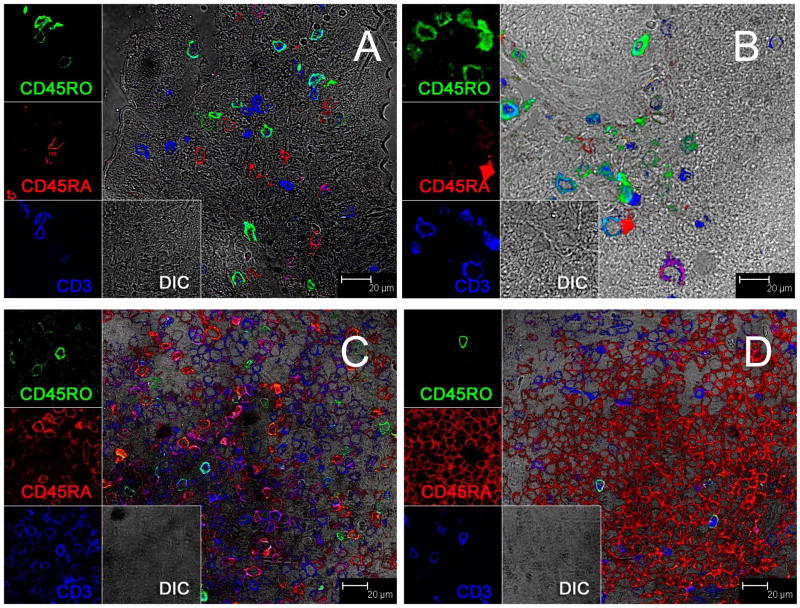
In tissues, essentially all of the OPD4+ (CD45RO+) cells were CD3 positive and lacked CD45RA expression (Fig. 5A and B). In addition, the vast majority of OPD4+ cells in the intestine were found in effector lymphoid tissues (lamina propria) with rare CD45RA+ (naïve) cells in this tissue (Fig. 5A–B). In contrast, organized lymphoid tissues harbored mainly naïve cells (CD45RA+) with rare CD45RO+ cells (Fig. 5C–D). Combined, these results are consistent with the interpretation that OPD4 selectively labels memory cells.
Figure 5.
Three-color immunofluorescent staining with CD45RO (green) CD45RA (red) and CD3 (blue) showing naïve/memory CD4 T cells in the jejunum (A) colon (B), and lymph nodes (C and D). Note that CD45RA and CD45RO do not co-localize indicating OPD4 is specific for memory cells. Also note that CD45RO+ cells are frequent in the intestinal lamina propria (A and B) but rare in organized lymphoid tissues of the lymph node (C and D) consistent with the specificity of this antibody for memory cells.
4. Discussion
The monoclonal antibody OPD4 is of particular interest for nonhuman primate research, because its expression is largely restricted to mature memory CD4 cells in human tissues [11]. Thus, having a single marker that reliably labels CD4+ memory cells in macaques could facilitate research into human diseases such as AIDS, malaria, tuberculosis, and others. Since OPD4 only reacts with an antigen of normal T cells and T-cell lines expressed on the helper/inducer phenotype, it could be considered as a “3 in 1” antibody, in that OPD4 positive cells are predominantly CD3+CD4+ memory T cells. However, a small proportion of OPD4+ cells were CD4neg, CD8+ cells (Fig. 1) indicating that it does not exclusively label CD4+ T cells. Reactivity of this antibody in rhesus macaques of Indian origin by immunohistochemistry has previously been reported [1]. However, pigtailed macaques and rhesus macaques of Chinese origin are also widely used in research into these and other diseases. Thus, this study sought to determine the reactivity and specificity of this monoclonal antibody in these nonhuman primates.
Interestingly, in our study, the OPD4 monoclonal antibody only cross-reacted with 30 of 68 (44%) rhesus macaques of Indian origin examined from the TNPRC colony (Table 1). Furthermore, 20 of the macaques that were negative were tested by either flow cytometry or by immunohistochemistry on frozen tissue sections, indicating the lack of reactivity could not be due to antigen cross linking or other artifacts associated with fixation or techniques routinely used on formalin fixed samples. Interestingly, a much higher proportion of neonatal macaques (19 of 28 or 68%) were positive for OPD4 compared to the adult macaques (11 of 40 or 28%). However, the significance of this is not yet known.
The other macaques tested were more uniform in their response. Most of the pigtailed macaques (6 of 8) reacted well with the OPD4 mAb, and the two that didn’t were only tested on formalin fixed tissues. In contrast, none of the Chinese origin rhesus macaques reacted with the antibody, even though all (n = 28) were tested by flow cytometry on unfixed tissues.
In macaques in which this antibody reacted, we also confirmed that, as in humans, the antibody was specific for memory CD4+ T cells. Flow cytometry demonstrated that, unlike CD95, OPD4 staining was fairly selective for CD3+CD4+ cells, though some CD8+ T cells were also positive (Fig. 1). Regardless, almost all OPD4+ cells in both adult and neonatal macaques also co-expressed CD95, indicating it was selective for memory cells (Fig. 2–3). Finally, the distribution of OPD4+ cells was consistent with that of memory cells, as most of the OPD4+ cells in intestinal tissues were localized in effector sites (lamina propria) with fewer cells in organized lymphoid tissues, such as lymph nodes and spleen (Fig. 4). Combined, these results suggest that, similar to human, OPD4 reacts with CD45RO expressed on the helper/inducer memory CD4+ T cells in macaques.
In summary, the OPD4 monoclonal antibody appears to specifically label CD4+ memory cells in macaque tissues, but only in certain species, and in these, not all members of that species examined were positive. In pigtails, the antibody may be very useful, as it appears to cross-react with most individuals. In contrast, fewer than half of the Indian origin macaques tested in this study reacted with the antibody, and thus its utility in research may be restricted to pre-selected animals. Finally, it appears to be useless in rhesus macaques of Chinese origin, suggesting that there are genetic differences between the CD45 isotypes between these macaques.
Acknowledgments
We wish to thank Linda Green, Janell LeBlanc, and Maryjane Dodd for technical assistance and Calvin Lanclos and Julie Bruhn for flow cytometry assistance. This work was supported by NIH grants AI49080, AI062410, and RR00164.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Li Q, Duan L, Estes JD, et al. Peak SIV replication in resting memory CD4(+) T cells depletes gut lamina propria CD4(+) T cells. Nature. 2005 doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 2.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4(+) T cells in multiple tissues during acute SIV infection. Nature. 2005 doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 3.Veazey RS, Tham IC, Mansfield KG, et al. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000;74(1):57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200(6):761–70. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200(6):749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186(9):1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamann D, Baars PA, Hooibrink B, van Lier RW. Heterogeneity of the human CD4+ T-cell population: two distinct CD4+ T-cell subsets characterized by coexpression of CD45RA and CD45RO isoforms. Blood. 1996;88(9):3513–21. [PubMed] [Google Scholar]
- 8.Merkenschlager M, Beverley PC. Evidence for differential expression of CD45 isoforms by precursors for memory-dependent and independent cytotoxic responses: human CD8 memory CTLp selectively express CD45RO (UCHL1) Int Immunol. 1989;1(4):450–9. doi: 10.1093/intimm/1.4.450. [DOI] [PubMed] [Google Scholar]
- 9.Wallace DL, Beverley PC. Phenotypic changes associated with activation of CD45RA+ and CD45RO+ T cells. Immunology. 1990;69(3):460–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Wills MR, Carmichael AJ, Weekes MP, Mynard K, Okecha G, Hicks R, Sissons JG. Human virus-specific CD8+ CTL clones revert from CD45ROhigh to CD45RAhigh in vivo: CD45RAhighCD8+ T cells comprise both naive and memory cells. J Immunol. 1999;162(12):7080–7. [PubMed] [Google Scholar]
- 11.Yoshino T, Mukuzono H, Aoki H, et al. A novel monoclonal antibody (OPD4) recognizing a helper/inducer T cell subset. Its application to paraffin-embedded tissues. Am J Pathol. 1989;134(6):1339–46. [PMC free article] [PubMed] [Google Scholar]
- 12.Schuurman HJ, van Baarlen J, Huppes W, Lam BW, Verdonck LF, van Unnik JA. Immunophenotyping of non-Hodgkin’s lymphoma. Lack of correlation between immunophenotype and cell morphology. Am J Pathol. 1987;129(1):140–51. [PMC free article] [PubMed] [Google Scholar]
- 13.Serra-Pages C, Morimoto C, Schlossman SF, Saito H, Streuli M. Proceedings of the 5th International Workshop and Conference. In: Schlossman SF, Bournsell L, Gilks W, Harlan JM, Kishimoto T, Morimoto C, editors. 5th International Workshop Oxford University Press; 1993. [Google Scholar]
- 14.Poppema S, Lai R, Visser L. Monoclonal antibody OPD4 is reactive with CD45RO, but differs from UCHL1 by the absence of monocyte reactivity. Am J Pathol. 1991;139(4):725–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Rasmussen T, Pahar B, Poonia B, Alvarez X, Lackner AA, Veazey RS. Massive infection and loss of CD4+ T cells occurs in the intestinal tract of neonatal rhesus macaques in acute SIV infection. Blood. 2007;109(3):1174–81. doi: 10.1182/blood-2006-04-015172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling B, Veazey RS, Luckay A, Penedo C, Xu K, Lifson JD, Marx PA. SIV(mac) pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans. Aids. 2002;16(11):1489–96. doi: 10.1097/00002030-200207260-00005. [DOI] [PubMed] [Google Scholar]
- 17.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168(1):29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 18.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–83. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]