Abstract
Neuronal plasticity plays a very important role in brain adaptations to environmental stimuli, disease, and aging processes. The kainic acid model of temporal lobe epilepsy was used to study the long-term anatomical and biochemical changes in the hippocampus after seizures. Using Northern blot analysis, immunocytochemistry, and Western blot analysis, we have found a long-term elevation of the proconvulsive opioid peptide, enkephalin, in the rat hippocampus. We have also demonstrated that an activator protein-1 transcription factor, the 35-kDa fos-related antigen, can be induced and elevated for at least 1 year after kainate treatment. This study demonstrated that a single systemic injection of kainate produces almost permanent increases in the enkephalin and an activator protein-1 transcription factor, the 35-kDa fos-related antigen, in the rat hippocampus, and it is likely that these two events are closely associated with the molecular mechanisms of induction of long-lasting enhanced seizure susceptibility in the kainate-induced seizure model. The long-term expression of the proenkephalin mRNA and its peptides in the kainate-treated rat hippocampus also suggests an important role in the recurrent seizures of temporal lobe epilepsy.
Kainic acid is a rigid analog of the excitatory amino acid glutamate that binds to and activates ionotropic glutamate receptors. Kainate-induced epileptic seizures have been widely used as a model for studying human temporal lobe epilepsy (1–6). A single systemic injection of a convulsive dose of kainate results in both short-term and long-term effects on the rat central nervous system. Within 1 hr of its administration to rats, the neuronal circuitry of the hippocampus is activated and later the animal undergoes robust and recurrent seizures. Within 3–4 days after kainate injection, pyramidal cells in the CA1 and CA3 fields of the hippocampus begin to degenerate (4, 5, 7, 8). One or 2 weeks later, spontaneous convulsions can be observed in kainate-treated rats. The neuronal excitation caused by kainate leads to increases in the expression of a variety of genes including immediate-early genes, growth factors, and opioid peptides. The short-term effects of kainate in the rat brain have been well characterized (9–14). However, the long-term effects of kainate, especially on the opioid peptides, have not been reported.
The opioid peptides derived from the proenkephalin (PENK) and prodynorphin (PDYN) precursors occur at high levels in the central nervous system. Upon stimulation, opioid peptides and their precursors are released and can function as neuromodulators. Activation of opioid receptors by opioid peptides exerts a broad spectrum of effects on physiological and pathological processes (for reviews see refs. 15 and 16). Earlier studies by Sonnenberg et al. (17) suggested the involvement of the activator protein-1 (AP-1) transcription factors in the regulation of expression of PENK. Comb and coworkers (18) have defined important regulatory sequences in the human PENK promoter, which share similarities to the AP-1 recognition sequences. AP-1-like regulatory sequences have also been reported in the promoter region of the PDYN gene (19).
These studies were designed to determine the long-term effects of kainate on the expression of PENK and AP-1 transcription factors in regions of the rat brain known to be important in kindling and epilepsy, such as the dentate gyrus and entorhinal cortex. We have demonstrated that a single injection of kainate leads to the up-regulation of PENK mRNA and its protein products in the rat hippocampus for up to 1 year. We have characterized the 35-kDa fos-related antigen (FRA) and JunD in the rat hippocampus 1 year after kainate treatment. The close spatial and temporal correlation of the expression of these two AP-1 transcription factors with the up-regulation of PENK mRNA suggests that the long-term expression of PENK is regulated by the long-term elevated AP-1 transcription factors, i.e., 35-kDa Fra and JunD. These studies demonstrate that a single injection of kainate can alter, almost permanently, the biochemistry and physiology of the adult brain.
MATERIALS AND METHODS
Animals and Treatments.
Adult male Fischer 344 rats (225–250 g body weight) were used throughout the study. Control animals (n = 8) were injected s.c. with physiological saline. Experimental animals were injected with kainate (Sigma, 7.25 mg/kg s.c.). The animals were rated according to the scale devised by Racine (20) for the initial 4 hr after the kainate injection. Only animals with full limbic seizures (forelimb clonus with rearing, stage 4) were chosen for further studies. Animals were perfused with 4% paraformaldehyde in phosphate-buffed saline (PBS) (pH 7.4) at different time points.
Immunocytochemistry.
The brains were sectioned at 35 μm on a sliding microtome. The sections were incubated in 4% normal goat serum in PBS for 30 min at room temperature to block nonspecific immunostaining. After three washes with PBS (pH 7.4), the sections were incubated overnight in PBS containing 0.025% Triton X-100/1% normal goat serum and the primary antiserum at 4°C. The avidin–biotin immunoperoxidase method with 3,3′-diaminobenzidine tetrahydrochloride as the chromogen was used to visualize immunoreactive cells as described (21). The FRA antiserum donated by M. Iadarola (National Institutes of Health, Bethesda) was used at a 1:2,000 dilution. This antiserum was raised against the M peptide region of c-Fos, which is conserved among the FRA proteins (22).
Northern Blot Analysis.
Northern blot analysis as described in detail by Bing et al. (23) was used to detect PENK mRNA. Briefly, total RNA was isolated from immediately frozen brain tissue according to the Tri Reagent protocol (Molecular Research Center, Cincinnati) and electrophoresed through 1.5% agarose/formaldehyde gels (1× SSPE/1 mM EDTA/6% formaldehyde). After transfer onto nylon membranes (Hybond-N, Amersham) with 20× SSC/3.0 M NaCl/0.3 M sodium citrate, the filters were baked at 80°C under vacuum. The vectors containing the cDNA for PENK (courtesy of Steven Sabol, National Institutes of Health, Bethesda), PDYN (courtesy of James Douglass, Amgen), and cyclophilin (courtesy of James Douglass) were linearized and transcribed using α-[32P]UTP (DuPont/NEN) as the radioactive nucleotide. The filters were hybridized with the radiolabeled cRNA probes in hybridization buffer (40 mM NaPO4/1 mM EDTA/50% formamide/2% SDS/10 mg/ml salmon sperm DNA) for 16 hr at 55°C, washed twice in a solution containing 2× SSC/0.1% SDS at room temperature, and washed twice with 0.1× SSC/0.1% SDS for 30 min at 60°C. Filters were exposed to x-ray film (Hyperfilm; Amersham) with intensifying screens. The relative densities of the autoradiographic bands were quantified by laser densitometry. To correct for possible variations in the amounts of total RNA loaded in different lanes, PENK RNA values were corrected for cyclophilin RNA levels, a constitutively expressed gene.
In Situ Hybridization.
The expression of PENK mRNA was analyzed by in situ hybridization histochemistry. The hybridizations were performed using free-floating sections. After perfusion with 4% paraformaldehyde, the frozen rat brain sections were cut on a sliding microtome. The 35-μm sections were collected in 24-well culture dishes containing a cryopreservative solution. Every 12th section was used for each specific probe. 35S-Labeled cRNA probes were generated from vectors containing the cDNA for PENK and PDYN. The sections were hybridized with 35S-labeled probes with a specific activity greater than 1 × 109 cpm/μg at 50°C for 16 hr. For post-hybridization washing, the sections were rinsed twice in 2 × SSC/50% formamide/10 mM DTT, treated with RNase, and washed again with 2 × SSC/50% formamide/10 mM DTT, followed with 0.25 × SSC/10 mM DTT at 55°C for 30 min. The sections were mounted on slides coated with polylysine and exposed to x-ray film for 3–4 days. The sections were then dipped in Kodak NTB-2 emulsion (2:1 with water) and exposed for 1 week before being developed in D-19 developer solution.
Western Blot Analysis.
Hippocampi from control and treated rats were homogenized and nuclear protein extracts were prepared as described (24). The FRA antiserum used at a dilution of 1:500 (from M. Iadarola, National Institutes of Health) recognizes a conserved sequence of the M peptide and cross-reacts with all known FRAs (22). JunD and c-Fos antiserum, purchased from Santa Cruz Biotechnology, were used at a dilution of 1:1,000. Each nuclear extract was obtained from one hippocampus. Forty micrograms of protein extract were separated on SDS/10% polyacrylamide gels, transferred onto a nitrocellulose membrane, and the resulting blot was blocked in PBS containing 3% skim milk for 30 min. Blots were incubated overnight at 4°C with the antiserum against FRA at a 1:500 dilution. After washing in PBS, the membranes were incubated with a biotinylated goat anti-rabbit antiserum for 1 hr followed by incubation with avidin–biotin-conjugated horseradish peroxidase (Vectorstain kit, Vector Laboratories) at room temperature. After washing, the blots were incubated in enhanced chemiluminescence reagent (Amersham), placed against Amersham Hyperfilm, and the film was processed.
RESULTS
Behavioral and Morphological Effects of Kainate Treatment.
All animals were closely observed and rated for seizure behavior according to the scale developed by Racine (20). Thirty minutes after treatment, the animals began to display wet dog shakes. Two hours after injection full limbic motor seizures were observed, including rearing and loss of postural control. After 2.5–3 hr, the animals showed profuse salivation, circling and jumping, and status epilepticus. Hyperexcitability was seen in most animals throughout the period of the experiment.
Changes in the Expression of PENK mRNA Revealed by Northern Blot Analysis After Kainate Injection.
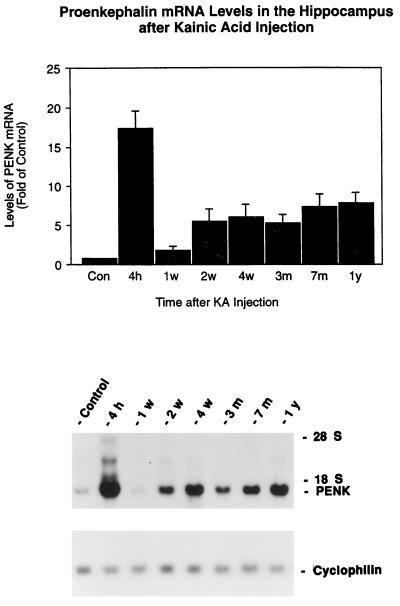
Because the major changes in the expression of PENK and PDYN mRNAs were in the hippocampi of the kainate-treated rats, the hippocampal tissues were dissected for Northern blot analysis. The major findings of this study were that kainate produces biphasic increases in PENK mRNAs in the rat hippocampus. The results showed that PENK mRNA levels were increased about 16-fold at 6 hr after kainate injection (Fig. 1). These increases returned by 7 days to the control levels, but they rose again at 2 weeks and persisted at this higher level for at least 1 year, the longest time point studied after kainate injections.
Figure 1.
Time course of the expression of PENK mRNA in the rat hippocampus after kainate injection. Rats were injected with kainate (7.25 mg/kg) s.c.. Hippocampi (n = 4) were collected at different time points and analyzed with Northern blots. (Upper) The autoradiographic signals corresponding to PENK and cyclophilin mRNA were quantified by laser densitometry. Ratios of PENK/cyclophilin mRNA density were calculated. Data represent means ± SEM. P < 0.01 at all time points compared with control except at 1 week (two-way analysis of variance, Bonferroni–Dunn post hoc tests were performed for between groups comparison). Control represents the mean of different time points from 1 week, 3 months, 7 months, and 1 year after a single injection of saline. There was no significant difference in the levels of PENK mRNA from these of control hippocampi. (Lower) Representative autoradiogragh of a Northern blot probed with 32P-labeled PENK and cyclophilin cRNAs showing the effects at 4 hr, 1 week, 2 weeks, 1 month, 3 months, 7 months, and 1 year after kainate treatment. The control sample was from the 3-month time point.
Changes in the Expression of PENK mRNA Revealed by in Situ Hybridization After Kainate Injection.
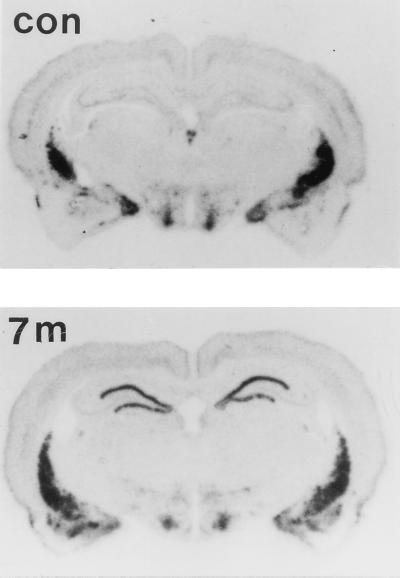
PENK mRNA expression as revealed by in situ hybridization is shown in Fig. 2. The increases in PENK mRNA compared with the control were primarily in the granule cell layer of the dentate gyrus 7 months after kainate treatment. The mRNAs visualized in the striatum, hypothalamus, and entorhinal cortex did not changes 7 months after kainate injection.
Figure 2.
Autoradiographs of in situ hybridization for PENK mRNA on coronal sections of the rat brain. Representative autoradiographs from four independent experiments were taken from a control animal 7 months after saline injection and 7 months after kainate treatment. Autoradiographs were made from x-ray film 7 days after exposure to brain sections hybridized with 35S-labeled probes.
The specificity of the riboprobes for PENK was studied with a sense probe from the same region as the antisense probes. The sense probe revealed virtually no hybridization signals in the hippocampus 7 months after kainate injections.
Expression of Enkephalin (ENK)-Immunoreactivity (IR) in the Rat Hippocampus After Kainate Treatment.
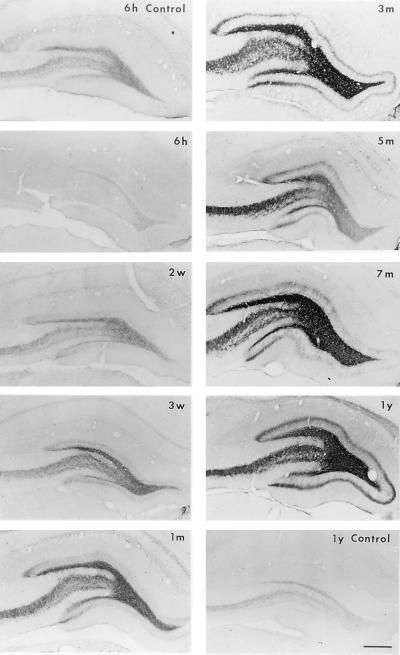
To determine whether the levels of ENK-like opioid peptides correlated with the persistent expression of PENK mRNA levels, alternate sections were processed for immunocytochemistry for ENK. Systemic administration of kainate resulted in the general reduction of PENK-derived peptide in the hippocampus 6 hr after treatment, suggesting a dramatic release of stored pools (Fig. 3). However, the ENK-IR rose again 3 weeks after kainate treatment and lasted for at least 1 year. This increase of ENK immunostaining was consistently prominent in the mossy fibers of hippocampus. Interestingly, ENK-IR in the hippocampus changed with the same temporal and spatial pattern as that observed for zinc with the Timm’s silver method (unpublished observation), which is commonly used to document mossy fiber sprouting. Ectopic ENK-IR was observed in the inner one-third of the molecular layer of the dentate gyrus 3 weeks after the kainate injections, and the immunoreactivity thickened progressively (Fig. 3).
Figure 3.
Expression of ENK-IR in the rat hippocampus after kainate treatment. Representative immunocytochemical photomicrographs from three separate experiments are shown. Hippocampal immunostainings for ENK-IR from saline-treated rats at different time points were similar; photomicrographs from 6 hr (6 h) and 1 year (1 y) are shown. The kainate-treated groups include 6 hr (6 h), 2 weeks (2 w), 3 weeks (3 w), 1 month (1 m), 3 months (3 m), 7 months (7 m), and 1 year (1 y). Dramatic increases in ENK-IR were observed at 3 weeks after kainate treatment and persisted for up to 1 year. Note the progressive increase in the density of ENK-IR in the inner molecular layer of the dentate gyrus (sprouting of mossy fibers) in kainate-treated animals. (Scale bar = 400 μm.)
Expression of FRA and JunD Immunoreactivity in the Rat Hippocampus After Kainate Treatment.
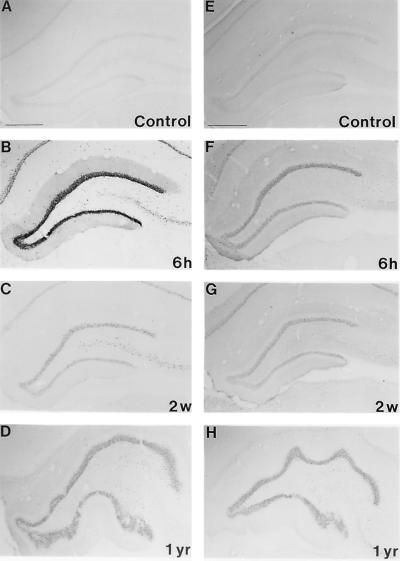
Previous reports from our laboratory and by others have shown that most of immediate-early genes, such as c-fos, fra 1, fra 2, c-jun, and junB, were only induced transiently in the rat brain (8, 25–31). To examine the possibility that the 35-kDa FRA and JunD are involved in the regulation of the long-term changes in opioid peptide levels, we processed alternate sections with FRA and JunD antisera and compared their spatiotemporal changes with those of PENK. The short-term changes in FRA-IR in the hippocampal formation have been reported (8, 32–36). Fig. 4 shows the dramatic increase in FRA-IR 6 hr after kainate treatment, but the most unexpected and interesting finding was the long-term increase of FRA-IR in the brain, particularly in limbic structures 2 weeks and 1 year after kainate treatment (Fig. 4). The dentate granule cell layer, which is spared from kainate-induced neurotoxicity, is the only structure examined that persistently expressed FRA-IR for at least 1 year. Compared with FRA-IR, the changes of JunD-IR after kainate injection appeared to be less dramatic. However, JunD-IR in the granular layer of the dentate gyrus after kainate injection was much denser than in the controls and this elevation persisted for at least 1 year.
Figure 4.
Representative photomicrographs from three separate experiments of FRA-like (A–D) and JunD-like (E–H) immunoreactivity in the hippocampus. The immunostained sections are from rats 6 hr (6 h), 2 weeks (2 w), and 1 year (1 yr) after treatment with kainate. Immunostaining for control from a 2-week (Control) time point is shown. There are no significant differences in the levels of FRA and JunD immunoreactivities between hippocampi 6 hr, 2 weeks, and 1 year after saline injection. Note the long-lasting changes in the intensity of the staining.
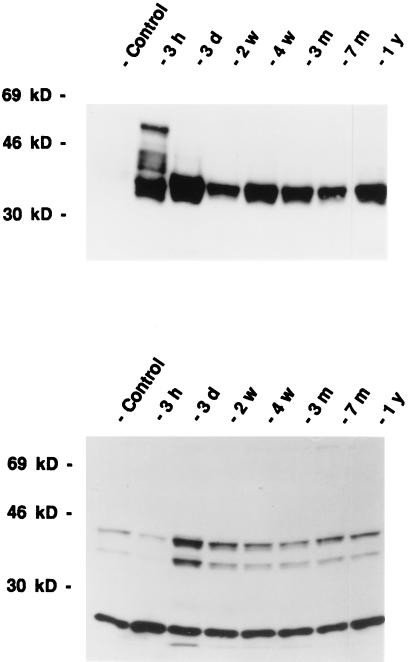
To characterize the 35-kDa FRA-like immunoreactivity, and to precisely define the composition of the AP-1 complex, we carried out Western blot analysis with FRA and JunD antisera. Western blot analysis with Fra antisera showed that the FRA immunoreactive protein bands at about 55, 46, and 35 kDa were markedly increased in intensity 3 hr after kainate treatment compared with the controls (Fig. 5). However, 2 weeks after kainate treatment, the 35-kDa FRA was the only immunoreactive FRA-like protein expressed and it was expressed at high levels for up to 1 year, and this 35-kDa band can be completely competed out with M peptides, which contain the conserved region from all of the known FRA. Because FRA have to dimerize with a Jun family member to bind to the regulatory sequences in the promoter region of the opioid peptide genes, we studied the expression of all known Jun family members, i.e., c-Jun, JunB, and JunD. The expression of c-Jun and JunD only lasted a few hours after kainate treatment (data not shown). JunD, however, appeared to be consistently expressed at high levels after kainate treatment (Fig. 5). There were three bands, 43-, 39-, and 28-kDa proteins, recognized by JunD antisera on Western blot analysis and all were competed out with the JunD peptide (data not shown). The 28-kDa band appears to be constitutively expressed at all time points. However, both the 39- and 43-kDa bands were expressed at lower levels in the control rats and 3 hr after kainate treatment. JunD-IR was consistently expressed at high levels 3 days or longer after kainate treatment.
Figure 5.
Time course of the expression of FRA-like (Upper) and JunD-like (Lower) immunoreactivity after kainate administration as revealed by Western blot analysis. Representative photomicrographs from four separate experiments are shown. Immunostaining for a control rat from a 3-month (Control) time point is shown. There were no significant differences in the levels of FRA and JunD immunoreactivity from the control hippocampi at different time points. The kainate-injected groups were sacrificed after 3 hr (3 h), 3 days (3 d), 2 weeks (2 w), 4 weeks (4 w), 3 months (3 m), 7 months (7 m), and 1 year (1 y). Note that at least four bands of protein were recognized by the FRA antiserum at 3 hr after kainate treatment, but only one 35-kDa FRA was detected at 2 weeks after kainate treatment. JunD antiserum appears to recognize three bands of immunoreactivity at 43, 39, and 28 kDa.
DISCUSSION
The major focus of this study was to understand the long-term consequences of altering gene expression in the nervous system and the mechanisms by which gene expression is regulated. This study demonstrates that a single injection of kainate induced a long-lasting up-regulation of PENK mRNA and its protein products in the rat hippocampus. Importantly, this elevation persisted for up to 1 year, the longest time point we investigated. We also characterized 35-kDa FRA, JunD, and their AP-1 DNA-binding activities (data not shown) in the rat hippocampus 1 year after kainate treatment. These two factors are colocalized in almost every granule cell of the dentate gyrus, which expresses the PENK mRNA. The close spatial and temporal correlation of the expression of these two AP-1 transcription factors with PENK suggest that the long-term expression of PENK is regulated by the long-term elevated AP-1 factors, i.e., the 35-kDa FRA and JunD. Because ENK is known to have proconvulsant properties in the hippocampus (37), the long-term increase of PENK mRNA and ENK-IR in the mossy fibers of the CA3 and CA4 regions and the prominent supragranular sprouting of ENK-containing mossy fibers may contribute to the development and expression of spontaneous seizures, which occur weeks to months after a single injection of kainate. The long-term expression of the ENK in the kainate-treated rat hippocampus suggests an important role in the recurrent seizures of human temporal lobe epilepsy.
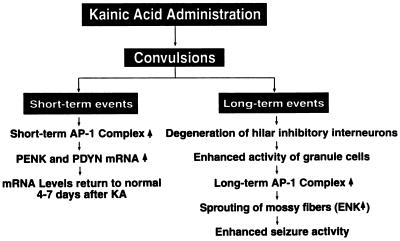
One of the most interesting findings from this study was the unexpectedly prolonged expression of AP-1 transcription factors in the granule cells of the hippocampal formation after a single injection of kainate. This is very different from the earlier reports, which indicated that the changes in AP-1 DNA-binding activity and the levels of FRA were transient (30, 31). Our previous time course studies revealed that kainate caused increases in AP-1 DNA-binding activity in the hippocampus and that there were differences in the components of AP-1 complexes at different stages after kainate treatment (38). We speculate that the degeneration of the hilar inhibitory interneurons after kainate treatment may be closely associated with this phenomenon. Loss of these inhibitory neurons causes a disinhibition of the granule cells in the hippocampus, which may induce the expression of FRAs and the AP-1 DNA-binding activity. The increased AP-1 activity may in turn trigger the expression of some target genes that are involved in the sprouting of the mossy fibers to innervate the granule cells. This positive feedback loop may further excite the granule cells and exacerbate the sprouting phenomenon. Because PENK is expressed in granule cells, it is likely that the long-lasting increase in the expression of this opioid peptide gene after kainate treatment is stimulated by the increase in AP-1 activity. The significance of the persistent increase in the levels of this opioid peptide after kainate treatment is not clear. The long-lasting increase in PENK may be associated with the permanent change in hippocampal excitability after kainate treatment. It is possible that the short-term and long-term AP-1 complexes play different roles in the regulation of neuronal function. The short-term AP-1 complex may mediate the induction of certain genes that respond to stimuli in an acute fashion ranging from minutes to hours. On the other hand, the long-term AP-1 complex may be associated with the changes in neuronal plasticity that often require protracted time periods ranging from days to months. We hypothesize that the persistent increase in the AP-1 factors underlies the long-lasting changes in opioid peptides and even activates the regeneration of neuronal processes, such as the sprouting of the mossy fibers in the dentate gyrus after kainate injection. The following schematic drawing illustrated our current hypothesis (Fig. 6).
Figure 6.
Flow diagram of the biochemical and morphological changes in the hippocampus after treatment with kainate. Single systemic injections of kainate result in both short-term and long-term changes in the hippocampus. The short-term event is reflected by the rapid induction of AP-1 transcription factors, which may underlie the rapid induction of PENK and PDYN in the hippocampus. Elevated expression of the opioid peptides in the hippocampus lasted for a few days and returned to normal levels. This is the end of the short-term event. The long-term event after the kainate injection may ultimately lead to increases in the seizure susceptibility of the animals. Degeneration of the hilar inhibitory interneurons is the key event, which sets up the following events. Disinhibition of dentate granule cells increases changes of granule cell activity. This may activate the long-term AP-1 transcription factor. The loss of the targets of the mossy fibers will result in sprouting. These may induce the second phase of the increase of PENK expression in the granule cells. All of these events will result in increases of seizure activities.
To our surprise, after the initial increases of PENK mRNA induced by kainate had returned to baseline 3–4 days later, there was a second increase of the message in the hippocampus beginning 2 weeks after kainate treatment. These increases persisted for up to 1 year after kainate treatment, the longest period that we have investigated. Kainate not only increases the expression of PENK mRNA, but more importantly, it produces morphological changes in the ENK-containing pathway in the dentate gyrus. In normal rats, ENK immunostaining is only observed in the mossy fiber pathway. Two or 3 weeks after kainate treatment, immunostaining for ENK begins to appear in the supragranular layer of the dentate gyrus, indicating sprouting of the mossy fibers to innervate the dendritic region of the granule cells. This phenomenon is similar to human temporal lobe epilepsy (39, 40) and provides further confirmation that kainate-induced convulsions are an excellent model for this human disease.
The functional reorganization of the hippocampus following seizure activity has been characterized immunocytochemically by light and electron microscopy and electrophysiologically by voltage clamp (41, 42). We hypothesize that the long-term expression of ENK in the mossy fibers and their supragranular sprouting may contribute in part to the development of the spontaneous seizures occurring weeks to months after a single injection of kainate. The functional significance of the sprouting of ENK containing fibers has to be established and thus needs further study.
The neural mechanisms underlying the permanent changes in the hippocampus following seizure have not been clearly defined. Recently, several investigators have shown that once the acute seizure episodes have been induced by a single convulsive dose of kainate, the animals maintain a long-lasting increase in their seizure susceptibility (43, 44). Understanding the cellular and molecular mechanisms responsible for induction of this phenomenon may provide insight into potential therapeutic strategies for treating temporal lobe epilepsy. Further understanding of both the mechanisms of regulation and functions of opioid peptides should provide additional avenues for designing therapeutic interventions for neurological diseases.
Acknowledgments
We would like to thank Dr. Bill Wetsel for the critical reading of the manuscript.
ABBREVIATIONS
- FRA
fos-related antigen
- AP-1
activator protein-1
- ENK
enkephalin
- PENK
proenkephalin
- PDYN
prodynorphin
- ENK-IR
ENK-immunoreactivity
References
- 1.Sloviter R S, Dempster D W. Brain Res Bull. 1985;15:39–60. doi: 10.1016/0361-9230(85)90059-0. [DOI] [PubMed] [Google Scholar]
- 2.Represa A, Ben-Ari Y. Epilepsy Res Suppl. 1992;7:261–269. [PubMed] [Google Scholar]
- 3.Tauck D L, Nadler J V. J Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadler J V, Okazaki M M, Gruenthal M, Ault B, Armstrong D R. Adv Exp Med Biol. 1986;203:673–686. doi: 10.1007/978-1-4684-7971-3_51. [DOI] [PubMed] [Google Scholar]
- 5.Sutula T, Cavazos J, Golarai G. J Neurosci. 1992;12:4173–4187. doi: 10.1523/JNEUROSCI.12-11-04173.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Represa A, Tremblay E, Ben Ari Y. Adv Exp Med Biol. 1990;268:419–424. doi: 10.1007/978-1-4684-5769-8_46. [DOI] [PubMed] [Google Scholar]
- 7.Coyle J T, Ferkany J W, Zaczek R. Neurobehav Toxicol Teratol. 1983;5:617–624. [PubMed] [Google Scholar]
- 8.Bing G, McMillian M, Kim H, Pennypacker K, Feng Z, Qi Q, Kong L, Iadarola M, Hong J. Neuroscience. 1996;73:1159–1174. doi: 10.1016/0306-4522(96)00053-x. [DOI] [PubMed] [Google Scholar]
- 9.Gall C. J Neurosci. 1988;8:1852–1862. doi: 10.1523/JNEUROSCI.08-06-01852.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong J S, McGinty J F, Grimes L, Kanamatsu T, Obie J, Mitchell C L. NIDA Res Monogr. 1988;82:48–66. [PubMed] [Google Scholar]
- 11.McGinty J F, Kanamatsu T, Obie J, Hong J S. NIDA Res Monogr. 1986;71:89–101. [PubMed] [Google Scholar]
- 12.Kanamatsu T, Obie J, Grimes L, McGinty J F, Yoshikawa K, Sabol S, Hong J S. J Neurosci. 1986;6:3094–3102. doi: 10.1523/JNEUROSCI.06-10-03094.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pennypacker K R, Walczak D, Thai L, Fannin R, Mason E, Douglass J, Hong J S. J Neurochem. 1993;60:204–211. doi: 10.1111/j.1471-4159.1993.tb05839.x. [DOI] [PubMed] [Google Scholar]
- 14.Gall C, Lauterborn J, Isackson P, White J. Prog Brain Res. 1990;83:371–390. doi: 10.1016/s0079-6123(08)61263-7. [DOI] [PubMed] [Google Scholar]
- 15.Hong J S, Grimes L, Kanamatsu T, McGinty J F. Toxicology. 1987;46:141–157. doi: 10.1016/0300-483x(87)90124-7. [DOI] [PubMed] [Google Scholar]
- 16.Angulo J A, McEwen B S. Brain Res Rev. 1994;19:1–28. doi: 10.1016/0165-0173(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 17.Sonnenberg J L, Rauscher F J d, Morgan J I, Curran T. Science. 1989;246:1622–1625. doi: 10.1126/science.2512642. [DOI] [PubMed] [Google Scholar]
- 18.Comb M, Mermod N, Hyman S E, Pearlberg J, Ross M E, Goodman H M. EMBO J. 1988;7:3793–3805. doi: 10.1002/j.1460-2075.1988.tb03264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douglass J O, Iadarola M J, Hong J S, Garrett J E, McMurray C T. NIDA Res Monogr. 1991;111:133–148. [PubMed] [Google Scholar]
- 20.Racine R, Okujava V, Chipashvili S. Electroencephalogr Clin Neurophysiol. 1972;32:295–299. doi: 10.1016/0013-4694(72)90178-2. [DOI] [PubMed] [Google Scholar]
- 21.Bing G, Stone E A, Zhang Y, Filer D. Brain Res. 1992;592:57–62. doi: 10.1016/0006-8993(92)91658-2. [DOI] [PubMed] [Google Scholar]
- 22.Young S T, Porrino L J, Iadarola M J. Proc Natl Acad Sci USA. 1991;88:1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bing G, Chen S, Zhang Y, Hillman D, Stone E A. Neurosci Lett. 1992;140:260–264. doi: 10.1016/0304-3940(92)90116-o. [DOI] [PubMed] [Google Scholar]
- 24.Sonnenberg J L, Macgregor Leon P F, Curran T, Morgan J I. Neuron. 1989;3:359–365. doi: 10.1016/0896-6273(89)90260-2. [DOI] [PubMed] [Google Scholar]
- 25.Popovici T, Represa A, Crepel V, Barbin G, Beaudoin M, Ben Ari Y. Brain Res. 1990;536:183–194. doi: 10.1016/0006-8993(90)90024-6. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Martin C, Diego I D, Crespo D, Fairen A. Dev Brain Res. 1992;68:83–95. doi: 10.1016/0165-3806(92)90250-z. [DOI] [PubMed] [Google Scholar]
- 27.Gass P, Herdegen T, Bravo R, Kiessling M. Neuroscience. 1992;48:315–324. doi: 10.1016/0306-4522(92)90493-l. [DOI] [PubMed] [Google Scholar]
- 28.Hsu C Y, An G, Liu J S, Xue J J, He Y Y, Lin T N. Stroke. 1993;24:178–188. [Google Scholar]
- 29.McKitrick D J, Krukoff T L, Calaresu F R. Brain Res. 1992;599:215–222. doi: 10.1016/0006-8993(92)90394-o. [DOI] [PubMed] [Google Scholar]
- 30.Morgan J I, Cohen D R, Hempstead J L, Curran T. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- 31.Morgan J I, Curran T. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 32.Marksteiner J, Wahler R, Bellmann R, Ortler M, Krause J E, Sperk G. Neuroscience. 1992;49:383–395. doi: 10.1016/0306-4522(92)90104-a. [DOI] [PubMed] [Google Scholar]
- 33.Sonnenberg J L, Mitchelmore C, Macgregor Leon P F, Hempstead J, Morgan J I, Curran T. J Neurosci Res. 1989;24:72–80. doi: 10.1002/jnr.490240111. [DOI] [PubMed] [Google Scholar]
- 34.Smeyne R J, Schilling K, Oberdick J, Robertson L, Luk D, Curran T, Morgan J I. Adv Neurol. 1993;59:285–291. [PubMed] [Google Scholar]
- 35.Stone E A, John S M, Bing G, Zhang Y. Brain Res Bull. 1992;29:285–288. doi: 10.1016/0361-9230(92)90058-6. [DOI] [PubMed] [Google Scholar]
- 36.Pennypacker K R, Thai L, Hong J S, McMillian M K. J Neurosci. 1994;14:3998–4006. doi: 10.1523/JNEUROSCI.14-07-03998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong J S, McGinty J F, Lee P H K, Xie C W, Mitchell C L. Prog Neurobiol. 1993;40:507–528. doi: 10.1016/0301-0082(93)90020-s. [DOI] [PubMed] [Google Scholar]
- 38.Feng, Z., Zhang, W., Bing, G. & Hong, J. S. (1997) Mol. Brain Res., in press. [DOI] [PubMed]
- 39.de Lanerolle N C, Kim J H, Robbins R J, Spencer D D. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- 40.Houser C R. Epilepsy Res Suppl. 1992;7:223–234. [PubMed] [Google Scholar]
- 41.Okazaki M M, Nadler J V. J Comp Neurol. 1995;352:515–534. doi: 10.1002/cne.903520404. [DOI] [PubMed] [Google Scholar]
- 42.Bausch S B, Chavkin C. J Neurosci. 1997;17:477–492. doi: 10.1523/JNEUROSCI.17-01-00477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gall C, Lauterborn J, Bundman M, Murray K, Isackson P. Epilepsy Res Suppl. 1991;4:225–245. [PubMed] [Google Scholar]
- 44.Turski L, Cavalheiro E A, Schwarz M, Turski W A, De Moraes Mello L E, Bortolotto Z A, Klockgether T, Sontag K H. Brain Res. 1986;370:294–309. doi: 10.1016/0006-8993(86)90484-1. [DOI] [PubMed] [Google Scholar]