Abstract
The occurrence of cortical plasticity during adulthood has been demonstrated using many experimental paradigms. Whether this phenomenon is generated exclusively by changes in intrinsic cortical circuitry, or whether it involves concomitant cortical and subcortical reorganization, remains controversial. Here, we addressed this issue by simultaneously recording the extracellular activity of up to 135 neurons in the primary somatosensory cortex, ventral posterior medial nucleus of the thalamus, and trigeminal brainstem complex of adult rats, before and after a reversible sensory deactivation was produced by subcutaneous injections of lidocaine. Following the onset of the deactivation, immediate and simultaneous sensory reorganization was observed at all levels of the somatosensory system. No statistical difference was observed when the overall spatial extent of the cortical (9.1 ± 1.2 whiskers, mean ± SE) and the thalamic (6.1 ± 1.6 whiskers) reorganization was compared. Likewise, no significant difference was found in the percentage of cortical (71.1 ± 5.2%) and thalamic (66.4 ± 10.7%) neurons exhibiting unmasked sensory responses. Although unmasked cortical responses occurred at significantly higher latencies (19.6 ± 0.3 ms, mean ± SE) than thalamic responses (13.1 ± 0.6 ms), variations in neuronal latency induced by the sensory deafferentation occurred as often in the thalamus as in the cortex. These data clearly demonstrate that peripheral sensory deafferentation triggers a system-wide reorganization, and strongly suggest that the spatiotemporal attributes of cortical plasticity are paralleled by subcortical reorganization.
The occurrence of cortical plastic reorganization following peripheral sensory deafferentation in adult animals has been demonstrated in every mammalian species investigated (1–4). Because this phenomenon has been observed in both sensory and motor cortical areas (5, 6), the ability to undergo functional reorganization seems to be a general property of the entire adult neocortex. Nevertheless, the mechanisms underlying cortical reorganization in adult animals remains the focus of an intense debate. Thus, whereas corticocortical connections have been implicated in the genesis of this phenomenon (7, 8), considerable experimental data also support the notion that reorganization at subcortical levels can contribute to the occurrence of cortical plasticity (9–14). Recently, a few studies carried out in the somatosensory (15, 16) and visual (17) systems have suggested that cortical plasticity may result exclusively from synaptic alterations in intrinsic cortical circuitry. These findings have rekindled the debate by arguing against any fundamental role for subcortical structures in the process of cortical reorganization.
Part of the difficulty in unequivocally establishing the contribution of subcortical structures to cortical plasticity lies in the fact that the evidence for subcortical reorganization has been usually obtained independently, without simultaneous characterization of the presumptive changes that it may have caused at cortical level. In this study, we have approached this issue by investigating how a peripheral sensory deafferentation affects simultaneously both subcortical and cortical relays of the rat somatosensory system. To achieve this goal, the activity of large populations of single neurons at the cortical, thalamic, and brainstem levels was simultaneously monitored before and after the induction of a reversible sensory deactivation obtained by a subcutaneous injection of the local anesthetic lidocaine. These experiments revealed that fundamental aspects of cortical plasticity, such as changes in neuronal receptive fields, total spatial extent of the cortical reorganization, and latency variations in sensory responses, are all accompanied by parallel modifications in thalamic and brainstem nuclei. These results indicate that subcortical plasticity plays a fundamental role in the process of cortical reorganization triggered by a peripheral sensory deafferentation.
MATERIALS AND METHODS
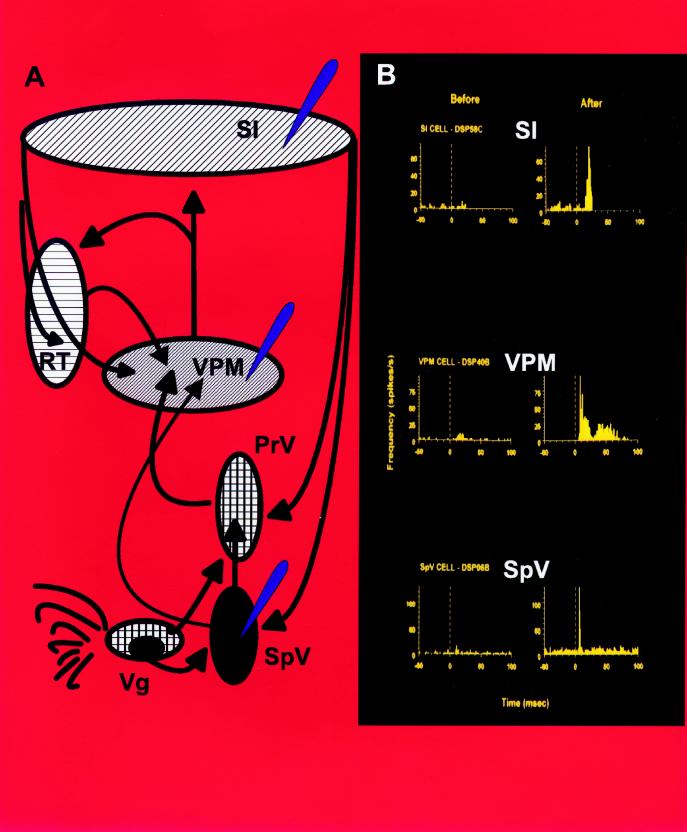
Twelve adult Long–Evans rats were used in our experiments. The short-term plastic effects of a reversible sensory deactivation were quantified by carrying out simultaneous recordings of the extracellular activity of single neurons distributed across cortical and subcortical relays of the rat trigeminal somatosensory pathway. Fig. 1A summarizes the connectivity of the neural structures from which simultaneous neuronal ensemble recordings were obtained in our experiments (see blue arrows).
Figure 1.
Simultaneous, multi-level sensory reorganization in the rat trigeminal somatosensory system. (A) Schematic organization of the processing levels and connectivity of the rat trigeminal somatosensory system. Notice the existence of parallel projections from the brainstem (PrV and SpV) to the VPM thalamus. The reticular (RT) nucleus provides the only source of GABAergic projections to VPM neurons. Dense corticothalamic and corticobulbar feedback projections are also depicted in this scheme. Blue arrows depict the main cortical and subcortical structures from which simultaneous neural ensemble recordings were obtained. (B) PSTHs were used to quantify the simultaneous unmasking of novel responses in the SpV nucleus, VPM nucleus, and SI cortex following a subcutaneous injection of lidocaine. Notice than before the injection these three simultaneously recorded neurons did not exhibit a significant response to the stimulation of whisker C3. Minutes after the induction of the lidocaine block, the same neurons responded vigorously to stimulation of that same whisker. Stimulus onset is at 0 ms. Stimulus duration is 100 ms. Response magnitude is depicted in spikes/s (Hz). Vg, trigeminal ganglion; PrV, principal nucleus of the trigeminal brainstem complex.
A full description of the surgical and recording procedures employed in this study has been published elsewhere (18, 19). Briefly, under pentobarbital anesthesia, up to three electrode arrays, each of which contained 8–16 Teflon-coated, stainless steel microwires (50 μm in diameter, NB Labs, Dennison, TX), were surgically implanted in the whisker representation area of the primary somatosensory (SI) cortex, the ventral posterior medial (VPM) nucleus of the thalamus, and in the pars interpolaris of the spinal trigeminal complex (SpV). A week after the surgery for electrode implantation, the animals were anesthetized again (50 mg/kg of pentobarbital, i.p.) and then transferred to a recording chamber. Using a multi-neuronal acquisition processor (Spectrum Scientific, Dallas), action potentials derived from cortical and subcortical neurons were identified. A series of voltage and time windows, combined with a real-time principal component algorithm (18, 19), were then used to isolate the units and to demarcate the spike timing. In the control phase of the experiments, the sensory responses of all single neurons were simultaneously characterized by using a computer-controlled probe to produce mechanical deflection of all facial whiskers (deflection amplitude = 3–5 degrees, stimulus duration = 100 ms, stimulus frequency = 1 Hz), one at a time in random order. A few selected skin sites in the face were also stimulated using the same stimulus protocol.
Poststimulus time histograms (PSTHs) and cumulative frequency histograms (CFHs) were used to identify the occurrence of statistically significant sensory responses and also to quantify their magnitude and latency. Statistical significance of sensory responses was assessed by applying a one-way Kolmogorov–Smirnov test to CFHs depicting each neuron’s response to a given stimulus (18, 19). Data derived from this analysis were used for the definition of the normal and reorganized neuronal receptive fields (RFs) and for plotting the somatosensory maps defined by neuronal ensembles. Immediately after the control phase was completed, a reversible sensory deafferentation was induced by injecting small volumes of the local anesthetic lidocaine (0.04 ml, 1% solution in saline) in the subcutaneous tissue of the maxillary gum behind the upper incisors, in the whisker pad, or in different regions of the upper lip. In each experiment, lidocaine was injected in only one of these locations. Immediately following the injection, the same single whiskers and facial regions were stimulated as described above.
Sensory responses derived from this experimental phase were then compared with data obtained during the control phase. Because the same large set of neurons was held throughout this experiment, we were able to quantify the spatiotemporal nature of the reorganization process not only by measuring changes in single neuron RFs but also by reconstructing the spatial extent of reorganization somatosensory maps located in cortical, thalamic, and brainstem structures. This latter estimate was obtained by measuring the number of whiskers for which unmasked neuronal responses were observed after the peripheral deactivation.
RESULTS
In this study, a total of 1,022 cortical, thalamic, and brainstem neurons were recorded in 12 adult rats. In each of four animals, simultaneous recordings were obtained from up to 135 neurons located in the SI cortex, VPM thalamus, and SpV nucleus of the trigeminal brainstem complex. Simultaneous recordings from the SI cortex and VPM nucleus were obtained in seven other rats. In one last animal, only cortical neurons were recorded. In every animal, the characterization of single neuron responses was performed before (i.e., control phase) and after the induction of a peripheral lidocaine block. During the control phase, single facial whiskers were randomly stimulated to quantitatively define the receptive fields of the simultaneously recorded populations of cortical and subcortical neurons. The results obtained during these control experiments matched previous descriptions of large multiwhisker receptive fields in the SpV, VPM thalamus, and SI cortex (20). The main effect induced by the lidocaine injection on these RFs is illustrated in Fig. 1B. Three to 5 min after the lidocaine injection, cortical and subcortical neurons with RFs around the injection site began to respond to the stimulation of facial whiskers, which did not elicit significant neuronal responses during the control phase of the experiment (Fig. 1B). Concurrent multi-site recordings revealed that this immediate unmasking of sensory responses occurred almost simultaneously in the SpV nucleus, the VPM thalamus, and the SI cortex (Fig. 1B). In fact, in all the experiments carried out, no clear sequence for the establishment of these novel sensory responses was observed. Instead, once the reorganization process started, it appeared at once at subcortical and cortical levels of the somatosensory system.
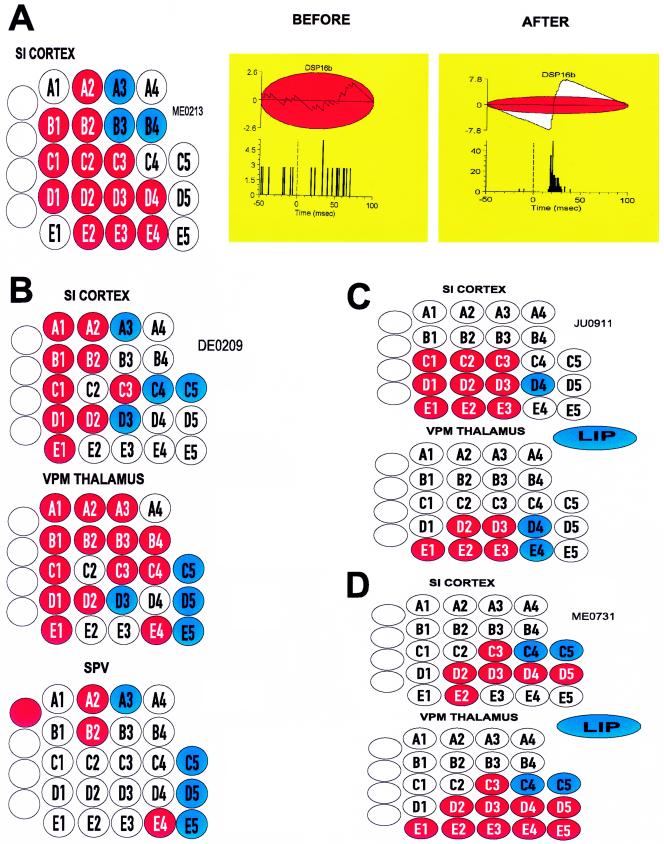
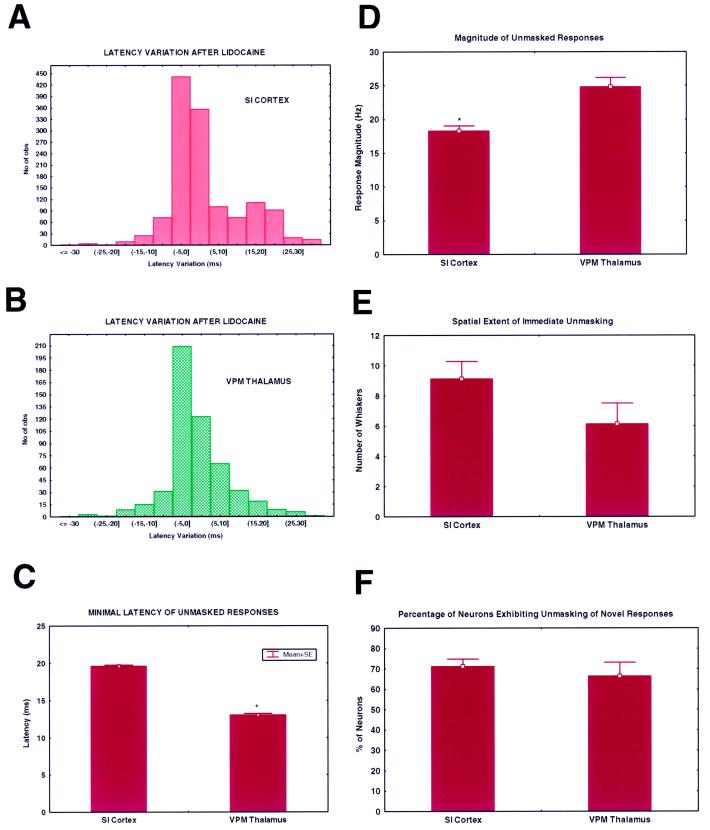
Because a large tissue area (around 2 mm2) was sampled in each of the neural structures investigated, we were able to provide an estimate of the total spatial extent of the reorganization process throughout the trigeminal system. Fig. 2 depicts four representative examples observed in our experiments. In these plots, blue circles identify the regions anesthetized by the lidocaine injection. Red circles, on the other hand, depict the region of unmasked responses observed at each level of the pathway. Thus, each red dot indicates that at least one neuron in the simultaneously recorded neuronal population exhibited a new (unmasked) sensory response to the stimulation of that particular whisker after the lidocaine injection. This analysis revealed that the reorganization process was marked by a considerable spatial overlap in the SI cortex, VPM thalamus, and to a lesser degree in the SpV nucleus (Fig. 2 B–D). Thus, similar sets of whiskers formed regions of unmasked sensory responses in cortical and subcortical sensory maps. This overlap was consistently observed in all animals used in this study. In fact, even in the case of SpV, where a smaller area of reorganization was observed, the unmasked region corresponded to a subset of the unmasked regions observed in the VPM thalamus and SI cortex (Fig. 2B). Fig. 2 B–D also illustrates that the unmasked region observed in the brainstem or thalamus could include whiskers which did not belong to the cortical unmasked region (see alpha whisker in SpV in Fig. 2B, and whisker E1 in the VPM in Fig. 2D), a finding that suggests that not all components of the subcortical reorganization process were necessarily reflected in the SI cortex. Even though individual cortical neurons exhibited slightly larger receptive field expansions (2.3 ± 0, 12 whiskers) than VPM neurons (1.7 ± 0.13 whiskers) after the lidocaine block, no statistical difference was observed when we compared the spatial extent of the unmasked zones in the SI cortex (9.1 ± 1.2 whiskers, mean ± SE) and in the VPM thalamus (6.1 ± 1.6 whiskers, mean ± SE; see Fig. 3E). Moreover, the percentage of cortical neurons exhibiting unmasked responses (71.1 ± 5.2%, mean ± SE) was the same as the percentage of VPM neurons (66.4 ± 10.7%) exhibiting the same effect. On the other hand, the magnitude (in spikes per second) of unmasked responses was significantly greater (P < 0.01, t test) in the VPM nucleus (24.8 ± 2.2 spikes/s, mean ± SE) than in the SI cortex (18.3 ± 0.8 spikes/s). Taken together, these results indicate the existence of a close relationship between the reorganization process in the VPM nucleus and the SI cortex.
Figure 2.
The spatial extent of the sensory reorganization process was measured by quantifying the number of stimulated whiskers (red circles) that induced unmasked (new) sensory responses at each level of the trigeminal pathway after the peripheral lidocaine block. In these plots, blue circles indicate the whiskers anesthetized by the lidocaine injection. These whisker, when stimulated produced no neural response. (A) The spatial extent of the cortical reorganization in one adult rat is illustrated. A total of 13 facial whiskers were found to induce novel responses in the SI cortex of this animal after an injection of lidocaine in the A3 whisker. An example of the criterion used to defined the occurrence of an unmasked sensory response is illustrated by the pairwise comparison of PSTHs and CFHs, obtained by stimulating whisker C3 and recording the sensory response of neuron DSP16b before (no significant response) and after (highly significant response) the peripheral lidocaine injection. (B) Similar analysis carried out for multi-level recordings revealed a high degree of spatial overlap for the simultaneous sensory reorganization process observed in the SI cortex, VPM thalamus, and SpV brainstem nucleus. In this animal lidocaine was injected in the gum. (C and D) Reorganization in the SI cortex reflected similar changes observed in the VPM thalamus. However, in some instances cortical reorganization involved a larger territory (C). Conversely, not all whiskers (see E1 and E3–E5 in D) that defined the unmasked zone in the VPM thalamus belonged to the corresponding cortical unmasked region. Lidocaine injection in C and D was in the animals’ upper lip.
Figure 3.
Comparison of the spatial and temporal attributes of the simultaneous reorganization observed in the SI cortex and VPM nucleus of the thalamus. (A and B) Quantitative analysis of the distributions of neuronal latency variation after the peripheral lidocaine injection revealed similar effects in the SI cortex (A) and the VPM nucleus of the thalamus (B). The only exception was the larger component of longer latencies (15–25 ms) observed for novel cortical responses. Further analysis (C) revealed that this could explained by the fact that unmasked cortical responses had significantly longer latencies than their thalamic counterparts. (D) The magnitude of these unmasked responses, on the other hand, was higher in the VPM nucleus than the SI cortex. No statistical difference was observed between the thalamic and the cortical reorganization processes when the spatial extent of the reorganization at the neuronal population level was measured (in number of whiskers, E) and when the percentage of neurons exhibiting unmasked sensory responses after the lidocaine injection (F) was calculated.
Confirming previous observations in the VPM nucleus (10), the process of sensory reorganization induced by the peripheral lidocaine block was not limited to the emergence of unmasked sensory responses in the cortex and brainstem. In fact, increases and decreases in sensory response latencies were also observed throughout the trigeminal sensory pathway. Once again, a significant degree of overlap was observed when histograms depicting the distribution of latency variations in the SI cortex and VPM thalamus were analyzed (Fig. 3 A and B), suggesting that similar mechanisms were responsible for these effects. The only major difference observed in this analysis was that neurons in the SI cortex exhibited a larger proportion of long-latency responses than VPM neurons. These turned out to be derived from a subgroup of unmasked responses (see Fig. 3C), whose minimal latencies were beyond the usual range observed in the SI cortex (7–10 ms). In fact, the average minimal latency of unmasked cortical responses (19.6 ± 0.3 ms, mean ± SE) was significantly longer than the average latency of unmasked VPM responses (13.1 ± 0.6 ms, t test, P < 0.01). This difference in latency (around 6 ms) was much longer than the normal time delay (around 2–3 ms) observed between thalamic and cortical responses (layer V) to the same tactile stimuli. The reasons for this difference remains unclear.
As previously described (10, 12), the reorganization process described here was entirely reversible. Four hours after the lidocaine injection, most cortical and subcortical neurons had lost their unmasked responses and returned to express their original RFs.
DISCUSSION
The results obtained in this study indicate that a reversible peripheral sensory deactivation, induced by a subcutaneous injection of the local anesthetic lidocaine, triggers an immediate and simultaneous sensory reorganization in the SI cortex, VPM thalamus, and SpV nucleus of the rat trigeminal brainstem complex. This system-wide reorganization was characterized by the emergence of unmasked sensory responses whose spatial domains overlapped significantly at cortical and subcortical levels. In fact, when the percentage of neurons exhibiting novel responses and the spatial extent of the unmasked regions were quantified, no statistical difference was found between the reorganization process observed in the SI cortex and in the VPM thalamus. Although the average latency of unmasked cortical responses was significantly longer than equivalent thalamic responses, distributions depicting latency variations for populations of cortical and thalamic neurons also overlapped considerably. Taken together, these findings strongly support the hypothesis that fundamental aspects of the process of cortical plasticity, particularly the ones involved in the early phases of the reorganization process, depend upon concomitant subcortical reorganization. These findings are also in agreement with recent observations that demonstrated that even long-term plastic changes in the primate somatosensory cortex are paralleled by thalamic and brainstem reorganization (14).
It is important to emphasize that even though short-term cortical, thalamic, and brainstem reorganization had been independently reported in distinct animal samples (10–13, 21–24), in this study we were able to quantify the simultaneous occurrence of this reorganization process at cortical and subcortical levels of the somatosensory system, and to identify potential correlations between effects in these different structures. This was only possible because we opted to employ large matrix-like arrays of electrodes (19), which provided a large spatial sampling in each of the structures of interest, and consequently allowed a more quantitative description of the reorganization process at several levels of the somatosensory system.
One surprising finding in this study was the lack of any consistent ascending (i.e., from brainstem to cortex) or descending (cortex to brainstem) sequence for the establishment of the sensory reorganization at multiple levels of the trigeminal system. Indeed, all of the experimental evidence suggests that the reorganization is established almost simultaneously at cortical and subcortical levels. We interpret this finding as evidence in favor of our hypothesis (10) that a peripheral deafferentation triggers very fast modifications in the balance of excitation and inhibition across the entire somatosensory system. This would account for the occurrence of almost immediate changes in receptive fields at all levels of the pathway. Another possibility is that the existence of both ascending and descending pathways within a highly parallel system, such as the somatosensory system, could have prevented us from detecting an extremely fast sequence (in the order of hundreds of milliseconds) for the establishment of the reorganization. Overall, our data seem to suggest that cortical plasticity is likely to be both guided and constrained by reorganization in subcortical structures; nevertheless, our results also point to the possibility that the occurrence of subcortical plasticity may not necessarily precede the establishment of cortical reorganization by much. Instead, these two processes may take place almost concomitantly.
Recently, several groups have described the occurrence of cortical plasticity without concomitant reorganization at the thalamic level (15–17). The discrepancy between these studies, which support an intrinsic cortical process, and our results cannot be completely resolved because of the differences in experimental paradigms used to induce cortical plasticity. In fact, a great deal of evidence suggests that the central modifications observed after a peripheral lidocaine block are very similar to those observed immediately after the induction of a more permanent peripheral deafferentation (e.g., nerve cut or digit amputation) in which tissue degeneration is produced (12, 24, 25). There are several possible factors, however, that could explain why no thalamic reorganization was observed in these studies. First, more exhaustive sampling of the area of interest may be required to demonstrate the occurrence of thalamic plasticity when nonquantitative mapping procedures are employed. Usually, due to technical difficulties, fewer penetrations are used to map the thalamus than the neocortex. For instance, Darian-Smith and Gilbert (17) used 30–40 penetrations to map the primary visual (VI) cortex and only 8–13 penetrations (5 in the silent zone) to map the entire lateral geniculate nucleus (LGN). Moreover, as shown here, thalamic reorganization may involve small changes in firing, and unmasking of long-latency responses which are difficult to detect in classic mapping procedures. Therefore, a more quantitative analysis, based on statistical comparison of PSTHs is necessary to rule out the occurrence of subcortical plastic changes. In their study, Darian-Smith and Gilbert (17) reported short-term changes in the RFs of VI cortical neurons, but did not investigate the presence of similar alterations in the LGN. Our results suggest that the sizable cortical reorganization observed in the VI cortex should be paralleled at least by short-term modifications in the LGN. Finally, because the characterization of plastic reorganization was carried out long after the visual deafferentation (26 weeks to 1 year), Darian-Smith and Gilbert could not rule out the possibility that some of the subcortical reorganization process is transient and, consequently, outlived by long-lasting cortical plasticity. Nevertheless, the demonstration of long-term functional and anatomical (26) reorganization in the somatosensory (9, 26) and in the visual thalamus (27) following peripheral sensory deafferentations seems to argue against this latter hypothesis, and indicates that thalamic plasticity accompanies long-term cortical reorganization.
Our data also suggest that subcortical structures may contribute to the process of cortical reorganization observed when behaving animals are subjected to long-term changes in tactile experience (15, 16). Wang et al. (16) reported that only cortical reorganization was observed when monkeys were trained to respond to specific sequences of tactile stimuli. Nevertheless, studies in the auditory system indicate that both thalamic and cortical reorganization can occur when changes in sensory experience are induced during classical conditioning paradigms (28, 29). An interesting hypothesis is that the process of thalamic reorganization may have lagged the plastic modifications observed by Wang et al. (16) in the SI cortex. In fact, Diamond et al. (15) demonstrated that changes in sensory experience triggered by a whisker-pairing paradigm induced cortical reorganization, which is first observed in supragranular and infragranular layers of the SI cortex and only later alters sensory properties of layer IV neurons, the main target of thalamocortical projections. Long-term chronic and simultaneous thalamic and cortical recordings will be required to further test this hypothesis and to rule out the possibility that subcortical plasticity also occurred at earlier stages of whisker-pairing. In fact, the occurrence of thalamic reorganization should be expected, given the dense reciprocal connectivity between cortical areas and their principal thalamic relays. In this context, subcortical plasticity is likely to occur either concomitantly with the process of cortical reorganization or as a consequence of the transference of the cortical effects to the thalamus and brainstem through the dense network of corticofugal projections. In the latter scenario, plastic changes in the neocortex could precede the establishment of measurable subcortical reorganization.
In conclusion, our results do not support the hypothesis that alterations in intrinsic cortical circuits alone are responsible for the phenomenon of cortical plasticity. Although our evidence addresses specific paradigms involving peripheral sensory deafferentations, our results also suggest that changes in sensory experience do not induce cortical plasticity which is independent of any short- or long-term subcortical reorganization. By simultaneously characterizing cortical and subcortical reorganization and observing that these processes are very similar in nature, our results clearly implicate subcortical structures, particularly the thalamus, as fundamental contributors in the establishment of cortical reorganization.
Acknowledgments
We thank Harvey Wiggins and Alex Kirillov (Spectrum Scientific), Larry Andrews (NB Labs), Suzette Casal, Laura Fitzpatrick, Agnes Ju, and Dr. Laura M. O. Oliveira for their technical support, and William Hall, Sid Simon, and Asif Ghazanfar for their comments on the manuscript. This study was sponsored by grants from the National Institute of Dental Research (DE-111121-01) and the Whitehall Foundation.
ABBREVIATIONS
- VPM
ventral posterior medial nucleus of the thalamus
- SI
somatosensory cortex
- SpV
pars interpolaris of the spinal nucleus of the trigeminal brainstem complex
- PSTH
poststimulus time histogram
- CFH
cumulative frequency histogram
- RF
receptive field
References
- 1.Merzenich M M, Kaas J H, Wall J T, Nelson R J, Sur M, Felleman D J. Neuroscience. 1983;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- 2.Merzenich M M, Kaas J H, Wall J T, Nelson R J, Sur M, Felleman D J. Neuroscience. 1983;10:639–665. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- 3.Kaas J H, Merzenich M M, Killackey H P. Annu Rev Neurosci. 1983;6:325–356. doi: 10.1146/annurev.ne.06.030183.001545. [DOI] [PubMed] [Google Scholar]
- 4.Kaas J H. Annu Rev Neurosci. 1991;14:137–168. doi: 10.1146/annurev.ne.14.030191.001033. [DOI] [PubMed] [Google Scholar]
- 5.Sanes J N, Suner S, Lando J F, Donoghue J P. Proc Natl Acad Sci Usa. 1988;85:2003–2007. doi: 10.1073/pnas.85.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanes J N, Suner S, Donoghue J P. Exp Brain Res. 1990;79:479–491. doi: 10.1007/BF00229318. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs K M, Donoghue J P. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- 8.Donoghue J P. Curr Opin Neurobiol. 1995;5:749–754. doi: 10.1016/0959-4388(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 9.Garraghty P E, Kaas J H. NeuroReport. 1991;2:747–750. doi: 10.1097/00001756-199112000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Nicolelis M A L, Lin R C S, Woodward D J, Chapin J K. Nature (London) 1993;361:533–536. doi: 10.1038/361533a0. [DOI] [PubMed] [Google Scholar]
- 11.Pettit M J, Schwark H D. Science. 1993;262:2054–2056. doi: 10.1126/science.8266104. [DOI] [PubMed] [Google Scholar]
- 12.Shin H-C, Park S, Son J, Sohn J-H. NeuroReport. 1995;7:33–36. [PubMed] [Google Scholar]
- 13.Pettit M J, Schwark H D. J Neurophysiol. 1996;75:1117–1125. doi: 10.1152/jn.1996.75.3.1117. [DOI] [PubMed] [Google Scholar]
- 14.Florence S L, Kaas J H. J Neurosci. 1995;15:8083–8095. doi: 10.1523/JNEUROSCI.15-12-08083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diamond M E, Huang W, Ebner F F. Science. 1994;265:1885–1888. doi: 10.1126/science.8091215. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Merzenich M M, Sameshima K, Jenkins W M. Nature (London) 1995;378:71–75. doi: 10.1038/378071a0. [DOI] [PubMed] [Google Scholar]
- 17.Darian-Smith C, Gilbert C D. J Neurosci. 1995;15:1631–1647. doi: 10.1523/JNEUROSCI.15-03-01631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolelis M A L, Chapin J K. J Neurosci. 1994;14:3511–3532. doi: 10.1523/JNEUROSCI.14-06-03511.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicolelis M A L, Ghazanfar A A, Faggin B M, Votaw S, Oliveira L M O. Neuron. 1997;18:529–537. doi: 10.1016/s0896-6273(00)80295-0. [DOI] [PubMed] [Google Scholar]
- 20.Nicolelis M A L, Baccala L A, Lin R C S, Chapin J K. Science. 1995;268:1353–1358. doi: 10.1126/science.7761855. [DOI] [PubMed] [Google Scholar]
- 21.Calford M B, Tweedale R. Nature (London) 1988;332:446–448. doi: 10.1038/332446a0. [DOI] [PubMed] [Google Scholar]
- 22.Calford M B, Tweedale R. J Neurophysiol. 1991;65:178–187. doi: 10.1152/jn.1991.65.2.178. [DOI] [PubMed] [Google Scholar]
- 23.Calford M B, Tweedale R. Somatosensory Motor Res. 1991;8:249–260. doi: 10.3109/08990229109144748. [DOI] [PubMed] [Google Scholar]
- 24.Silva A C, Rasey S K, Wu X, Wall J T. J Comp Neurol. 1996;366:700–716. doi: 10.1002/(SICI)1096-9861(19960318)366:4<700::AID-CNE9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Garraghty P E, Muja N. J Comp Neurol. 1996;367:319–326. doi: 10.1002/(SICI)1096-9861(19960401)367:2<319::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 26.Rausell E, Cusick C G, Taub E, Jones E G. Proc Natl Acad Sci USA. 1992;89:2571–2575. doi: 10.1073/pnas.89.7.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eysel U T. Nature (London) 1982;299:442–444. doi: 10.1038/299442a0. [DOI] [PubMed] [Google Scholar]
- 28.Lennartz R C, Weinberger N M. Behav Neurosci. 1992;106:484–497. doi: 10.1037//0735-7044.106.3.484. [DOI] [PubMed] [Google Scholar]
- 29.Weinberger N M. Annu Rev Neurosci. 1995;18:129–158. doi: 10.1146/annurev.ne.18.030195.001021. [DOI] [PMC free article] [PubMed] [Google Scholar]