Abstract
Global long-term potentiation (LTP) was induced in organotypic hippocampal slice cultures by a brief application of 10 mM glycine. Glycine-induced LTP was occluded by previous theta burst stimulation-induced potentiation, indicating that both phenomena share similar cellular processes. Glycine-induced LTP was associated with increased [3H]α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid (AMPA) binding in membrane fractions as well as increased amount of a selective spectrin breakdown product generated by calpain-mediated spectrin proteolysis. Antibodies against the C-terminal (C-Ab) and N-terminal (N-Ab) domains of GluR1 subunits were used to evaluate structural changes in AMPA receptor properties resulting from glycine-induced LTP. No quantitative or qualitative changes were observed in Western blots from membrane fractions prepared from glycine-treated slices with C-Ab. In contrast, Western blots stained with N-Ab revealed the formation of a 98-kDa species of GluR1 subunits as well as an increased amount of immunoreactivity after glycine-induced LTP. The amount of spectrin breakdown product was positively correlated with the amount of the 98-kDa species of GluR1 after glycine treatment. Functional modifications of AMPA receptors were evaluated by determining changes in the effect of pressure-applied AMPA on synaptic responses before and after glycine-induced LTP. Glycine treatment produced a significant increase in AMPA receptor function after potentiation that correlated with the degree of potentiation. The results indicate that LTP induction produces calpain activation, truncation of the C-Ab domain of GluR1 subunits of AMPA receptors, and increased AMPA receptor function. They also suggest that insertion of new receptors takes place after LTP induction.
Keywords: hippocampus, plasticity, calpain, glutamate, spectrin
Long-term potentiation (LTP) is an attractive candidate for a cellular mechanism of learning and memory as it exhibits many features expected for such a mechanism. It is induced by patterns of stimulation similar to those found in animals exploring novel environments; it is very long lasting (weeks in chronic preparations), and its pharmacology matches that of learning and memory (1, 2). It is generally agreed that LTP induction requires N-methyl-d-aspartate (NMDA) receptor activation, resulting in increase in postsynaptic calcium concentration and in the activation of several biochemical cascades leading to increased synaptic efficacy (3, 4). Possible mechanisms for LTP expression, in particular whether LTP is expressed as an increase in neurotransmitter release or by modifications of postsynaptic elements, are still a matter of debate (5–7). Over the last 10–15 years, a large body of evidence has been accumulated supporting the hypothesis that LTP expression is due, at least in part, to selective modifications of postsynaptic glutamate α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid (AMPA) receptors.
In the dentate gyrus of animals sacrificed 1 hr after LTP induction in the perforant path, the degree of LTP was positively correlated with increased [3H]AMPA binding in the molecular layer (8). Allosteric effectors that increase AMPA receptor-mediated responses produced smaller increases in AMPA receptor-mediated responses after LTP induction (9, 10). Finally, the waveform of excitatory postsynaptic potentials (EPSPs) exhibited modifications consistent with a modification of AMPA receptor kinetics (11). Recent evidence suggests the existence in the hippocampus of a population of silent synapses, i.e., synapses that express NMDA receptor-mediated responses but no AMPA receptor-mediated responses (12, 13). After LTP induction, silent synapses were transformed into active synapses expressing functional AMPA receptors. The existence of such synapses could not only account for the controversial results and interpretations obtained from quantal analysis performed before and after LTP induction by several groups (14–16), but is also consistent with the idea that LTP is due, at least in part, to the transformation of nonfunctional AMPA receptors into functional receptors, an idea first proposed by Lynch and Baudry (17, 18).
Although the above-discussed results implicate postsynaptic AMPA receptor modifications as part of the mechanisms underlying LTP expression, the nature of the changes as well as of the processes responsible for them are still unknown. A large body of work supports a critical role for the calcium-dependent protease, calpain, in LTP induction. Calpain inhibitors block LTP induction in vitro in acute and cultured hippocampal slices (19–21), as well as in vivo (22). Furthermore, both theta-burst stimulation (TBS) and NMDA receptor stimulation produce calpain activation as evidenced by the accumulation of a selective spectrin breakdown product (SBDP) generated by calpain-mediated spectrin proteolysis (23, 24). Interestingly, calpain activation recently has been shown to directly modify the immunological properties of AMPA receptor subunits (25–27), particularly of the GluR1 subunit, which is essential for LTP expression (28). We previously showed that a brief application of high concentrations of glycine in acute hippocampal slices produced a long-lasting increase in EPSPs, indistinguishable from tetanus-induced LTP (29, 30). The present study used a similar approach in cultured hippocampal slices to analyze changes in AMPA receptor properties after “global LTP” induction by glycine. We first verified that glycine-induced LTP was occluded by tetanus-induced LTP. Several aspects of AMPA receptor properties, including ligand binding and immunological properties of GluR1 subunits, then were analyzed in glycine-treated slices. Finally, changes in AMPA receptor function were estimated by measuring the effects of pressure-applied AMPA before and after glycine-induced LTP. The results indicate that glycine-induced potentiation is accompanied by direct modifications of the structure and function of the AMPA receptors, possibly due to calpain activation.
MATERIALS AND METHODS
Preparation of Organotypic Hippocampal Slice Cultures.
Organotypic hippocampal cultures were prepared from 7–8-day-old Sprague–Dawley rat pups according to Stoppini et al. (31) with the following modifications. Hippocampi were dissected in a laminar flow hood under sterile conditions in minimum essential medium (MEM) (GIBCO no. 61100–061) containing 25 mM Hepes, 10 mM Tris-base, 10 mM d-glucose, and 3 mM MgCl2. Hippocampal slices (400 μm thick) were obtained using a McIllwain tissue chopper and explanted onto a membrane (Millicell-CM, 0.4 μm pore size) placed in 1 ml of MEM (GIBCO no. 41200–072) containing 3 mM glutamine, 30 mM Hepes, 5 mM NaHCO3, 30 mM d-glucose, 0.5 mM l-ascorbate, 2mM CaCl2, 2.5 mM MgSO4, 1 μg insulin, and 20% horse serum, including penicillin. Both cutting and incubation media were adjusted to pH 7.2. The membranes were placed in specialized wells and stored at 35°C in a humidified incubator containing oxygen and carbon dioxide-enriched air. Slices were kept on the interface membrane for 8–15 days for slice recovery and development. During this period, the incubation medium was changed every 48 hr. After this period, the whole membrane with the slices was transferred to a recording chamber or pharmacologically treated as described below for SDS/PAGE analysis.
Electrophysiological Recording in Cultured Hippocampal Slices Electrophysiological recordings were obtained from cultured slices (400 μm) as described in Stoppini et al.
(31). Slices were incubated for at least 60 min at 32°C in an interface chamber with an artificial cerebrospinal fluid containing 124 mM NaCl, 3 mM KCl, 1.25 mM KH2PO4, 2.5 mM CaCl2, 2.5 mM MgCl2, 26 mM NaHCO3, 10 mM glucose, and 2 mM l-ascorbate, constantly oxygenated with a mixture of O2:CO2 (95%:5%). A recording glass micropipette (≈2–5 MΩ containing 2 M NaCl) was placed in the cell body layer of CA1 or in stratum radiatum to record EPSPs generated by a stimulating bipolar electrode (0.2-msec pulse duration) placed in the pyramidal cell layer of CA3. EPSP amplitudes and slopes were recorded and stored in a computer for further analysis. In general, EPSP amplitudes were measured from EPSPs recorded in cell body layer, whereas EPSP slopes were measured from EPSPs recorded in stratum radiatum, as EPSP amplitudes were difficult to measure accurately in stratum radiatum due to the appearance of a population spike in the recordings (see Fig. 1 for examples of EPSPs recorded in cell body layer and stratum radiatum).
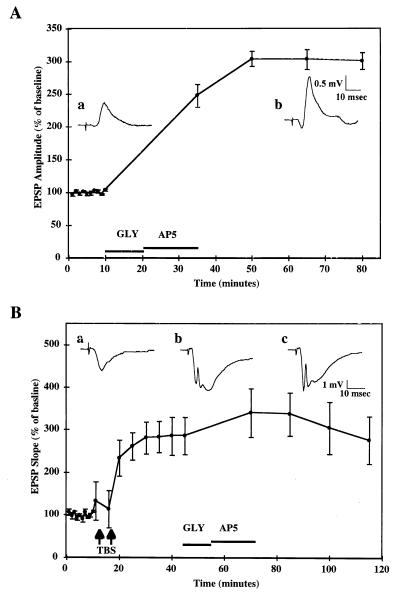
Figure 1.
Glycine-induced LTP in hippocampal slice cultures. (A) Cultured hippocampal slices were prepared as described in Materials and Methods and maintained in culture for 8–15 days before use. Field EPSPs were evoked by stimulation of CA3 cell body layer and recorded in CA1 pyramidal cell body layer. After a 10-min baseline period, glycine (10 mM) was applied for 10 min followed by d,l-AP5 (20 μM) for at least 15 min. After glycine treatment, stimulation was resumed every 15 min to minimize the recurrence of seizures. Data represent EPSP amplitudes expressed as percent of the mean values obtained over the first 10 min of baseline recording (means ± SEM of 56 experiments). (Inset) Typical EPSPs recorded before (a) and after (b) glycine treatment. Note the occurrence of a population spike after glycine treatment. (B) After a 10-min baseline recording, two episodes of TBS were administered at 5-min intervals to induce maximal potentiation (286% ± 44% of baseline values). Thirty minutes later, glycine (10 mM) was applied for 10 min, followed by AP5 (20 μM) for at least 15 min. Data represent EPSP slopes expressed as percent of the mean values obtained over the first 10 min of baseline recording (means ± SEM of six experiments). (Inset) Typical EPSPs recorded before TBS (a), after TBS (b), and after glycine treatment (c).
After a 10-min baseline recording, stimulation was interrupted and glycine (10 mM) was perfused as previously described (30). After 10 min of glycine perfusion, 20 μM d,l-2-amino-5-phosphonovaleric acid was perfused for 15 min and in some cases longer to reduce the degree of seizures induced by glycine. After 15 min of 2-amino-5-phosphonovaleric acid perfusion, stimulation was resumed at 1 pulse/15 min only because slices were, in general, hyperexcitable. After glycine treatment, recording lasted for 45 min after which slices were collected in 0.32 M sucrose solution containing 0.1 mM EGTA and 0.1 mM leupeptin and stored at −70°C for future analysis. In a number of slices, glycine treatment did not result in potentiation of recorded EPSPs. These slices were collected and processed for binding and immunoblotting analysis and were referred to as Gly/No LTP in figures.
Occlusion of glycine-induced LTP by electrical stimulation was tested in cultured hippocampal slices. TBS consisted of three bursts of four pulses (0.25 msec duration) delivered at 100 Hz, administered at 5 Hz. TBS was administered twice at 5-min intervals to produce maximal potentiation. Thirty minutes after TBS administration, glycine treatment was performed as described above.
In experiments where AMPA receptor function was assessed, AMPA (100 μM) was dissolved in a buffer containing 124 mM NaCl, 3 mM KCl, 1.25 mM KH2PO4, 10 mM Hepes, 1.25 mM NaH2PO4, adjusted to pH 7.4. AMPA was applied through a glass electrode (≈5–20-μm tip) using a Narishige IM-200 microinjector pump set at 10 psi and for a duration sufficient to produce approximately a 50% reduction in the slope of the EPSP recorded from stratum lacunosum/moleculare of CA1 (generally 10–35 msec). For control experiments, the stimulation intensity delivered to the stimulation electrode was increased to produce a 100% increase in the evoked EPSP slope before the second pressure application of AMPA.
Preparation of Membrane Fractions.
After storage at −70°C, slices were thawed, and 8–10 slices from each group were collected and homogenized. Pellets of membrane fractions were obtained by centrifugation of homogenates at 48,000 g for 15 min and resuspension in 0.32 M sucrose solution containing 3 mM EGTA. Membranes were washed with ice-cold distilled water containing 3 mM EGTA and 0.1 mM leupeptin. Membranes were finally resuspended in 100 mM Tris-acetate buffer, pH 7.4 containing 0.1 mM EGTA, and aliquots were used for immunoblotting and binding assays.
Electrophoresis and Immunoblotting.
Aliquots from each sample were used for SDS/PAGE (5–10% polyacrylamide) as described by Laemmli (32). Proteins were transferred onto nitrocellulose membranes as described by Towbin et al. (33). Nitrocellulose membranes were incubated with antibodies against SBDP, antibodies against GluR1 C-terminal domain (C-Ab), or antibodies against GluR1 N-terminal domain (N-Ab). The bands corresponding to SBDP and GluR1 subunits were detected with an alkaline phosphatase-conjugated secondary antibody (Bio-Rad) as previously described (26–27). The nitrocellulose paper was scanned, and bands were quantified by measuring the gray scale value using an image quantification software.
[3H]AMPA Binding Assay.
Aliquots of membrane suspensions were incubated at 0°C for 45–60 min in Tris-acetate buffer containing 50 mM Tris/acetate, 0.05 mM EGTA, 50 mM KSCN, and 40 nM [3H]AMPA. Nonspecific binding was defined as the binding measured in the presence of 2 mM l-glutamate. Incubation was terminated by centrifugation for 10 min at 48,000 g. Pellets were washed with Tris-acetate buffer (100 mM; pH 7.4) containing 0.1 mM and 50 mM KSCN. Radioactivity in the pellet was determined by scintillation spectroscopy.
RESULTS
Effects of Glycine Treatment on EPSPs in Cultured Hippocampal Slices.
A brief application of a high concentration of glycine (10 mM) resulted in increased epileptiform activity in CA1, characterized by multiple episodes of bursts of firing that generally lasted more than 2 sec each. At the end of glycine application, 2-amino-5-phosphonovaleric acid was perfused for 15 min to terminate epileptiform activity. In the majority of slices exhibiting prolonged and repeated episodes of seizures during glycine application, a long-lasting increase in the amplitude and slope of EPSPs recorded in CA1 after electrical stimulation in CA3 slowly developed; the increase reached a plateau after 30 min of glycine application and persisted for the duration of the experiment (Fig. 1A). In a number of cases, the same glycine treatment was followed by seizure activity, which led to a depression of EPSPs or to a recovery without potentiation. Control slices from the same pup were simultaneously incubated in artificial cerebrospinal fluid in an adjacent recording chamber for the duration of the experiment and did not exhibit significant modifications in EPSPs slope during the duration of the recording session. Control and glycine-treated slices exhibiting potentiation or not were collected for biochemical analyses.
The effect of glycine treatment on synaptic responses previously potentiated by TBS was investigated to determine whether TBS-induced LTP occluded glycine-induced potentiation (Fig. 1B). After a 10-min baseline recording, TBS was administered twice at 5-min intervals resulting in a 186% ± 44% increase in EPSP slope. Glycine treatment administered 30 min after TBS produced the same increase in epileptiform activity as in naive slices but did not result in any significant long-term changes in EPSP slope, suggesting that TBS- and glycine-induced potentiation share similar mechanisms.
Effects of Glycine-Induced Potentiation on Glutamate Receptor Properties.
Control and glycine-treated slices were collected, and 8–10 slices from each group were pooled and membrane fractions were prepared. [3H]AMPA binding was significantly increased in glycine-treated slices exhibiting LTP as compared with control slices (2.23 ± 0.36 pmol/mg protein versus 1.89 ± 0.32 pmol/mg protein, respectively; means ± SEM of five experiments; P < 0.05; Student’s paired t test). Although [3H]AMPA binding also was increased in glycine-treated slices that did not exhibit LTP (2.14 ± 0.40 pmol/mg protein versus 1.89 ± 0.32 pmol/mg protein; means ± SEM of five experiments; P = 0.32; Student’s paired t test), this effect did not reach statistical significance. This result is in agreement with previous studies showing an increase in [3H]AMPA binding after glycine treatment in acute hippocampal slices (29).
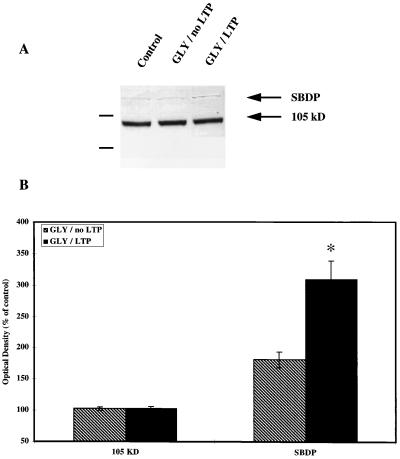
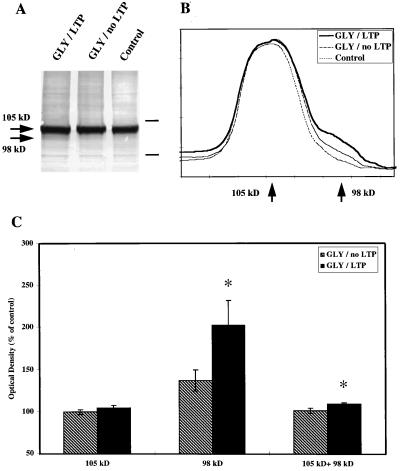
Aliquots from each membrane fraction also were used for immunoblotting. Two separate gels were run for each sample: one gel to analyze changes in immunological properties of GluR1 subunits of AMPA receptors using C-Ab and to evaluate calpain activation by determining the increase in SBDP using antibodies specific for the 155-kDa band, and the second to analyze changes in GluR1 immunoreactivity using N-Ab. Glycine treatment produced an increase in SBDP immunoreactivity as compared with control slices but did not result in any significant changes in the intensity of the band labeled with C-Ab (Fig. 2). The increase in SBDP correlated with the appearance of a second band labeled by N-Ab with a molecular mass about 7 kDa smaller than the control GluR1 subunits (98 kDa versus 105 kDa) (Fig. 3). The changes in GluR1 immunoreactivity were quantified by measuring the gray values of the 105-kDa and the 98-kDa species as well as the sum of the 105-kDa plus 98-kDa species (Fig. 3). Glycine treatment resulted in a significant increase in the 98-kDa species but did not modify the 105-kDa species labeled with N-Ab. It also resulted in a significant increase in total immunoreactivity (105- plus 98-kDa species) labeled with N-Ab. The changes in the 98-kDa band correlated with the degree of LTP obtained in the corresponding slices and with the increase in [3H]AMPA binding measured in aliquots from the same samples (P < 0.05) (data not shown).
Figure 2.
Effects of glycine-induced LTP on GluR1 (C-Ab) and SBDP immunoreactivity. Slices were collected at the end of the recording sessions (i.e., generally 75 min after glycine treatment) and processed for Western blots as described in Materials and Methods. (A) Representative blot stained with C-Ab (105 kDa) and antibodies against calpain-mediated SBDP. (Left) Molecular mass markers (118 and 86 kDa). (B) Densitometric analyses of Western blots similar to those shown in A. Optical densities of the 105-kDa band or of the 150-kDa SBDP band were measured, and data were expressed as percentage of control values (means ± SEM of six experiments). ∗, P < 0.01 as compared with control values (Student’s t test).
Figure 3.
Effects of glycine-induced LTP on GluR1 (N-Ab) immunoreactivity. Slices were collected at the end of the recording sessions (i.e., generally 75 min after glycine treatment) and processed for Western blots as described in Materials and Methods. (A) Representative blot stained with N-Ab. Arrows indicate the location of the 105-kDa and the 98-kDa bands labeled with N-Ab. Bars indicate the relative position of molecular mass markers: 118 and 86 kDa. (B) Representative profile of the scans obtained from blots similar to those shown in A. Note the increased amount of immunoreactivity migrating with an apparent molecular mass of 98 kDa in glycine-treated slices exhibiting LTP as compared with those exhibiting no LTP and control slices. (C) Quantitative analyses of gels similar to those shown in A. Areas corresponding to the 105- and 98-kDa peaks were integrated, as well as the total immunoreactivity (105 + 98 kDa). The data were expressed as percent of control values and are means ± SEM of six experiments. P < 0.05 (Student’s t test).
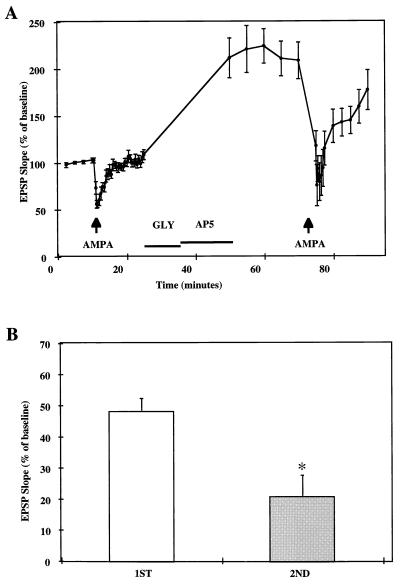
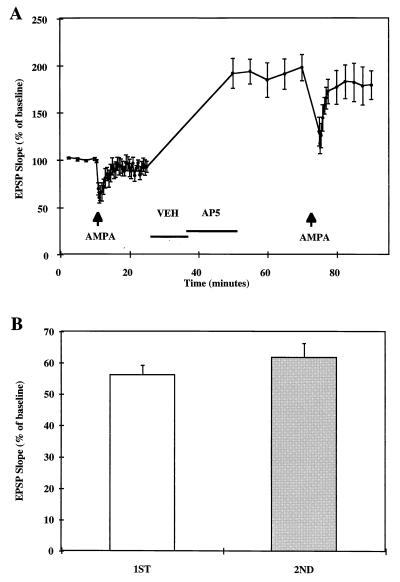
We used pressure application of AMPA to compare AMPA receptor function before and after glycine treatment in cultured hippocampal slices. After a 10-min baseline recording, 100 μM AMPA was pressure-applied to produce approximately a 50% reduction in evoked field EPSP slope measured in stratum lacunosum-moleculare of CA1 (Fig. 4A). After EPSPs recovered from AMPA application, treatment with 10 mM glycine for 10 min followed by a 15-min perfusion with 20 μM d,l-2-amino-5-phosphonovaleric acid resulted in EPSP potentiation. A second AMPA application for the same duration as the first application performed 40 min after glycine treatment resulted in a significantly greater percent reduction in evoked EPSP slope (Fig. 4B). The increase in AMPA-mediated reduction in evoked responses was correlated with the degree of potentiation induced by glycine treatment (P < 0.01) (data not shown). To eliminate the possibility that the effect of AMPA varied with EPSP size, stimulation intensity was increased after the first AMPA application to produce approximately a 100% increase in EPSP slope. Under these conditions, a second AMPA application resulted in the same proportional decrease in evoked EPSP slope as the first one (Fig. 5).
Figure 4.
Effects of pressure-applied AMPA on EPSP slopes before and after glycine-induced LTP. After a 10-min baseline recording, 100 μM AMPA was pressure-applied for 10–35 msec (arrow) to produce approximately 50% reduction in EPSP slope. Responses were allowed to recover for the next 15 min after which glycine (GLY, 10 mM) was perfused for 10 min followed by 20 μM AP5 (duration indicated by the horizontal bar). AMPA was applied again (same duration as in the first application) 40 min after glycine perfusion. (A) EPSP slopes were expressed as percent of average baseline values and represent means ± SEM of seven experiments. (B) Maximal reduction of EPSP slopes elicited by AMPA application was calculated as the percent of the means of EPSP slopes recorded for 10 min before the first (Left) and second (Right) AMPA application (means ± SEM of seven experiments). ∗, P < 0.005 (Student’s t test).
Figure 5.
Effects of pressure-applied AMPA on EPSP slopes before and after increasing stimulation intensity. After a 10-min baseline recording, 100 μM AMPA was pressure-applied for 10–35 msec to produce approximately 50% reduction in EPSP slope. Responses were allowed to recover for the next 15 min after which glycine vehicle (VEH) was perfused for 10 min followed by 20 μM AP5. At the end of AP5 perfusion, stimulation intensity was increased to produce approximately 100% increase in EPSP slope. AMPA was applied again (same duration as in the first application) 25 min later. (A) EPSP slopes were expressed as percent of average baseline values and represent means ± SEM of seven experiments. (B) Maximal reduction of EPSP slopes elicited by AMPA application was calculated as the percent of the means of EPSP slopes recorded for 10 min before the first (Left) and second (Right) AMPA application (means ± SEM of seven experiments).
DISCUSSION
A brief application of high concentration of glycine previously has been shown to produce a long-lasting potentiation of synaptic responses recorded in CA1 of acute hippocampal slices, and this effect was shown to be mediated by NMDA receptor activation (29, 30). The present results indicate that the same treatment also produces potentiation in cultured hippocampal slices. Furthermore, occlusion of glycine-induced potentiation by previous TBS-induced LTP suggests that both types of potentiation share similar mechanisms. In agreement with previous results in acute slice preparations (29), glycine-induced LTP in cultured slices was associated with increased [3H]AMPA binding to membrane fractions. It is not clear from these data whether this increase in binding is due to a higher affinity of the receptor for the agonist or to an increase in the number of AMPA receptors. Glycine treatment also resulted in calpain activation as indicated by the accumulation of SBDP. This biochemical marker has been used by several laboratories to demonstrate calpain activation, in particular after NMDA receptor activation (23, 34) or TBS-induced LTP in hippocampal slices in culture (24, 35). Interestingly, SBDP accumulation was more elevated in glycine-treated slices that exhibited potentiation as compared with those not showing potentiation, suggesting that calpain activation is a necessary event in LTP induction. We recently have shown that calpain treatment of synaptic membranes does not modify [3H]AMPA binding, indicating that the increase in [3H]AMPA binding resulting from glycine treatment is not a direct consequence of calpain-mediated truncation of GluR subunits (27). We previously showed that calcium treatment of frozen-thawed brain sections produces an increase in [3H]AMPA binding that was partly blocked by calpain inhibitors (36), and we proposed that this effect was the result of the insertion of receptors in membranes from an intracellular pool of receptors (37). Similarly, we recently showed that NMDA treatment of hippocampal slices in culture results in increased [3H]AMPA binding and calpain-mediated truncation of the C-terminal domain of GluR1 subunits (38). It is thus conceivable that the increase in [3H]AMPA binding resulting from glycine treatment is due to the insertion of receptors in synaptic membranes.
Results from Western blots obtained from glycine-treated slices indicated the formation of a new species of GluR1 subunits with a molecular mass of 98 kDa. This effect was not accompanied with significant changes in the amount of the 105-kDa species labeled with C-Ab or N-Ab. We recently showed that calpain activation in tissue sections or calpain treatment of synaptic membranes results in the truncation of a portion of the C-terminal domain of the GluR1 subunit, producing a decrease in amount of the 105-kDa species labeled with C-Ab and the formation of the 98-kDa species along with a decrease in the 105-kDa species in blots labeled with N-Ab (26, 27). Our results are therefore consistent with the idea that a fraction of GluR1 subunits has been truncated as a result of glycine-induced potentiation. The increase in total immunoreactivity labeled with N-Ab indicated that the total number of receptors increased in the membrane fraction. This could account for the lack of decrease in the 105-kDa species labeled with C-Ab, as insertion of intact receptors would mask the decrease due to truncation of existing receptors. Alternatively, it is conceivable that there is no truncation of existing receptors and only insertion of truncated receptors. In either case, increased number of membrane-associated AMPA receptors could account for increased [3H]AMPA binding after glycine application. Finally, our data further support a critical role for calpain in LTP induction as the truncation of GluR1 subunits observed after glycine application is similar to that observed in calpain-treated synaptic membranes.
The data obtained with pressure application of AMPA also suggest changes in AMPA receptor properties after glycine-induced potentiation. AMPA application activates AMPA receptors, resulting in neuronal depolarization, a reduced driving force across cell membranes, and consequently smaller evoked EPSPs. Alternatively, prolonged occupation of synaptic receptors by AMPA as well as desensitization might reduce the efficacy of endogenously released glutamate at AMPA receptors. Increasing stimulation intensity activates additional synapses and AMPA application under these conditions resulted, as predicted, in a proportionally equal reduction in EPSP slope. Local application of AMPA after glycine treament resulted in a proportionally greater reduction in evoked EPSP slope. If potentiation was solely due to the insertion of receptors with properties similar to those of existing receptors, the same relative effect of AMPA application would have been expected before and after glycine application. It is therefore more likely that the appearance of truncated synaptic receptors is responsible for the increased effect of AMPA application after glycine-induced LTP. This idea fits well with the positive correlations observed between the degree of synaptic potentiation and the degree of reduction in EPSP during AMPA application on one hand and with the increase in the amount of the 98-kDa species of the GluR1 subunit on the other hand. It also could account for the large difference in the increase in EPSP (about 200%) and those in AMPA receptor binding (18%) and receptor number as determined in Western blots (10%).
To summarize, we found that a brief period of glycine application produced a long-lasting potentiation of synaptic transmission in cultured hippocampal slices that was occluded by previous tetanus-induced LTP. Potentiation was accompanied by calpain activation and by increased AMPA receptors with GluR1 subunits truncated in their C-terminal domain. It also was associated with increased responsiveness to pressure-applied AMPA. We propose that truncation of the C-terminal domain of GluR1 by calpain results in increased AMPA receptor function and that this mechanism participates in LTP expression.
Acknowledgments
This research was supported by Grant NS 18427 from the National Institute of Neurological Disorders and Stroke to M.B. and a grant from Sankyo Pharmaceutical, Ltd.
ABBREVIATIONS
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid
- TBS
theta burst stimulation
- LTP
long-term potentiation
- SBDP
spectrin breakdown product
- N-Ab
antibodies against GluR1 N-terminal domain
- C-Ab
antibodies against GluR1 C-terminal domain
- NMDA
N-methyl-d-aspartate
- EPSP
excitatory postsynaptic potential
References
- 1.Maren S, Baudry M. Neurobiol Learning Memory. 1995;63:1–18. doi: 10.1006/nlme.1995.1001. [DOI] [PubMed] [Google Scholar]
- 2.Martinez J L, Derrick B E. Annu Rev Psych. 1996;47:173–203. doi: 10.1146/annurev.psych.47.1.173. [DOI] [PubMed] [Google Scholar]
- 3.Bliss T V, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 4.Bear M F, Malenka R C. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 5.Baudry M, Davis J L, editors. Long-Term Potentiation. III. Cambridge, MA: MIT Press; 1996. [Google Scholar]
- 6.Medina J H, Izquierdo I. Brain Res Rev. 1995;21:185–194. doi: 10.1016/0165-0173(95)00013-5. [DOI] [PubMed] [Google Scholar]
- 7.Edwards F A. Trends Neurosci. 1995;18:250–255. doi: 10.1016/0166-2236(95)80003-k. [DOI] [PubMed] [Google Scholar]
- 8.Maren S, Tocco G, Standley S, Baudry M, Thompson R F. Proc Natl Acad Sci USA. 1993;90:9654–9658. doi: 10.1073/pnas.90.20.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahi K, Baudry M. Proc Natl Acad Sci USA. 1992;89:6881–6885. doi: 10.1073/pnas.89.15.6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staubli U, Kessler M, Lynch G. Psychobiology. 1990;18:377–381. [Google Scholar]
- 11.Ambros-Ingerson J, Larson J, Xiao P, Lynch G. Synapse. 1991;9:314–316. doi: 10.1002/syn.890090406. [DOI] [PubMed] [Google Scholar]
- 12.Liao D Z, Hessler N A, Malinow R. Nature (London) 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 13.Isaac J T R, Nicoll R A, Malenka R C. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 14.Bekkers J M, Stevens C F. Nature (London) 1990;346:724–729. doi: 10.1038/346724a0. [DOI] [PubMed] [Google Scholar]
- 15.Malinow R, Tsien R W. Nature (London) 1990;346:177–180. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- 16.Foster T C, McNaughton B L. Hippocampus. 1991;1:79–91. doi: 10.1002/hipo.450010108. [DOI] [PubMed] [Google Scholar]
- 17.Lynch G, Baudry M. Science. 1984;224:1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- 18.Lynch G, Baudry M. Hippocampus. 1991;1:9–14. doi: 10.1002/hipo.450010103. [DOI] [PubMed] [Google Scholar]
- 19.Oliver M W, Baudry M, Lynch G. Brain Res. 1989;505:233–238. doi: 10.1016/0006-8993(89)91448-0. [DOI] [PubMed] [Google Scholar]
- 20.del Cerro S, Larson J, Oliver M W, Lynch G. Brain Res. 1990;530:91–95. doi: 10.1016/0006-8993(90)90660-4. [DOI] [PubMed] [Google Scholar]
- 21.Denny J B, Polan-Curtain J, Ghuman A, Wayner M J, Armstrong D L. Brain Res. 1990;534:317–320. doi: 10.1016/0006-8993(90)90148-5. [DOI] [PubMed] [Google Scholar]
- 22.Staubli U, Larson J, Baudry M, Thibault O, Lynch G. Brain Res. 1988;444:153–158. doi: 10.1016/0006-8993(88)90922-5. [DOI] [PubMed] [Google Scholar]
- 23.del Cerro S, Arai A, Kessler M, Bahr B A, Vanderklish P, Rivera S, Lynch G. Neurosci Lett. 1994;167:1–2. doi: 10.1016/0304-3940(94)91049-9. [DOI] [PubMed] [Google Scholar]
- 24.Vanderklish P, Saido T C, Gall C, Lynch G. Mol Brain Res. 1995;32:25–35. doi: 10.1016/0169-328x(95)00057-y. [DOI] [PubMed] [Google Scholar]
- 25.Bi X, Tocco G, Baudry M. NeuroReport. 1994;6:61–64. doi: 10.1097/00001756-199412300-00017. [DOI] [PubMed] [Google Scholar]
- 26.Bi X, Chang V, Molnar E, McIlhinney J, Baudry M. Neuroscience. 1996;73:903–906. doi: 10.1016/0306-4522(96)00157-1. [DOI] [PubMed] [Google Scholar]
- 27.Bi X, Chen J, Dang S, Wenthold R J, Tocco G, Baudry M. J Neurochem. 1997;68:1484–1494. doi: 10.1046/j.1471-4159.1997.68041484.x. [DOI] [PubMed] [Google Scholar]
- 28.Vanderklish P, Neve R, Bahr B A, Arai A, Hennegrif M, Larson J, Lynch G. Synapse. 1992;12:333–337. doi: 10.1002/syn.890120410. [DOI] [PubMed] [Google Scholar]
- 29.Shahi K, Baudry M. Brain Res. 1993;627:261–266. doi: 10.1016/0006-8993(93)90329-l. [DOI] [PubMed] [Google Scholar]
- 30.Shahi K, Marvizon J C, Baudry M. Neurosci Lett. 1993;149:185–188. doi: 10.1016/0304-3940(93)90767-f. [DOI] [PubMed] [Google Scholar]
- 31.Stoppini L, Buchs P A, Muller D. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts-Lewis J M, Savage M J, Marcy V R, Pinsker L R, Siman R. J Neurosci. 1994;14:3934–3944. doi: 10.1523/JNEUROSCI.14-06-03934.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saido T C, Yokota M, Nagao S, Yamaura I, Tani E, Tsuchiya T, Suzuki K, Kawashima S. J Biol Chem. 1993;268:25239–25243. [PubMed] [Google Scholar]
- 36.Tocco G, Massicotte G, Standley S, Thompson R F, Baudry M. Eur J Neurosci. 1992;4:1093–1103. doi: 10.1111/j.1460-9568.1992.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 37.Standley S, Bi X, Baudry M. In: Long-Term Potentiation. Baudry M, Davis J L, editors. III. Cambridge, MA: MIT Press; 1996. pp. 17–40. [Google Scholar]
- 38.Gellerman D, Bi X, Baudry M. J Neurochem. 1997;69:131–136. doi: 10.1046/j.1471-4159.1997.69010131.x. [DOI] [PubMed] [Google Scholar]