Abstract
HIV infection often involves the development of AIDS-related dementia complex, a variety of neurologic, neuropsychologic, and neuropathologic impairments. A possible contributor to AIDS-related dementia complex is the HIV envelope glycoprotein gp120, which damages neurons via a complex glutamate receptor- and calcium-dependent cascade. We demonstrate an endocrine modulation of the deleterious effects of gp120 in primary hippocampal and cortical cultures. Specifically, we observe that gp120-induced calcium mobilization and neurotoxicity are exacerbated by glucocorticoids, the adrenal steroids secreted during stress. Importantly, this deleterious synergy can occur between gp120 and synthetic glucocorticoids (such as prednisone or dexamethasone) that are used clinically in high concentrations to treat severe cases of the Pneumocystis carinii pneumonia typical of HIV infection. Conversely, we also observe that estradiol protects neurons from the deleterious actions of gp120, reducing toxicity and calcium mobilization.
A cornerstone of neuroendocrinology is the ability of hormones to influence the nervous system, including altering neurochemistry, electrophysiology, and behavior. Only recently has it been recognized that hormones can endanger or protect neurons, compromising or enhancing, respectively, the likelihood of a neuron surviving a coincident insult. Perhaps the best documented case of such endangerment involves the adrenal steroid hormones glucocorticoids (GCs), which are secreted in response to stress. Physiological concentrations of GCs impair the capacity of hippocampal, cortical, and striatal neurons to survive excitotoxic seizures, ischemia, and energy deprivation; moreover, stress itself can exacerbate the neurotoxicity of these insults (1, 2). As an example of protection, estrogenic steroids decrease excitotoxic or oxidative damage to hippocampal and cortical neurons (3, 4); in addition, postmenopausal estrogen replacement decreases the risk of late-onset Alzheimer disease (which primarily damages cortex and hippocampus) (5–7). In the present report, we document a new instance of GC endangerment and estrogenic protection that may be of clinical relevance.
About 20% of cases of HIV infection involve AIDS-related dementia complex (ADC), a variety of neurologic and neuropsychologic impairments accompanied by diffuse neuropathologic changes in cortical and subcortical regions (8–11); the neuropathologic changes are of sufficient magnitude to be thought to contribute to the functional impairments (9, 11). ADC was initially thought to be due to opportunistic central nervous system infections secondary to immunosuppression but is now recognized to arise directly from HIV infection of the brain. The virus appears to gain access to the brain through infected blood monocytes in a Trojan horse scenario (12). Within the brain, HIV targets macrophages and microglia, from which it damages neighboring neurons through a multistep cascade that appears to involve the release of an as yet unidentified excitatory amino acid. This leads to overactivation of potentially excitotoxic glutamatergic N-methyl-d-aspartate receptors and mobilization of excessive cytosolic calcium. These deleterious effects of HIV appear to involve its envelope glycoprotein gp120. In vitro and in vivo models demonstrate that gp120 can damage neurons and glia, and does so by activating N-methyl-d-aspartate receptors, mobilizing cytosolic calcium, and subsequently generating nitric oxide (13–17). Moreover, transgenic overexpression of gp120 in mice results in neuropathologic changes reminiscent of HIV infection (18). Although ADC is likely to involve other factors, particularly cytokines, gp120 remains central to thinking about the deleterious effects of HIV within the brain; thus, we tested the ability of GCs and of estrogen to modulate gp120-induced neurotoxicity. This represents one of the few neuroendocrine studies, to our knowledge, relevant to ADC.
MATERIALS AND METHODS
Preparation of Cultures.
Cells were dissociated with papain, rather than trypsin, filtered through an 80-μm cell strainer, and resuspended in a modified MEM (Univ. California, San Francisco, Tissue Culture Facility; ref. 19) supplemented with 10% horse serum (HyClone, Logan, Ut). Cells were plated at a density of 20,000 cells per cm2 on either 48-well tissue culture plates (for neurotoxicity studies) or on 12-mm glass coverslips (Carolina Biological, Burlington, NC; for calcium studies), both having been pretreated with poly-(d-lysine) (Sigma). Cells were maintained in this same medium for 11–14 days before use, at which time, immuncytochemical staining of neurons with an antibody (Sigma) against neuron-specific microtubule-associated protein 2 indicates the neuron/nonneuron ratio to be approximately 30:70.
Experimental Procedure for Toxicity Studies.
Cultures were incubated for 72 h in MEM containing 200 pM gp120 (HIVSF2pg120, dissolved in a citrate buffer, supplied through the National Institutes of Health AIDS Reagent Program, from Chiron) and/or indicated quantities of steroid hormone (obtained from either Steraloids, Wilton, NH, or Sigma; steroids were dissolved in 1 ml of ethanol for stock solutions, but 1 μl of the stock solution was diluted in 10 ml of DMEM). Experimental solutions were made just prior to each experiment by diluting appropriate amounts of stock gp120 and/or steroids in DMEM (GIBCO/BRL) to the required concentration; gp120 and steroidal controls were exposed to citrate or ethanol alone, respectively.
Cultures were exposed to gp120 and/or steroids either after complete removal of prior medium or after spiking medium with microliter amounts of solutions. After 72 h, medium was removed and cells were fixed for 24 h in 4°C methanol. Neuron death was then assessed by quantitative cell counts after cultures were stained for microtubule-associated protein 2 (Sigma). A transect across the center of the well was counted; only neurons with intact processes were counted.
Calcium Imaging.
After treatment for 24 h with or without 1 μM corticosterone, medium was removed and coverslips were treated with 2 μM fura 2AM (Molecular Probes) and 0.005% pluronic acid dissolved in Hanks’ balanced salt solution (GIBCO/BRL) with 50 mM Hepes buffer at pH 7.4 and 5 mM glucose (HBSSh) for 25 min. Coverslips were placed on a stage with continuous perfusion with HBSSh medium on an inverted Olympus microscope (Scientific Instruments, Sunnyvale, CA) with ×10 or ×20 objectives. gp120 was dissolved in HBSSh at a concentration of 200 pM. Five initial readings prior to gp120 exposure (arrow) were followed by 60 readings at 10-sec intervals afterward. Calcium concentrations in individual cells were determined from the ratio of light intensity at 340- and 380-mn excitation wavelengths by using standard imaging techniques and analysis with fluor and metafluor software from Universal Imaging (West Chester, PA).
The area under the curve of the increase of free cytosolic calcium concentrations above the averaged baseline was calculated (for 60 measures after gp120 exposure) by using an in-house macro program for excel spreadsheet. Results are expressed as nanomoles of calcium.
RESULTS
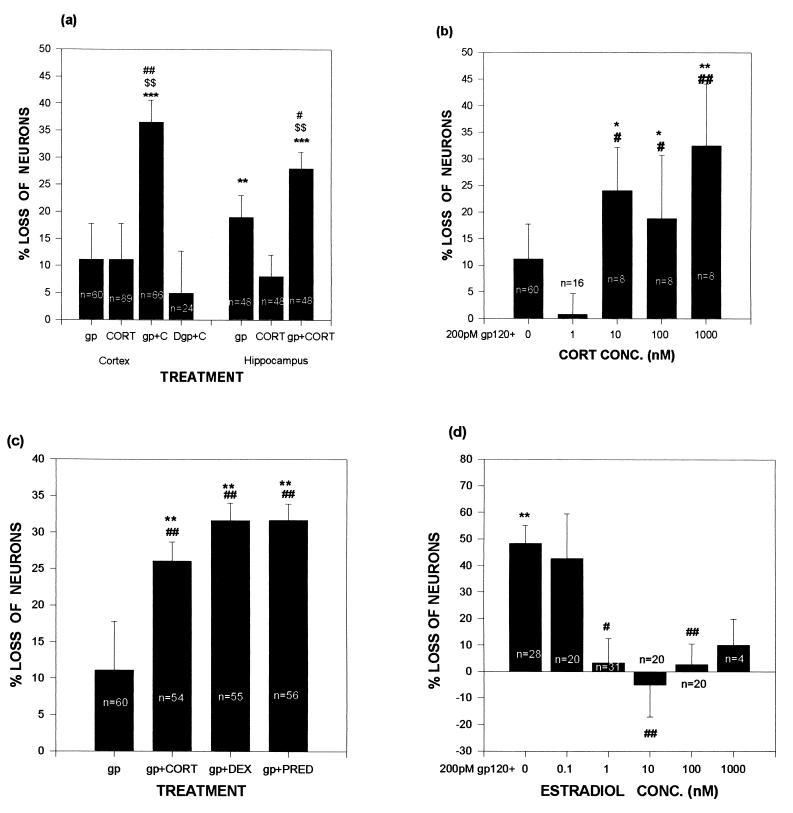
Cultures were prepared from 18-day fetal rats (20) and were studied after 11–14 days in culture, a time when synapses have formed and steroid responses are demonstrable (21, 22). In cortical cultures, no significant neuron loss occurred after administration of either gp120 or an upper physiological concentration of corticosterone (the GC of rats; Fig. 1A). However, a highly significant synergy occurred between the two. A synergy also occurred in hippocampal cultures, where gp120 alone was also neurotoxic. The gp120 contribution to the toxic synergy did not occur if the glycoprotein was heat-denatured (80°C for 30 min; data not shown).
Figure 1.
Exposure to GCs exacerbates gp120-induced cell death in neuronal cultures, whereas estradiol is protective. Data are expressed as percentage of numbers of neurons in control wells (no gp120 or steroid) in the same plate. (A) In both hippocampal and cortical cultures, neuron loss was more pronounced in cultures treated with gp120 and 1 μM corticosterone than in cultures treated with either agent alone. (B) gp120-induced neuron loss varied with corticosterone concentrations. Significant levels of gp120-induced neurotoxicity coincided with corticosterone concentrations above the Kd (approximately 5 nM) of the type II GC receptor. (C) gp120-induced neuron death was worsened by the 1 μM concentrations of the synthetic GCs dexamethasone and prednisone. (D) Treatment of cortical cultures with estradiol reduced gp120-induced neurotoxicity. ∗∗ and ∗∗∗ indicate significant difference at the P < .01 and .001 levels, respectively, when compared with control (0% neuron loss, derived from cultures exposed to neither gp120 nor any steroid); # and ## indicate significant difference at the P < .05 and .01 levels, respectively, when compared with gp120 alone; $$ indicates significant difference at the P < .01 level, when compared with hormone alone; Newman–Keuls post-hoc tests after ANOVA. gp, gp120 (200 pM); CORT, corticosterone; Dgp, denatured gp120; DEX, dexamethasone; PRED, prednisone.
We next tested the dose dependency of the corticosterone endangerment. Previous work showed that such endangerment is mediated by the low-affinity type II GC receptor (for review, see ref. 2); in agreement with this, the steroid augmented gp120 toxicity only when administered at concentrations above the Kd of that receptor (approximately 5 nM; Fig. 1B).
As will be discussed, synthetic GCs such as prednisone and dexamethasone are often used in extremely high concentrations to treat various features of HIV infection. We observed that those compounds, both type II receptor agonists, also augmented the toxicity of gp120 (Fig. 1C). The endangerment was GC-specific, in that equal amounts of testosterone (a non-GC steroid) did not augment gp120 toxicity (data not shown).
In the course of these steroid-specificity studies, we noted that estradiol not only did not endanger cortical neurons but protected them from gp120. There was a nonsignificant trend toward protection at 1 nM concentrations, which is well above the Kd of the ERβ estrogen receptor that is found in brain tissue (approximately 0.4 nM; ref. 23), although significant protection occurred at higher concentrations (Fig. 1D).
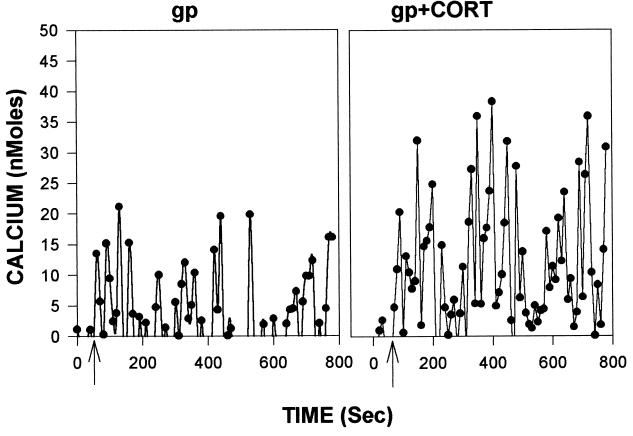
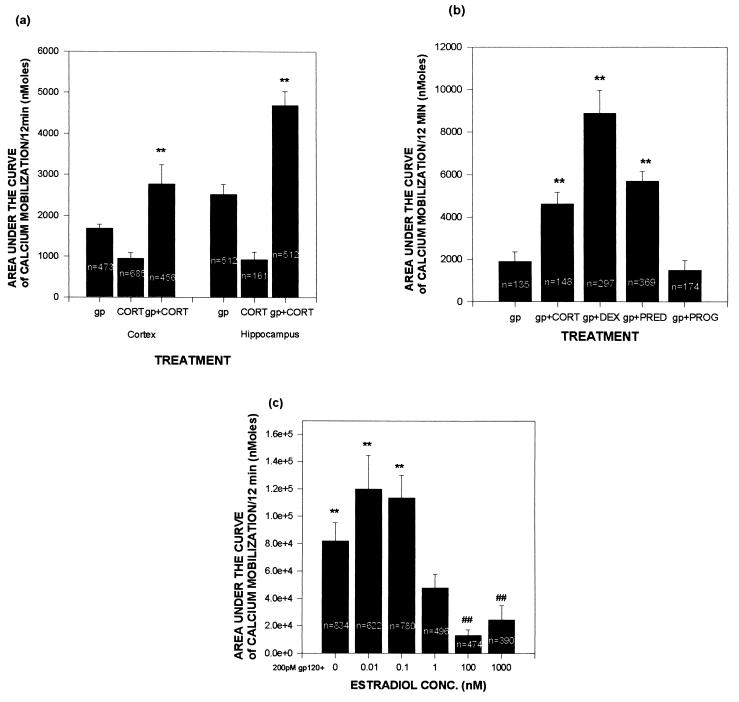
As noted, gp120-induced neurotoxicity appears to arise from its ability to mobilize excessive cytosolic calcium (13–16). We observed such increases after gp120 administration (see Fig. 2 for representative data from hippocampal cultures; similar responses were observed in cortical cultures); such mobilization was augmented by administration of corticosterone in both culture types (Figs. 2 and 3A). As with the toxicity data, the effects of the gp120 were eliminated when denatured glycoprotein was used (data not shown).
Figure 2.
Representative calcium response of a hippocampal neuron exposed to gp120 with (Right) or without (Left) prior exposure to 1 μM corticosterone for 24 h. Data are presented after subtraction of the average of the five initial basal readings (which averaged between 50 and 100 nM in these studies).
Figure 3.
Exposure to GCs increases gp120-induced mobilization of free cytosolic calcium, whereas estrogen reduces the effects of gp120. Cultures were incubated in MEM containing steroid hormone for 24 h prior to a 10-min exposure to 200 pM gp120. A total of 60 readings were taken over the final 10 min, and data presented represent area under the curve of those 60 measures after subtracting pre-gp120 basal values. (A) Calcium concentrations in hippocampal and cortical cultures treated acutely with gp120 and/or pretreated with 1 μM corticosterone. (B) Pretreatment with the GCs 100 mM corticosterone, dexamethasone, or prednisone exacerbates the mobilization of calcium; the non-GC steroid progesterone at 100 nM did not exacerbate damage. (C) Pretreatment with estradiol attenuates gp120-induced calcium mobilization. Experiments were carried out and analyzed as described in Fig. 2. ∗ and ∗∗ indicate significantly more calcium mobilization (P < .05 and .01, respectively) when compared with gp120 alone by t test (A) or by Newman–Keuls post-hoc test after ANOVA (B); # indicates significantly less calcium mobilization (P < .05) when compared with gp120 alone by post-hoc test.
The synthetic GCs prednisone and dexamethasone also significantly augmented gp120-induced calcium mobilization (Fig. 3B). In contrast, the non-GC steroid progesterone had no effect, although estradiol significantly decreased such calcium mobilization (Fig. 3C). As with the toxicity data, significant protection only occurred with estradiol concentrations well above the Kd of its receptor.
Further analysis of the data suggested differing mechanisms by which GCs and estradiol had their opposing effects on gp120-induced calcium mobilization (Table 1). GCs enhanced the gp120 effect both by increasing the percentage of neurons that responded to the glycoprotein with a rise in free cytosolic calcium concentrations and by potentiating the magnitude of the calcium oscillations in those neurons that were responsive. In contrast, estradiol blunted the effects of gp120 by decreasing the percentage of neurons that responded to the glycoprotein with a rise in cytosolic calcium concentrations; however, estradiol did not alter the size of the calcium response in those neurons that did respond.
Table 1.
Response of neurons to gp120
| % neurons failing to respond to gp120 | Ca rise, nM | |
|---|---|---|
| Vehicle | 9.6 | 2,814 ± 211 |
| Corticosterone | 0.0 | 6,585 ± 162* |
| Estradiol | 35.7 | 3,999 ± 220 |
The percentage of neurons that failed to respond to gp120 with a mobilization of free cytosolic calcium concentrations was measured. Glucocorticoid treatment, on the average, significantly reduced the percentage of neurons that were unresponsive to gp120, whereas estradiol had the opposite effect (P < 0.001 by χ2 analysis). Responsiveness consisted of an increase in cytosolic calcium concentrations after gp120, 200 pM, administration (n = 135, 148, and 378 for vehicle, 100 nM corticosterone, and 100 nM estradiol, respectively). The extent of calcium mobilization in neurons that responded to gp120 with a calcium rise was measured. Numbers represent data (mean ± SEM) taken from those neurons that were responsive to gp120 (i.e., those that were not included above).
P < 0.001 when compared to vehicle by Newman–Keuls post-hoc test after one-way ANOVA.
DISCUSSION
A considerable amount is understood about the mechanisms underlying GC endangerment of neurons, and some of these mechanisms are likely to be relevant to the present instance. The steroids decrease glucose transport in the hippocampus, producing an energy deficit that exacerbates the decline in ATP concentrations and mitochondrial function during insults. As a result, neurons are less capable of carrying out the costly task of removing glutamate from the synapse or sequestering cytosolic calcium, leading to elevated levels of both during insults. This elevation, in turn, results in exacerbation of calcium-dependent degenerative events, such as cytoskeletal proteolysis or oxygen radical accumulation (for review, see ref. 2). These GC actions will certainly be relevant to gp120, insofar as the glycoprotein appears to damage neurons through glutamate receptor- and calcium-dependent routes (13–16). Of additional relevance to the present findings, GCs act independently of energy status to enhance voltage-gated calcium currents in hippocampal neurons (24–26). Finally, GCs can blunt the expression of neurotrophins and inhibit injury-induced sprouting in the hippocampus (27–29), both possibly relevant to any compensatory responses to deleterious effects of gp120. Further work must clarify which of these mechanisms are relevant to the gp120/GC synergy and explain the roughly equal vulnerability of both hippocampal and cortical neurons, as the latter are typically less sensitive to most indices of GC endangerment (1, 2).
Less mechanistic understanding exists concerning the newer phenomenon of estrogenic protection of neurons, but the little known appears relevant to the present case. Estradiol decreases glutamate-induced calcium mobilization in the hippocampus (3), thus directly counteracting the glutamate-receptor-dependent calcium mobilization triggered by gp120. In the present case, this appears to be some sort of threshold phenomenon, in that estradiol decreased the percentage of neurons responding to gp120 with a rise in cytosolic calcium concentrations without changing the size of that rise in the responsive neurons; the mechanisms underlying that distinction await further study. Estrogens have antioxidant activity in cardiac tissue and endothelium (30–33), as well as in hippocampal neurons (3). Thus, estradiol might blunt the pathologic generation of oxygen radicals caused by gp120-induced calcium mobilization. Finally, estradiol induces expression of a number of neurotrophins in the hippocampus and cortex and, as a probable consequence of this, increases the density of dendritic spines (34). The antioxidant effects of estrogen are generally thought to occur in supraphysiologic concentrations, although the other effects are likely to be physiologic and receptor-mediated (35). The relatively high concentrations of estradiol required for reducing both toxicity and calcium mobilization suggest that the supraphysiologic antioxidant effects of the steroid may be responsible for protection; this awaits further study.
The present report is constrained by the obvious artificiality of the use of gp120 outside the context of other possible contributors to ADC. For example, although there is evidence that pyramidal neurons are predominately damaged by gp120, interneurons appear to be more susceptible to cytokine-induced damage (36) [of note, the cultures used in these studies are 85–90% pyramidal (21)]. Another constraint on the present report is the reliance upon an in vitro system. The impact of these hormones on in vivo models of HIV infection [e.g., the gp120 transgenic mouse model (18)] needs to be examined. Should these findings prove relevant to understanding the progression of ADC in humans, there are a number of implications. The estrogenic protection would generate the prediction that among HIV sufferers, men should have the greater extent of HIV neuropathology and a higher incidence or severity of ADC-related dysfunction; this has not yet been tested, to our knowledge. Of possibly greater importance, estrogen administration could conceivably be therapeutic for ADC-related dysfunction in HIV patients; such clinical trials are now in progress concerning the protective effects of estrogen against late-onset Alzheimer disease.
The GC endangerment might be of clinical relevance in a number of ways. (i) Despite initial reports of possible adrenal insufficiency in AIDS, adrenal GC secretory capacity remains intact, and there is, in fact, mild hypercortisolism in a significant subset of AIDS patients (37); of possible significance, gp120 stimulates the adrenocortical axis, and gp120 transgenic mice have elevated GC concentrations (38). (ii) Although the rate of depression in symptom-free HIV-positive individuals is similar to that in uninfected populations, there is a dramatic increase in the incidence of depressions in individuals with AIDS (39–42). An extensive literature demonstrates that about half of depressives have significantly elevated GC concentrations (43); although the rates of hypercortisolism in depressed individuals with AIDS have not been compared with uninfected depressed individuals, the demonstrated intactness of the adrenocortical axis in the former suggests that they should hypersecrete GCs at at least the same rate as other depressed populations. (iii) One of the most serious consequences of HIV infection is the frequent cases of Pneumocystis carinii pneumonia, and the current treatment of choice for serious cases involves administration of megadoses of synthetic GCs such as those used in this study (44–48). Although the number of severe cases of Pneumocystis carinii pneumonia is declining, best estimates are that thousands of individuals are still treated annually in this megadose range (e.g., 160 mg of methylprednisolone per day for 7 days). The present findings suggest that any of these routes of exposure to elevated GC concentrations might have adverse neurological consequences; this possibility remains to be tested in a clinical setting.
Acknowledgments
We thank David Brenneman, Lynn Pulliam, and Stuart Lipton for advice and Ogden BioServices Corporation for assistance in working with gp120. Support was provided by a Howard Hughes Grant for Undergraduate Research (R.C.) and the Adler Foundation and National Institutes of Health Grant RO1 MH53814 (R.S.). The estimate of the incidence of use of corticosteroids for Pneumocystis carinii pneumonia was derived from Dr. Jeff Jones of the Centers for Disease Control and Prevention, based on his >28,000 person years follow-up of AIDS patients from 1990 to 1994.
ABBREVIATIONS
- ADC
AIDS-related dementia complex
- GC
glucocorticoid
References
- 1.McEwen B. Prog Brain Res. 1992;93:365–382. doi: 10.1016/s0079-6123(08)64585-9. [DOI] [PubMed] [Google Scholar]
- 2.Sapolsky R. Stress. 1996;1:1–19. doi: 10.3109/10253899609001092. [DOI] [PubMed] [Google Scholar]
- 3.Goodman Y, Bruce A, Cheng B, Mattson M. J Neurochem. 1996;66:1836–1839. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- 4.Behl C, Widmann M, Trapp T, Holsboer F. Biochem Biophys Res Commun. 1995;216:473–477. doi: 10.1006/bbrc.1995.2647. [DOI] [PubMed] [Google Scholar]
- 5.Henderson V, Paganini-Hill A, Emanuel C, Dunn M, Buckwalter J. Arch Neurol. 1994;51:896–903. doi: 10.1001/archneur.1994.00540210068014. [DOI] [PubMed] [Google Scholar]
- 6.Mortel K, Meyer J. J Neuropsych Clin Neurosci. 1995;7:334–340. doi: 10.1176/jnp.7.3.334. [DOI] [PubMed] [Google Scholar]
- 7.Paganini-Hill A, Henderson V. Am J Epidemiol. 1994;140:256–269. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- 8.Budka H H. Brain Pathol. 1991;1:163–175. doi: 10.1111/j.1750-3639.1991.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 9.Wiley C, Masliah E, Morey M. Ann Neurol. 1991;29:651–662. doi: 10.1002/ana.410290613. [DOI] [PubMed] [Google Scholar]
- 10.Masliah E, Ge N, Morey M, DeTeresa R, Terry R, Wiley C. Lab Invest. 1992;66:285–293. [PubMed] [Google Scholar]
- 11.Lipton S, Gendelman H. N Engl J Med. 1995;332:934–939. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- 12.Haas A. Nature (London) 1986;322:130–133. [Google Scholar]
- 13.Dreyer E, Kaiser P, Offermann J, Lipton S. Science. 1990;248:364–366. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- 14.Lipton S. Neuron. 1991;7:111–118. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- 15.Lo T, Fallert C, Piser T, Thayer S. Brain Res. 1992;594:189–196. doi: 10.1016/0006-8993(92)91125-x. [DOI] [PubMed] [Google Scholar]
- 16.Nath A. Brain Res. 1995;678:200–208. doi: 10.1016/0006-8993(95)00185-s. [DOI] [PubMed] [Google Scholar]
- 17.Dawson V, Dawson T, Uhl G, Snyder S. Proc Natl Acad Sci USA. 1993;90:3256–3259. doi: 10.1073/pnas.90.8.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toggas S, Masliah E, Rockenstein E, Rall G, Abraham C, Mucke L. Nature (London) 1994;367:188–190. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- 19.Yu A, Hertz E, Hertz L. J Neurochem. 1984;42:951–958. doi: 10.1111/j.1471-4159.1984.tb12696.x. [DOI] [PubMed] [Google Scholar]
- 20.Elliott E, Sapolsky R. J Neurochem. 1992;59:1033–1039. doi: 10.1111/j.1471-4159.1992.tb08345.x. [DOI] [PubMed] [Google Scholar]
- 21.Banker G, Goslin K. Culturing Nerve Cells. Cambridge, MA: MIT Press; 1991. [Google Scholar]
- 22.Sapolsky R, Brooke S, Stein-Behrens B. J Neurosci Methods. 1995;58:1–15. doi: 10.1016/0165-0270(94)00155-a. [DOI] [PubMed] [Google Scholar]
- 23.Kuiper G, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson J. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 24.Kerr D, Campbell L, Hao S, Landfield P. Science. 1989;245:1505–1509. doi: 10.1126/science.2781293. [DOI] [PubMed] [Google Scholar]
- 25.Joels M, de Kloet E. Science. 1989;245:1502–1505. doi: 10.1126/science.2781292. [DOI] [PubMed] [Google Scholar]
- 26.Kerr D, Campbell L, Thibault O, Landfield P. Proc Natl Acad Sci USA. 1992;89:8527–8530. doi: 10.1073/pnas.89.18.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith M, Makino S, Kvetnansky R, Post R. J Neurosci. 1995;15:1768–1773. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueyama T, Nemoto K, Tone S, Senba E. Soc Neurosci Abstr. 1995;21:126.2. [Google Scholar]
- 29.DeKosky S, Scheff S, Cotman S. Neuroendocrinology. 1984;38:33–39. doi: 10.1159/000123862. [DOI] [PubMed] [Google Scholar]
- 30.Keaney J, Shwaery G, Xu Z. Circulation. 1994;89:2251–2259. doi: 10.1161/01.cir.89.5.2251. [DOI] [PubMed] [Google Scholar]
- 31.Lacort M, Leal A, Liza M. Lipids. 1995;30:141–147. doi: 10.1007/BF02538267. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz-larrea M, Leal A, Liza M. Steroids. 1994;59:383–390. doi: 10.1016/0039-128x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 33.Subbiah M, Kessel B, Agrawal M. J Clin Endocrinol Metab. 1993;77:1095–1101. doi: 10.1210/jcem.77.4.8408459. [DOI] [PubMed] [Google Scholar]
- 34.McEwen B, Woolley C. Exp Gerontol. 1994;29:431–439. doi: 10.1016/0531-5565(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 35.Chan R, Huey E, Maecker H, Cortopassi K, Howard S, Iyer A, McIntosh L, Ajilore O, Brooke S, Sapolsky R. Brain Pathol. 1996;6:481–489. doi: 10.1111/j.1750-3639.1996.tb00877.x. [DOI] [PubMed] [Google Scholar]
- 36.Masliah E, Ge N, Mucke L. Crit Rev Neurobiol. 1996;10:57–67. doi: 10.1615/critrevneurobiol.v10.i1.30. [DOI] [PubMed] [Google Scholar]
- 37.Sellmeyer D, Grunfeld C. Endocr Rev. 1996;17:518–533. doi: 10.1210/edrv-17-5-518. [DOI] [PubMed] [Google Scholar]
- 38.Raber J, Toggas S, Lee S, Bloom F, Epstein C, Mucke L. Soc Neurosci Abstr. 1996;22:791.5. [Google Scholar]
- 39.Summers J, Zisook S, Atkinson J, Sciolla A, Whitehall W, Brown S, Patterson T, Grant I. J Nerv Ment Dis. 1995;183:384–389. doi: 10.1097/00005053-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Lyketsos C, Hoover D, Guccione M, Dew M. Am J Psychiatry. 1996;153:1430–1437. doi: 10.1176/ajp.153.11.1430. [DOI] [PubMed] [Google Scholar]
- 41.Pugh K, Riccio M, Jadresic D, Burgess A. Psychol Med. 1994;24:897–904. doi: 10.1017/s0033291700028981. [DOI] [PubMed] [Google Scholar]
- 42.Kemeny M, Fahey J, Schneider S, Taylor S, Weiner H, Visscher B. Psychsom Med. 1989;51:224–246. [Google Scholar]
- 43.APA Taskforce on Laboratory Tests in Psychiatry. Am J Psychiatry. 1987;144:1253–1266. doi: 10.1176/ajp.144.10.1253. [DOI] [PubMed] [Google Scholar]
- 44.Bozzette S, Sattler F, Chin J, Wu A, Gluckstein D, et al. N Engl J Med. 1990;323:1451–1456. doi: 10.1056/NEJM199011223232104. [DOI] [PubMed] [Google Scholar]
- 45.el-Sadr W, Sidhu G, Diamond G. AIDS Res. 1986;2:349–358. doi: 10.1089/aid.1.1986.2.349. [DOI] [PubMed] [Google Scholar]
- 46.Gagnon S, Boota A, Fischl M, Baier H, Kirksey O, Lavoie L. N Engl J Med. 1990;323:1444–1447. doi: 10.1056/NEJM199011223232103. [DOI] [PubMed] [Google Scholar]
- 47.MacFadden D, Hyland R, Inouye T, Edelson J, Rodriguez C, Rebuck A. Lancet. 1987;i:1477–1478. doi: 10.1016/s0140-6736(87)92219-7. [DOI] [PubMed] [Google Scholar]
- 48.Rankin J, Pella J. Am Rev Respir Dis. 1987;136:182–189. doi: 10.1164/ajrccm/136.1.182. [DOI] [PubMed] [Google Scholar]