Abstract
Two isoforms of the substance P (SP) receptor, differing in the length of the cytoplasmic carboxyl-terminus by ≈8 kDa, have been detected previously in rat salivary glands and other tissues. The binding and functional properties of these two isoforms have been investigated using full-length (407 amino acids) and carboxyl-terminally truncated (324 amino acids) rat SP receptors transfected stably into Chinese hamster ovary cells. Both the full-length and the truncated receptor bound radiolabeled SP with a similar Kd (≈0.1 nM). The average number of high affinity SP binding sites per cell was 1.0 × 105 and 0.3 × 105 for the full-length and the truncated SP receptor, respectively. In both cell lines, SP induced a rapid but transient increase in cytosolic calcium concentration ([Ca2+]i), which consisted of the release of Ca2+ from intracellular stores and the influx of extracellular Ca2+. Both components are dependent on phospholipase C activation. Although the full-length and the truncated receptor utilize the same calcium pathways, they differ in their EC50 values (0.28 nM for the full-length; 0.07 nM for the truncated). These differences in responsiveness may be related to the observed differences in receptor desensitization. The truncated receptor, in contrast to the full-length receptor, does not undergo rapid and long-lasting desensitization. Cells possessing the short isoform of the SP receptor would thus be expected to exhibit a prolonged responsiveness.
Substance P (SP), a member of the tachykinin family of peptides, is involved in many physiological processes, including exocrine gland secretion, vasodilation, pain transmission, and neurogenic inflammation (1). Molecular cloning established that the receptor for SP belongs to the G protein-coupled receptor family that possesses seven putative transmembrane domains (2, 3). Results from crosslinking and reconstitution studies have provided evidence that the SP receptor, also known as the neurokinin-1 receptor, couples to a subgroup of G proteins, Gq/11 (4, 5). It has been shown in many tissues that SP activates phospholipase Cβ (PLCβ) which results in a transient increase in intracellular inositol 1,4,5-trisphosphate (IP3) and cytosolic calcium concentration (6–9). The SP receptor, when stably expressed in Chinese hamster ovary (CHO) cells, also couples to phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis (10, 11).
Biochemical studies in which the SP receptors in a membrane preparation of rat submaxillary glands were covalently labeled with a photoreactive SP analog (12, 13) have demonstrated the presence of two SP receptor isoforms that differ in the length of the cytoplasmic carboxyl-terminus by approximately 8 kDa. The photolabeling of the full-length SP receptor isoform (molecular mass, 46 kDa following deglycosylation) and the short isoform (molecular mass, 37 kDa following deglycosylation) are inhibited by SP with the same IC50 (1 nM), indicating that both receptor isoforms bind SP with a similar affinity. A cDNA encoding a carboxyl-terminally truncated SP receptor with a calculated molecular weight of 35,797 has been cloned (14). When expressed in COS cells, this truncated receptor, in contrast to the receptor detected by photoaffinity labeling, has a binding affinity at least 10-fold less than that of the full-length receptor. Therefore, it is unlikely that the short receptor isoform identified in the rat salivary gland is derived from this spliced variant.
To study the functional properties of the short SP receptor isoform identified in the rat salivary gland by photoaffinity labeling, a rat SP receptor mutant (324 amino acids; molecular weight, 37,360) carboxyl-terminally truncated to approximate the size of this short receptor isoform (13) was stably expressed in CHO cells (15). In this report, we have compared the binding and signaling properties of this truncated receptor mutant with those of the full-length receptor (407 amino acids) also expressed in CHO cells (10).
MATERIALS AND METHODS
Materials.
Alpha-modified MEM (α-MEM) and SP were purchased from Sigma. Fetal bovine serum (FBS) and Geneticin (G418) were obtained from Life Technologies. 125I-labeled Bolton–Hunter SP [125I-(BH)-SP and 125I-(BH)-Bpa8-SP] were prepared as described (12, 16). The molecular weight standards were from Bio-Rad and Rainbow markers were from Amersham. Endoglycosidase F was from Boehringer Mannheim. Glass coverslips (9 × 22 mm) were from Bellco Glass. Fura-2/AM and pluronic acid were from Molecular Probes. SK&F 96365 (1-{(-[3-(4-methoxyphenyl)propoxy]-4-methoxyphenethyl}-1H-imidaxole hydrochloride) and nimodipine were obtained from Biomol Research Laboratories (Plymouth Meeting, PA). 8-(N,N-diethylamino)octyl 3,4,5-trimethoxybenzoate (TMB-8 HCl) was from Research Biochemicals (Natick, MA). U73122 (1-[6-[[17(-3-Methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl-1H-pyrrole-2,5-dione), U73343 (1-[6-[[17(-3-Methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl-2,5-pyrrolidine-dione), and GF 109203X (2-[1-(3-dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3-yl)maleimide) were from Calbiochem. All other reagents were from Fisher.
Cell Transfection and Culture.
The generation of CHO cells stably expressing the full-length rat SP receptor or the truncated receptor lacking the last 83 amino acids at its carboxyl-terminal tail has been described (10, 15). The transfected CHO cells were maintained in α-MEM containing 10% FBS and 0.8 mg/ml Geneticin in 5% CO2 at 37°C.
Photoaffinity Labeling of Transfected CHO Cells.
The transfected CHO cells were harvested and resuspended (107 cells/ml) in Hepes buffer (20 mM Hepes/120 mM NaCl/5 mM KCl/2.2 mM MgCl2/1 mM CaCl2/33 mM glucose, pH 7.4) containing 0.6% BSA. 125I-(BH)-Bpa8-SP (≈1 nM) was added and the cells were incubated for 60 min at 4°C to reach equilibrium. The cells were irradiated for 30 min at a distance of 6 cm from a 100-W long-wave (365 nm) ultraviolet lamp (Blak-Ray, San Gabriel, CA). After photolysis, the cells were collected and washed twice with Hepes buffer, followed by homogenization in 5 mM Tris⋅HCl containing 1 mM EDTA (pH 7.0). The photolabeled membranes were then centrifuged at 40,000 × g for 30 min and the pellet was stored at −20°C.
Deglycosylation Treatment.
The photolabeled membranes were treated with 1% SDS at 60°C for 5 min, and diluted in 50 mM sodium phosphate buffer (pH 6.0) containing 1% Nonidet P-40 to adjust the final SDS concentration to 0.2%. Endoglycosidase F was added to the solution at 0.5 units/mg protein and incubated overnight at 37°C.
SDS/PAGE and Autoradiography.
Photoaffinity-labeled and deglycosylated membranes were denatured at 60°C for 5 min and resolved by SDS/PAGE (10% acrylamide) according to the method of Laemmli (17). Gels were then dried and autoradiographed using Kodak x-ray films (XAR 5) with an intensifying screen. The molecular weights of the receptor proteins were determined by using molecular weight standards from Bio-Rad and Rainbow markers from Amersham.
Receptor Binding Assay.
For equilibrium binding experiments, CHO cells were resuspended in Hepes buffer and incubated with increasing concentrations of 125I-(BH)-SP (0.1–1 nM) for 1 hr at 4°C. A mixture of protease inhibitors (3 μg/ml chymostatin/5 μg/ml leupeptin/30 μg/ml bacitracin) was added to prevent peptide degradation. Binding was terminated by the addition of 5 ml ice-cold Hepes buffer and rapid filtration through a glass fiber filter (Whatman GF/C) which had been soaked for at least 2 hr in poly(ethylenimine) (0.1%). The radioactivity on the filter was determined with a Nuclear Enterprises NE 1600 γ-counter at a counting efficiency of 70%. Nonspecific binding was determined by the addition of excess unlabeled SP (1 μM). Both Kd and Bmax calculated from the Scatchard transformation of the binding data.
To determine the ligand dissociation rate at 37°C, the transfected CHO cells were briefly exposed to 10 nM 125I-(BH)-SP in Hepes buffer for 30 sec. The specific binding was determined at different times following the addition of an excess of unlabeled SP (1 μM) using the filtration method described above.
Measurement of [Ca2+]i.
CHO cells were plated onto 9 × 22 mm glass coverslips in a tissue culture dish and maintained in α-MEM containing 10% FBS and 0.8 mg/ml Geneticin in 5% CO2 at 37°C for 48 hr before experiments. The coverslips were washed and incubated with 4 μM of the calcium indicator, Fura-2/AM and 0.01% pluronic acid (9:1 vol/vol) in Hepes-buffered saline (HBS) solution (10 mM Hepes/140 mM NaCl/5 mM KCl/1 mM MgSO4/1.8 mM CaCl2/10 mM glucose; pH7.4) for 25 min at room temperature. The coverslip was then placed into a cuvette containing 2 ml HBS at an incident angle of 30° to the fluorometer light source. Fluorescence measurement of [Ca2+]i changes was performed at 37°C on a Hitachi F-2000 fluorescence spectrophotometer. Emission was measured at 510 nm with excitation wavelengths of 340 and 380 nm (Fura-2 bound and unbound to calcium, respectively) (18). The ratio of the fluorescence at the two excitation wavelengths, which is proportional to [Ca2+]i, was calculated.
To determine the contribution of the entry of extracellular Ca2+ to the increase in [Ca2+]i, calcium measurements were also performed in calcium-free HBS buffer containing EGTA (1 mM). To examine the desensitization of the SP-induced Ca2+ response, cells were exposed to SP at a concentration of 10 nM for 30 sec, followed by three washes. Preliminary results established that this concentration of SP is optimal for observing desensitization. After a 10-min recovery period in Ca2+-contain HBS buffer, cells were exposed to a second application of SP (10 nM).
To characterize the Ca2+ channels, cells were incubated with a series of Ca2+ channel blockers: nimodipine (3 μM), or ω-conotoxin GVIA (1 μM), or ω-agatoxin IVA (1 μM), or SK&F 96365 (20 μM) for 5 min before application of SP. To inhibit the release of Ca2+ from intracellular stores, cells were pretreated with TMB-8 (1 mM) for 5 min and washed three times before SP addition. To examine the role of PLCβ-catalyzed PIP2 hydrolysis in the Ca2+ response, cells were preincubated with 10 μM U73122 (an inhibitor of PLCβ), or U73343 (an inactive analog of U73122) and washed three times before addition of SP. Cells were also treated with 5 μM GF 109203X [a protein kinase C (PKC) inhibitor] for 5 min, followed by three washes with HBS buffer and stimulation by SP.
RESULTS
Photoaffinity Labeling of the Full-Length and the Truncated SP receptor.
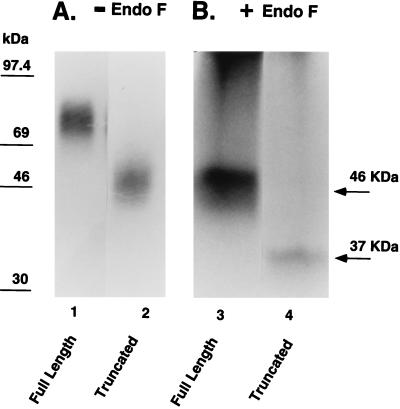
The transfected CHO cells were photoaffinity labeled using 125I-(BH)-Bpa8-SP and analyzed by SDS/PAGE and autoradiography. The full-length and the truncated receptor migrated as diffuse bands, centered at ≈80 and 50 kDa, respectively (Fig. 1). The diffuseness of the bands is due to the heterogeneous glycosylation of the SP receptor when expressed in CHO cells (19). After treatment with endoglycosidase F, the deglycosylated receptors migrated as a 46-kDa band (full-length receptor) and a 37-kDa band (truncated receptor). The Mr of the deglycosylated truncated receptor mutant is the same in size as that predicted from the primary sequence and is the same, within experimental error, as that determined for the short receptor isoform reported in rat salivary glands (13).
Figure 1.
Deglycosylation of photoaffinity-labeled SP receptors. The full-length and the truncated SP receptor expressed in CHO cells were covalently and specifically labeled with 125I-(BH)-Bpa8-SP and analyzed by SDS/PAGE and autoradiography. (A) the full-length (lane 1) and the truncated receptor (lane 2) migrated diffuse bands, centered at ≈80 kDa and 50 kDa, respectively. (B) After treatment with endoglycosidase F, the deglycosylated receptors migrated as a 46-kDa band (the full-length, lane 3) and a 37-kDa band (the truncated receptor, lane 4).
Binding Properties of the SP Receptors.
Scatchard analysis of equilibrium binding data established that the two receptors bind 125I-(BH)-SP with a similar high affinity (Kd, ≈0.1 nM), and a Bmax of 1 × 105 sites per cell for the full-length receptor and 0.3 × 105 sites per cell for the truncated receptor (data not shown).
SP-Induced Ca2+ Responses in Transfected CHO Cells.
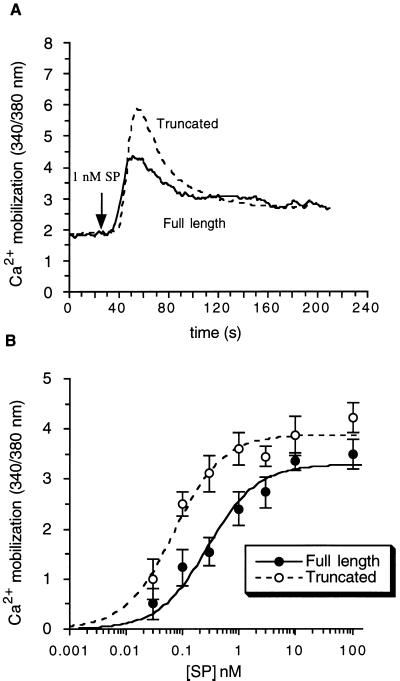
In both cell lines, SP evoked an initial rapid increase in [Ca2+]i, followed by a decline to a plateau higher than the basal level (Fig. 2A). However, cells expressing the truncated receptor, despite having fewer receptors per cell, had a greater peak height value for [Ca2+]i at submaximal concentration of SP. Analysis of the concentration dependence of the peak [Ca2+]i to SP showed an EC50 value of 0.28 nM for the full-length receptor and 0.07 nM for the truncated receptor, although both cell lines have a similar maximum Ca2+ response (Fig. 2B).
Figure 2.
SP-induced increase in [Cai in transfected CHO cells. Upon SP stimulation, light emission from cells was measured at 510 nm with excitation wavelengths of 340 and 380 nm at 37°C. The ratio of the fluorescent emissions at the two excitation wavelengths is proportional to the change in [Ca2+]i. (A) Time course. Cells were exposed to SP (1 nM) and the change in [Ca2+]i was recorded in cells expressing either the full-length (—) or the truncated receptor (- - -). (B) Concentration dependency of the SP-induced increase. Cells were exposed to SP at a concentration range from 0.03 to 100 nM and the maximum peak height of [Ca2+]i was measured at each SP concentration. The EC50 value for the full-length receptor (•) is 0.28 nM and for the truncated receptor (○) is 0.07 nM. Results are expressed as the mean ± SE from at least four experiments.
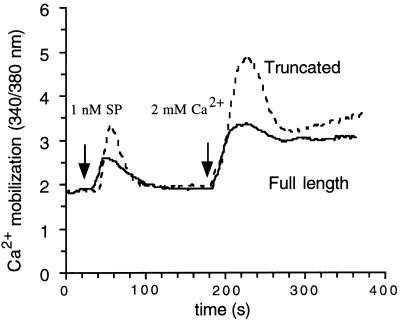
To study the basis for the difference in responsiveness of the two receptors, we first examined the SP-induced Ca2+ response in the absence of extracellular Ca2+. Under Ca2+-free condition, SP (1 nM) induced a rapid and transient rise in [Ca2+]i in both cell lines. When extracellular Ca2+ concentration was restored to 2 mM 150 sec after SP application, a rapid Ca2+ influx was observed, followed by a prolonged decline to a plateau in both cell lines (Fig. 3). This experiment demonstrated that the Ca2+ response evoked by SP consists of two components: the release of Ca2+ from intracellular stores and the influx of extracellular Ca2+ through membrane-bound Ca2+ channels. For both components of the [Ca2+]i increase, the response mediated by the truncated receptor was greater than that by the full-length receptor.
Figure 3.
The SP-induced increase in [Ca2+]i in the absence of extracellular Ca2+. SP (1 nM) was added to cells in Ca2+-free HBS (containing 1 mM EGTA) buffer as indicated and the calcium release from its intracellular store was measured. Extracellular Ca2+ level was restored to 2 mM at 180 sec and the calcium influx was measured as the change in [Ca2+]i. Addition of Ca2+ (2 mM) to the Ca2+-free HBS buffer in the absence of SP causes only a slight increase in basal level of [Ca2+]i. The traces are from representative experiments repeated at least three times.
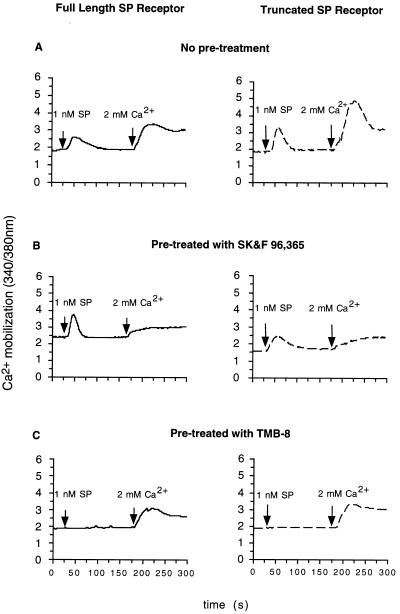
To further characterize the Ca2+ responses in both cell lines, the two Ca2+ pathways were studied using a series of pharmacological reagents. Preincubation with various blockers of voltage-gated Ca2+ channels: nimodipine (L-type), ω-conotoxin GVIA (N-type), and ω-agatoxin IVA (P/Q type), had no effect on the SP-induced Ca2+ response in either cell lines (data not shown). Treatment of both cell lines with SK&F 96365, a compound that is reported to inhibit a class of voltage-independent Ca2+ channels linked to receptor activation (20), reduced the extracellular Ca2+ influx by >90%, but had no effect on Ca2+ release from intracellular stores (Fig. 4B). On the other hand, when both cell lines were treated with TMB-8, which blocks Ca2+ release from intracellular stores (21–23), the Ca2+ release was completely inhibited, but the Ca2+ influx was unaffected (Fig. 4C).
Figure 4.
Effects of pharmacological reagents on each component of the SP-induced increase in [Ca2+]i. Cells expressing the full-length receptor (—) or the truncated receptor (- - -) were pre-treated with (A) buffer, or (B) SK&F 96,365 (20 μM), or (C) TMB-8 (1 mM). Following SP (1 nM) stimulation, the changes in [Ca2+]i were measured in Ca2+-free HBS buffer (1 mM EGTA). Extracellular Ca2+ level was restored to 2 mM at 180 sec and the calcium influx was recorded. The traces are from representative experiments repeated at least three times.
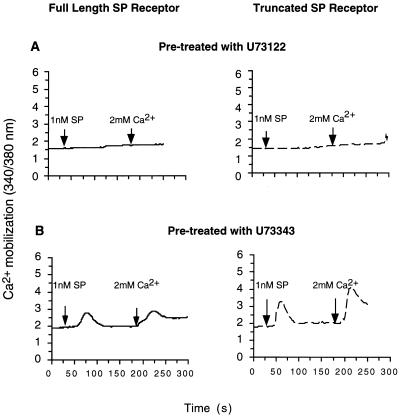
To examine whether PIP2 hydrolysis by PLCβ is necessary also for the activation of the Ca2+ channel, the cells were pretreated with U-73122, an amino-steroid inhibitor of PLCβ. Pre-incubation with U-73122 (10 μM) prevented extracellular Ca2+ influx, as well as intracellular Ca2+ release (Fig. 5A). In contrast, treatment with U-73343, an inactive analog of U-73122, had no effect on either pathways (Fig. 5B). In addition to IP3, diacylglycerol (DAG) is generated by activation of PLCβ and hydrolysis of PIP2. To explore whether DAG, via activation of PKC, is involved in Ca2+ channel activation, we used a selective inhibitor of PKC, GF 109203X (24). Treatment of both cell lines with GF 109203X (10 μM) had no effect on either component of the Ca2+ response (data not shown).
Figure 5.
Effect of PLCβ inhibition on the SP-induced [Ca2+]i change. Cells expressing the full-length receptor (—) or the truncated receptor (- - -) were preincubated with (A) U73122 (10 μM), or (B) U73343 (10 μM). In calcium-free HBS buffer (containing 1 mM EGTA), changes in [Ca2+]i were measured upon application of SP (1 nM), followed by addition of Ca2+ (2 mM) to the buffer. The traces are from representative experiments repeated at least three times.
Desensitization of the SP-Induced Ca2+ Responses.
Because it has been reported by Sasakawa et al. (25) that a series of deletion mutants of the SP receptor with different lengths of the carboxyl-terminal tail have modified desensitization properties, we compared the desensitization of the truncated receptor mutant with that of the full-length receptor (Fig. 6A). Following a brief exposure to SP (10 nM), a second application of SP 10 min later elicited only a minimal Ca2+ response in cells expressing the full-length receptor. In contrast, cells expressing the truncated receptor exhibited a sizable Ca2+ response upon a second application of SP. This difference in desensitization is not due to a difference in the duration of receptor occupancy by SP between the full-length and the truncated SP receptor, since following brief incubation (30 sec) at 37°C, radiolabeled SP dissociates from both receptors with a similar rate constant (t1/2 ≈ 1 min) (data not shown).
Figure 6.
Desensitization of the SP-induced Ca2+ response. Cells expressing the full-length receptor (—) or the truncated receptor (- - -) were exposed briefly (30 sec) to SP (10 nM) in the presence (A), or the absence (B) of extracellular Ca2+, followed by rapid removal of the ligand by multiple wash steps. After a 10 min incubation in Ca2+-containing HBS buffer, the cells were exposed to a second application of SP (10 nM) in the presence (A), or the absence (B) of extracellular Ca2+. In the Ca2+-free experiments (B), extracellular Ca2+ level was restored to 2 mM at the indicated time. The traces are from representative experiments repeated at least three times.
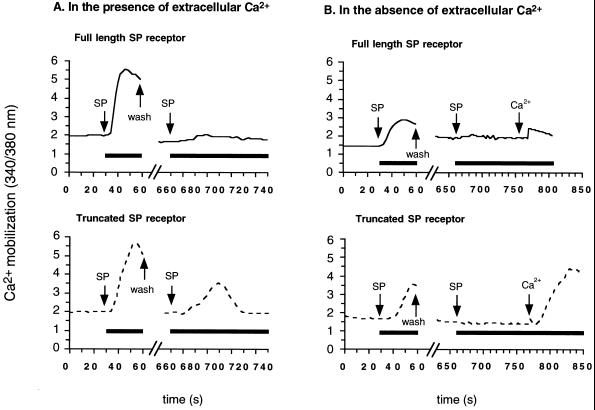
To assess receptor desensitization with regard to each component of the Ca2+ response, desensitization experiments were performed in the absence of extracellular Ca2+ (Fig. 6B). As expected, cells expressing the full-length receptor showed no Ca2+ release upon the second exposure to SP in Ca2+-free solution. Interestingly, Ca2+ release from intracellular stores after a second application of SP was also not observed in cells transfected with the truncated receptor. However, when extracellular Ca2+ was restored during the second exposure to SP, cells expressing the truncated receptor, but not the full-length receptor, exhibited a significant Ca2+ influx. The magnitude of this Ca2+ influx was the same as that observed in cells which had not been previously exposed to SP (see Fig. 3). Thus, unlike the full-length receptor, the truncated SP receptor mutant does not undergo a rapid and long-lasting desensitization.
DISCUSSION
Cells expressing the truncated SP receptor respond to the application of SP with an increase in [Ca2+]i at lower concentrations of SP than cells expressing the full-length receptor. The truncated receptor, in contrast to the full-length receptor, does not undergo rapid and long-lasting desensitization. This lack of desensitization of the truncated receptor may contribute to the increase in receptor-mediated Ca2+ response observed at low SP concentrations.
In agreement with previous findings (26), our results demonstrate that the Ca2+ response evoked by SP in CHO cells expressing the SP receptor consists of the Ca2+ release from intracellular stores and the influx of extracellular Ca2+. The mechanism by which the generation of IP3 leads to the Ca2+ release from intracellular stores by SP is well understood (27, 28). However, the identity and activation mechanism of the membrane bound Ca2+ channels involved in receptor-mediated Ca2+ entry remain unknown. The lack of effect by blockers of various types of voltage-gated calcium channel rules out the involvement of this class of Ca2+ channels in the SP-induced Ca2+ response. Several voltage-independent mechanisms of Ca2+ entry have been proposed: direct activation of channels by a G protein, calcium release-activated or calcium depletion-activated calcium channel, IP3- or IP4-operated calcium channels (28–31). TMB-8 has been used to block agonist-induced Ca2+ release from intracellular stores in several receptor systems (21–23). In this study, TMB-8 completely inhibited the SP-induced Ca2+ release in transfected CHO cells, but did not affect influx of extracellular Ca2+ (Fig. 4C). This finding suggests that the release of Ca2+ from intracellular stores is not required for activation of the Ca2+ channel and thus rules out the involvement of calcium-release-activated or calcium depletion-activated calcium channel. Furthermore, the observation that an inhibitor of PLCβ (U73122) abolished both intracellular Ca2+ release and extracellular Ca2+ influx indicates that both components of the Ca2+ response are dependent on PLCβ activation (Fig. 5). Thus, it is unlikely that calcium influx is due to direct activation of Ca2+ channels by a G-protein. In addition to generating IP3, activation of PLCβ converts PIP2 to DAG which is known to regulate multiple cellular functions via activation of PKC. Since neither component of the [Ca2+]i increase was affected by GF 109203X, a specific inhibitor of calcium and DAG-dependent PKC isoforms, DAG and PKC do not appear to be involved in activation of Ca2+ channels. Taken together, the results from these pharmacological studies are consistent with a model in which SP receptor activation leads to Ca2+ influx via a membrane-bound Ca2+ channel that is regulated by IP3 and/or its metabolites. In fact, the presence of an IP3-dependent Ca2+ channel has been detected in CHO cells expressing the SP receptor by patch clamp recording (31). Moreover, SP has been shown to induce a rapid but transient elevation in intracellular IP3 in transfected CHO cells, which parallels the time course of the observed Ca2+ responses (10, 31–33).
Previous studies have demonstrated that the SP-induced [Ca2+]i elevation and IP3 increase desensitize rapidly (7, 8, 34). In the present study, we have shown that the truncated receptor mutant, unlike the full-length receptor, is resistant to desensitization (Fig. 6A). In cells transfected with the truncated receptor, upon a second application of SP, the magnitude of Ca2+ entry was the same as that observed in cells not previously exposed to SP, but no intracellular Ca2+ release was observed (Fig. 6B). This observed activation of Ca2+ channels indicates that the truncated receptor did not undergo a long-lasting desensitization. Thus, the absence of Ca2+ release from intracellular stores upon a second application of SP is not due to receptor desensitization, but rather must involve downstream components in the signaling pathway, such as inactivation of the IP3 receptor on the endoplasmic reticulum membrane (35), or depletion of intracellular Ca2+ stores (26).
The desensitization of G protein-coupled receptors is associated with receptor phosphorylation and subsequent uncoupling of receptors from G proteins (36). This agonist-dependent phosphorylation occurs at specific regions of the receptor carboxyl tail and is mediated by a class of serine/threonine kinases, G protein-coupled receptor kinases (37). Nearly 30% of the residues on the cytoplasmic carboxyl-terminal tail of the full-length SP receptor are serine and threonine residues, potential sites for phosphorylation by G protein-coupled receptor kinases. In fact, it has been shown that the SP receptor, when reconstituted in phospholipid vesicles, can be phosphorylated by G protein-coupled receptor kinases 2 and 3 (β-adrenergic receptor kinase 1 and 2) (5). Thus, it is possible that the full-length receptor, but not the truncated receptor, can be desensitized by a G protein-coupled receptor kinases-catalyzed phosphorylation. In addition, the truncated SP receptor mutant (324 amino acids) expressed in epithelial cells has been reported to have impaired internalization properties, suggesting that internalization may contribute to desensitization of the SP-induced Ca2+ response (38). However, in our experiment, the duration of exposure to SP is too short (30 sec) for significant internalization to occur.
How the short receptor isoform detected in the rat salivary gland arises is not known. Despite the acceptance of the notion by some investigators that it is derived from a spliced variant of the SP receptor mRNA, we feel this is unlikely because this even shorter form (311 amino acids) when expressed in COS cells has a diminished binding affinity compared with the full-length receptor and the truncated mutant studied in this report. Thus it seems more likely that the truncated isoform detected in salivary gland arises by a posttranslational modification of the full-length receptor (13). The fact that the binding affinity of the truncated mutant studied here is the same as the short isoform detected in salivary gland, supports the choice of this truncation mutant to evaluate the functional properties of the short receptor isoform found in vivo. The truncated SP receptor mutant is more responsive to SP stimulation than the full-length receptor and resistant to desensitization. Cells expressing the short isoform of the SP receptor would thus be expected to exhibit a prolonged responsiveness. In fact, a study on cells isolated from the rat parotid gland which contains both receptor isoforms showed an incomplete desensitization (39), which is likely to be due to the presence of the short receptor isoform.
Acknowledgments
We thank Dr. Isabel Mintz for providing ω-conotoxin GVIA and ω-agatoxin IVA toxins. This work is supported by National Institutes of Health Grants NS 31346 (N.D.B.), NS 30791 (B.E.S.), and AG09525 (J.K.B.).
ABBREVIATIONS
- SP
substance P
- PLCβ
phospholipase Cβ
- PIP2
phosphatidylinositol 4,5-bisphosphate
- IP3, inositol 1
4,5-trisphosphate
- DAG
diacylglycerol
- PKC
protein kinase C
- [Ca2+]i
cytosolic calcium concentration
- TMB-8 HCl
8-(N,N-diethylamino)octyl 3,4,5-trimethoxybenzoate
- HBS
Hepes-buffered saline
- FBS
fetal bovine serum
- BH
Bolton–Hunter
- CHO
Chinese hamster ovary
References
- 1.Otsuka M, Yoshioka K. Physiol Rev. 1993;73:229–307. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- 2.Yokota Y, Sasai Y, Tanaka K, Fujiwara T, Tsuchida K, Shigemoto R, Kakizuka A, Ohkubo H, Nakanishi S. J Biol Chem. 1989;264:17649–17652. [PubMed] [Google Scholar]
- 3.Hershey A D, Krause J E. Science. 1990;247:958–962. doi: 10.1126/science.2154852. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald S G, Dumas J J, Boyd N D. Biochemistry. 1996;35:2909–2916. doi: 10.1021/bi952351+. [DOI] [PubMed] [Google Scholar]
- 5.Kwatra M M, Schwinn D A, Schreurs J, Blank J L, Kim C M, Benovic J L, Krause J E, Caron M G, Lefkowitz R J. J Biol Chem. 1993;268:9161–9164. [PubMed] [Google Scholar]
- 6.Berridge M J. Biochem J. 1984;220:345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMillian M K, Soltoff S P, Talamo B R. Biochem Biophys Res Commun. 1987;148:1017–1024. doi: 10.1016/s0006-291x(87)80233-4. [DOI] [PubMed] [Google Scholar]
- 8.Menniti F S, Takemura H, Oliver K G, Putney J W. Mol Pharmacol. 1991;40:727–733. [PubMed] [Google Scholar]
- 9.Taylor S W, Merritt J E, Putney J W, Rubin R P. Biochem Biophys Res Commun. 1986;136:362–368. doi: 10.1016/0006-291x(86)90919-8. [DOI] [PubMed] [Google Scholar]
- 10.Takeda Y, Blount P, Sachais B S, Hershey A D, Raddatz R, Krause J E. J Neurochem. 1992;59:740–745. doi: 10.1111/j.1471-4159.1992.tb09430.x. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima Y, Tsuchida K, Negishi M, Ito S, Nakanishi S. J Biol Chem. 1992;267:2437–2442. [PubMed] [Google Scholar]
- 12.Boyd N D, White C F, Cerpa R, Kaiser E T, Leeman S E. Biochemistry. 1991;30:336–342. doi: 10.1021/bi00216a005. [DOI] [PubMed] [Google Scholar]
- 13.Kage R, Leeman S E, Boyd N D. J Neurochem. 1993;60:347–351. doi: 10.1111/j.1471-4159.1993.tb05857.x. [DOI] [PubMed] [Google Scholar]
- 14.Fong T M, Anderson S A, Yu H, Huang R R C, Strader C D. Mol Pharmacol. 1992;41:24–30. [PubMed] [Google Scholar]
- 15.Blount P, Krause J E. J Biol Chem. 1993;268:16388–16395. [PubMed] [Google Scholar]
- 16.Liang T, Cascieri M A. J Neurosci. 1981;10:1133–1140. doi: 10.1523/JNEUROSCI.01-10-01133.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Grynkiewicz G, Poenie M, Tsien R Y. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 19.Kage R, Hershey A D, Krause J E, Boyd N D, Leeman S E. J Neurochem. 1995;64:316–321. doi: 10.1046/j.1471-4159.1995.64010316.x. [DOI] [PubMed] [Google Scholar]
- 20.Merritt J E, Armstrong W P, Benham C D, Hallam T J, Jacob R, Jaxa-Chamiec A, Leigh B K, McCarthy S A, Moores K E, Rink T J. Biochem J. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grune S, Engelking L R, Anwer M S. J Biol Chem. 1993;268:17734–17741. [PubMed] [Google Scholar]
- 22.Gandhi C R, DeBuysere M S, Olson M S. Biochim Biophys Acta. 1992;1136:68–74. doi: 10.1016/0167-4889(92)90086-q. [DOI] [PubMed] [Google Scholar]
- 23.Palmer F B, Byers D M, Spence M W, Cook H W. Biochem J. 1992;286:505–512. doi: 10.1042/bj2860505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 25.Sasakawa N, Sharif M, Hanley M R. FEBS Lett. 1994;347:181–184. doi: 10.1016/0014-5793(94)00532-x. [DOI] [PubMed] [Google Scholar]
- 26.Garland A M, Grady E F, Lovett M, Vigna S R, Frucht M M, Krause J E, Bunnett N W. Molecular Pharmacol. 1996;49:438–446. [PubMed] [Google Scholar]
- 27.Berridge M J. Nature (London) 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 28.Putney J W J. In: The Tachykinin Receptors. Buck S H, editor. Totowa, NJ: Humana; 1994. pp. 257–283. [Google Scholar]
- 29.Clapham D E. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 30.Berridge M J. Ann NY Acad Sci. 1995;766:31–43. doi: 10.1111/j.1749-6632.1995.tb26646.x. [DOI] [PubMed] [Google Scholar]
- 31.Mochizuki-Oda N, Nakajima Y, Nakanishi S, Ito S. J Biol Chem. 1994;269:9651–9658. [PubMed] [Google Scholar]
- 32.Mochizuki-Oda N, Nakajima Y, Nakanishi S, Ito S. Regul Pept. 1993;46:450–452. doi: 10.1016/0167-0115(93)90116-p. [DOI] [PubMed] [Google Scholar]
- 33.Sanders M A, LeVine H., III J Neurochem. 1996;67:2362–2372. doi: 10.1046/j.1471-4159.1996.67062362.x. [DOI] [PubMed] [Google Scholar]
- 34.Sugiya H, Tennes K A, Putney J W. Biochem J. 1987;244:647–653. doi: 10.1042/bj2440647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hajnoczky G, Thomas A P. Nature (London) 1994;370:474–477. doi: 10.1038/370474a0. [DOI] [PubMed] [Google Scholar]
- 36.Freedman N J, Lefkowitz R J. Recent Prog Horm Res. 1996;51:319–351. [PubMed] [Google Scholar]
- 37.Sterne-Marr R, Benovic J L. Vitam Horm (San Francisco) 1995;51:193–234. doi: 10.1016/s0083-6729(08)61039-0. [DOI] [PubMed] [Google Scholar]
- 38.Bohm S K, Khitin L M, Smeekens S P, Grady E F, Payan D G, Bunnett N W. J Biol Chem. 1997;272:2363–2372. doi: 10.1074/jbc.272.4.2363. [DOI] [PubMed] [Google Scholar]
- 39.Putney J W J, Bird G S J, Horstman D A, Hughes A R, Menniti F S, Nogimori K, Obie J, Oliver K G, Sugiya H, Takemura H. Ann NY Acad Sci. 1991;632:94–102. doi: 10.1111/j.1749-6632.1991.tb33097.x. [DOI] [PubMed] [Google Scholar]