Abstract
The lateral superior olive (LSO) is the primary auditory nucleus for processing of interaural sound level differences, which is one of the major cues for sound localization. During development, survival and maturation of LSO neurons critically depend on synaptic activity and intracellular calcium signaling. Before hearing onset, glutamatergic synaptic inputs from the cochlear nucleus (CN) to the LSO activate group I metabotropic glutamate receptors (mGluRs), which leads to calcium release from intracellular stores and large calcium influx from the extracellular milieu. Here, we investigated the nature of the mGluR-activated membrane channel that mediates the influx of ex-tracellular calcium. Using Fura-2 calcium imaging in brain stem slices of neonatal and juvenile mice, we found that this calcium channel is blocked by Ni2+, La3+, and 2-aminoethoxydiphenylborane (2-APB), known antagonists of transient receptor potential (TRP) channels. During postnatal development, the contribution of extracellular calcium influx to mGluR-mediated Ca2+ responses gradually decreased and was almost abolished by the end of the third postnatal week. Over this period, the contribution of Ca2+ release from internal stores remained unchanged. The developmental decrease of TRP-like channel-mediated calcium influx was significantly less in congenitally deaf waltzer mice, suggesting that early auditory experience is necessary for the normal age-dependent downregulation of functional TRP channels.
INTRODUCTION
The lateral superior olive (LSO) is a binaural auditory brain stem nucleus involved in the processing of interaural sound level differences. LSO neurons receive excitatory, glutamatergic inputs from the ipsilateral cochlea by the anteroventral cochlear nucleus (AVCN) and inhibitory, glycinergic inputs from the contralateral cochlea by the medial nucleus of the trapezoid body (MNTB) (Boudreau and Tsuchitani 1968; Oertel 1999; Tollin 2003). Both inputs are tonotopically organized and aligned, which enables LSO neurons to process binaural inputs in a frequency-specific manner (Sanes and Rubel 1988; Tollin 2003).
During development, the LSO circuit undergoes a number of morphological and functional changes that include growth and refinement of dendritic arbors (Rietzel and Friauf 1998; Sanes et al. 1992), functional and structural refinement of the MNTB–LSO pathway (Kandler and Gillespie 2005; Kim and Kandler 2003; Sanes and Siverls 1991; Sanes and Takacs 1993), and a switch in neurotransmitter phenotype (Gillespie et al. 2005; Kotak et al. 1998; Nabekura et al. 2004). Many of these processes occur before hearing onset but nevertheless depend on neuronal activity and intracellular Ca2+ signaling (Friauf and Lohmann 1999; Kotak and Sanes 2000; Lohmann et al. 1998; Sanes and Siverls 1991; Sanes and Takacs 1993).
Before hearing onset, spontaneous neuronal activity is present in the form of high-frequency bursts at various levels in the auditory pathway (Glowatzki and Fuchs 2000; Gummer and Mark 1994; Jones et al. 2001; Kotak and Sanes 1995; Kros et al. 1998; Lippe 1994; Romand and Ehret 1990). In the prehearing mouse, activation of glutamatergic cochlear nucleus (CN) inputs onto LSO neurons elicits postsynaptic Ca2+ responses that are mediated by ionotropic and metabotropic glutamate receptors (mGluRs). The recruitment of specific types of glutamate receptors depends on the spatial and temporal patterns of synaptic activation (Ene et al. 2003). Burstlike synaptic activity activates group I and group II mGluRs, which in turn generate postsynaptic Ca2+ responses with a typical biphasic profile. The initial phase of mGluR-elicited Ca2+ responses in immature LSO neurons is mediated by Ca2+ release from intracellular stores, whereas the later and prolonged phase is mediated by an influx of extracellular Ca2+ through membrane Ca2+ channels.
The identity of mGluR-activated membrane Ca2+ channels in LSO neurons is unclear, although their dependency on intracellular Ca2+ release suggests that they belong to the family of transient receptor potential channels (TRPCs) (Clapham 2003; Montell 2005), which can be activated by mGluRs (Bengtson et al. 2004; Gee et al. 2003; Kim et al. 2003; Tozzi et al. 2003). In this study, we investigated the developmental changes of mGluR-mediated Ca2+ responses in mouse LSO neurons and characterized the pharmacological properties of the mGluR-activated Ca2+ channel. We found that the pharmacological profile of mGluR-activated Ca2+ channels is consistent with the characteristics of a TRPC. During the first three postnatal weeks, group I mGluR-evoked Ca2+ responses decreased, with the most pronounced changes occurring around the onset of hearing when mGluRs ceased to trigger extracellular Ca2+ influx. These developmental changes were delayed in congenitally deaf waltzer mice, suggesting that developmental downregulation of TRP-like Ca2+ responses is influenced by normal auditory experience.
METHODS
Animals
Experiments were performed in C57BL/6J and homozygote waltzer Cdh23 mice (C57BL/6J-Cdh23v−2J) of both genders (Jackson Laboratory, Bar Harbor, ME; Charles River, Wilmington, MA), ages between postnatal day 0 (P0: the day of birth) and P20. Waltzer homozygotes were identified by their rollover behavior deficits (Wada et al. 2001). Heterozygote mice were obtained by breeding homozygote/wild-type pairs. All experimental procedures were in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Slice preparation
Animals between P0 and P7 were anesthetized by hypothermia and animals older than P7 were anesthetized using isoflurane. Animals were quickly decapitated and the brains were removed and placed into cold (4–8°C) artificial cerebrospinal fluid with kynurenic acid [ACSF with KA, composition (in mM): NaCl 124, NaHCO3 26, glucose 10, KCl 5, KH2PO4 1.25, MgSO4 1.3, CaCl2 2, and kynurenic acid 1; pH 7.4 when aerated with 95% O2-5% CO2]. Transversal, 200- to 300- μm-thick slices of the brain stem were cut with a vibrotome (DTK-1500E, Ted Pella, Redding, CA) and slices containing the LSO were used for Fura-2 labeling.
Fura-2 labeling
Slices from P0 to P7 animals were labeled with an acetoxymethyl ester of Fura-2 (Fura-2 AM), using bulk-labeling, as described previously (Ene et al. 2003). In slices from animals older than P7 LSO neurons labeled poorly or not at all, using the bulk-labeling method, most likely resulted from poor penetration of Fura-2 AM through the extracellular matrix. Therefore slices from P9 to P20 were labeled using a novel spin-labeling procedure. Slices were placed on filter paper (12-μm pores; Corning Life Sciences, Acton, MA) in an interface-type chamber and after 15–30 min were transferred into a microcentrifuge tube equipped with a 10-kDa cutoff molecular filter (Amicon; Millipore, Bedford, MA). Slices were covered with 100 μM Fura-2 AM in ACSF and aerated with 95% O2-5% CO2. The micro-centrifuge tube was centrifuged for 15–20 min at about 430 g (IEC Clinical Centrifuge; International Equipment, Needham Heights, MA), forcing the Fura-2 AM solution to pass through the slice. Slices were then removed from the microcentrifuge tube, immediately washed with freshly aerated ACSF, and kept in an interface chamber in the dark at room temperature (22–25°C) until used for Ca2+ imaging.
Calcium imaging
Ca2+ imaging was performed using an inverted epifluorescence microscope (Nikon Eclipse TE200) equipped with ×10 and ×20 air objectives [numerical aperture (NA): 0.5 and 0.75, respectively] as previously described (Ene et al. 2003). Slices were continuously superfused with oxygenated ACSF at 30–32°C (perfusion rate 2–3 ml/min) containing 100 μM Trolox to minimize photodamage (Scheenen et al. 1996). Fluorescence images were acquired every 5–10 s with a 12-bit, cooled, interline-transfer CCD camera (IMAGO; T.I.L.L. Photonics, Martinsried, Germany) using alternating excitation (duration 20–50 ms) at 340 and 380 nm (Polychrome II; T.I.L.L. Photonics).
In some experiments, Fura-2 fluorescence was converted to intra-cellular Ca2+ concentrations ([Ca2+]i) as previously described (Ene et al. 2003) using the equation: [Ca2+]i = Kdβ(R − Rmin)/(Rmax − R), where Rmin is the fluorescence ratio of Ca2+ free Fura-2, Rmax is the ratio of Ca2+ bound Fura-2, β is the ratio of the fluorescence intensity of Ca2+ free Fura-2 at 380 nm to the fluorescence intensity of Ca2+-bound Fura-2 at 380 nm (Grynkiewicz et al. 1985). The Ca2+ Kd value for Fura-2 was taken as 224. Rmin and Rmax were determined by incubating the slices first in Ca2+-free ACSF (Ca2+ was replaced with an equimolar concentration of Mg2+) with 2 mM EGTA and 4 β M Ca2+ ionophore Br-A23187 (Alomone Labs, Jerusalem, Israel). CaCl2 (10 mM) was then added to determine Rmax. The imaging system was calibrated repeatedly over the course of this study and Rmin varied from 0.25 to 0.31, Rmax varied from 1.22 to 1.8, and β varied from 2.3 to 3.7.
Drug application
Drugs from concentrated stock solutions were dissolved in ACSF and delivered by bath application. Group I mGluRs were stimulated using the specific agonist (S)-3,5-dihydroxyphenylglycine (DHPG 20–50 μM). All experiments were performed in the presence of antagonists of ionotropic GluRs, glycine receptors, and μ-aminobutyric acid type A (GABAA) receptors. Ionotropic GluRs were blocked by 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX, 20 μM; Tocris, Ballwin, MO) and D-2-amino-5-phosphonopentanoic acid (D-APV, 100 μM, Tocris). Glycine receptors were blocked by strychnine (10 μ M; Sigma, St. Louis, MO) and GABAA receptors were blocked by bicuculline (10 μM; Tocris). Tetrodotoxin (TTX, 1 μM; Alomone Labs) was added to block glutamate release resulting from spontaneous action potentials. For TRPC antagonists we used Ni2+, La3+ (Sigma, St. Louis, MO), and 2-APB (Calbiochem, San Diego, CA).
Data analysis
Series of 340- and 380-nm paired images were low-pass filtered (Gaussian 3 ×3 kernel) and the background was subtracted using the program Tillvision (T.I.L.L. Photonics) as described previously (Ene et al. 2003). Ca2+ responses were monitored and measured from the soma of neurons. Cells with high resting [Ca2+]i (>250 nM, indicating unhealthy cells) (Zirpel and Rubel 1996), cells in which the Fura-2 signal was saturated ([Ca2+]i >1.5 μM, because Fura-2 does not faithfully report [Ca2+]i >1.5–2 μM), and cells in which [Ca2+]i did not return to baseline after stimulation were excluded from analysis. Changes in [Ca2+]i that exceeded 2 SDs of the baseline and changes in ΔR/R > 0.1 were considered as responses. Excel (Microsoft, Redmond, WA), Origin (OriginLab, Northampton, MA), and Matlab (The MathWorks, Natick, MA) were used for data analysis.
Responses elicited by the specific group I mGluR agonist DHPG (50 μM, 90 s) were quantified and categorized using the following parameters: peak amplitude, plateau amplitude, duration, and area under the curve. Traces were aligned to the onset of the response that was considered time 0. Baseline [Ca2+]i was determined as the average of five data points before response onset (in the window −50 to 0 s). Peak amplitude was measured in the time window 0 to 40 s. Plateau amplitude was determined by averaging three to five data points in the window 80 to 100 s. Response duration was measured from the onset of the response until the response returned to baseline. Based on these measurements, responses were classified into three response types (Table 1, nFig. 2): peak and plateau (pp), peak small plateau (psp), and peak no plateau (pnp). In pp responses the peak response was followed by a clear plateau (peak amplitude 236 ± 50 nM; plateau amplitude 107 ± 22 nM, = 287 cells), with a duration that always exceeded the 90-s duration of drug application (182 ± 13 s, n = 287 cells). In pnp responses the plateau phase was completely missing, the peak amplitude was small (60 ± 8 nM, n = 177 cells), and the duration was always shorter than the duration of drug application (70 ± 3 s, n = 177 cells). The intermediate group of psp responses was characterized by a gradually decreasing plateau phase, which always exceeded the duration of drug application (141 ± 15 s, n = 124 cells).
TABLE 1.
Parameters describing mGluR-elicited calcium responses in developing LSO neurons
| Peak and Plateau (pp) Group | Peak and Small Plateau (psp) Group | Peak and No Plateau (pnp) Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | P0–P5 | P9–P12 | P13–P16 | P17–P19 | P9–P12 | P13–P16 | P17–P19 | P9–P12 | P13–P16 | P17–P19 |
| N | 222 | 178 | 132 | 54 | 178 | 132 | 54 | 178 | 132 | 54 |
| n | 222 | 46 | 15 | 4 | 75 | 42 | 7 | 59 | 75 | 43 |
| n/N, % | 100 | 24 | 11 | 7 | 42 | 32 | 12 | 32 | 57 | 80 |
| Peak amplitude, nM | 304 ± 15 | 247 ± 27 | 304 ± 51 | 90 ± 6 | 106 ± 13 | 138 ± 10 | 84 ± 1.5 | 56 ± 9 | 76 ± 6 | 51 ± 5 |
| Plateau amplitude, nM | 157 ± 7 | 97 ± 11 | 121 ± 20 | 51 ± 3 | 51 ± 4 | 46 ± 6 | 65 ± 13 | — | — | — |
| Duration, s | 190 ± 3 | 157 ± 5 | 167 ± 6 | 215 ± 8 | 129 ± 3 | 122 ± 4 | 172 ± 22 | 65 ± 3 | 76 ± 2 | 65 ± 3 |
| Area, mM · s | 25.9 ± 1.0 | 16.0 ± 1.8 | 26. ± 3 4.2 | 11.6 ± 0.3 | 8.3 ± 0.7 | 9.2 ± 0.7 | 11.9 ± 1.5 | 2.1 ± 0.3 | 3.1 ± 0.3 | 2.5 ± 0.4 |
Values are means ± SE. N = total number of cells tested; n = number of cells in a response group.
FIG. 2.
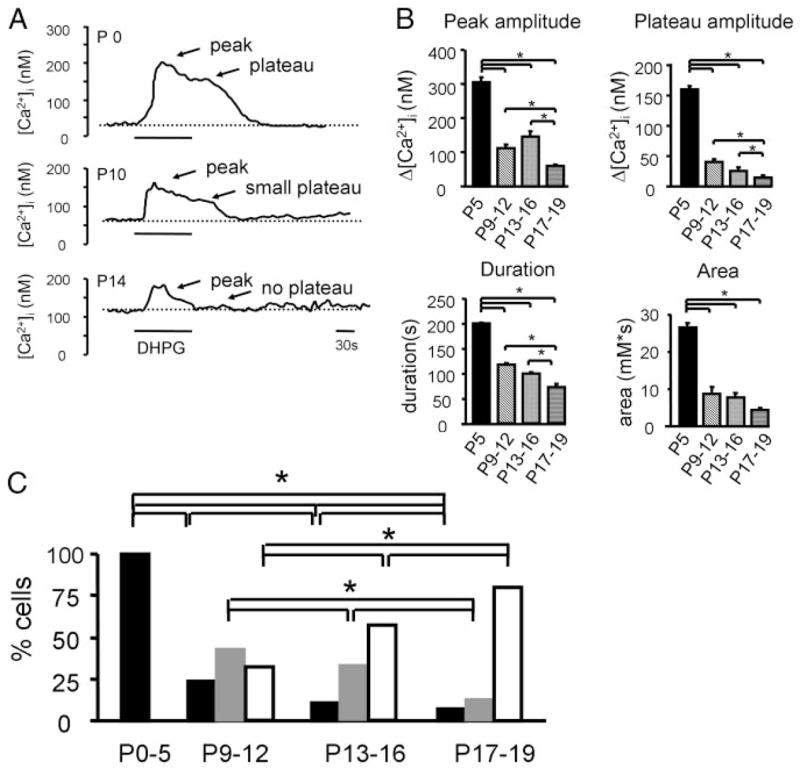
Developmental changes of group I metabo-tropic glutamate receptor (mGluR)–mediated Ca2+ responses in LSO neurons. A: example of DHPG-elicited (50 μM, 90 s) Ca2+ responses in 3 LSO neurons, each from a P0, P10, and P14 mouse. Responses undergo a transition from a profile characterized by peak and plateau (pp) to a profile characterized by a smaller peak and a smaller plateau (psp), and finally to a profile characterized by a peak only [peak and no plateau (pnp)]. Dotted line indicates baseline Ca2+ levels. B: group data of peak amplitudes, plateau amplitudes, area, and duration of DHPG-evoked Ca2+ responses at different ages. Number of cells in each age group: P0–P5: 222; P9–P12: 165; P13–P16: 135; P17–P19: 54 (asterisks: P < 0.05, ANOVA). C: developmental changes in the percentage of pp-type (black bars), psp-type (gray bars), and pnp-type (white bars) responses (asterisks: P < 0.05, chi-square test). Number of cells in each age group: P0–P5: pp n = 222; P9–P12: pp n = 46; psp n = 75, pnp n = 59; P13–P16: pp n = 15, psp n = 42, pnp n = 75; P17–P19: pp n = 4, psp n = 7, pnp n = 43.
Statistical analysis
Statistical significance was analyzed using paired t-test, ANOVA followed by Student–Newman–Keuls post hoc test, Fisher’s exact test, linear (Pearson) correlation test, Mann–Whitney nonparametric statistical test, Kolmogorov–Smirnov (KS) test, and chi-square test. Values of P < 0.05 were considered significant. Throughout the text, values are expressed as means > SE.
RESULTS
Group I mGluR-elicited Ca2+ responses were analyzed in 738 LSO neurons from 51 C57Bl6 mice (ages between P0 and P19), in 60 LSO neurons from six heterozygote waltzer mice, and in 156 LSO neurons from 14 waltzer Cdh23 homozygote mice (ages between P11 and P20). All cells included in the analysis responded to KCl-evoked depolarizations (60 mM, 30 s) with peak amplitudes ranging from about 100 nM to Fura-2–saturating responses (>1.5 μM).
Spin-labeling of LSO neurons in older slices
To overcome the poor labeling with Fura-2 AM of slices from animals older than P7 we developed a new method, spin-labeling, which uses centrifugation to force Fura-2 AM into the slice. To evaluate whether this new method affects Ca2+ responses of LSO neurons, the two labeling methods were compared in slices from P0–P5 animals. At this age, both methods produced a similar number of cells loaded with Fura-2 (bulk-labeling: n = 7.39 ± 0.92 cells/100 μm2, n = 7 slices; spin-labeling: n = 7.98 ± 0.37 cells/100 μm2, n = 3 slices; t-test P > 0.05) and a similar somatic fluorescence intensity at 360 nm, the isosbestic point of Fura-2, measured with identical settings (bulk-labeling: 338 ± 67 AU, n = 7 slices; spin-labeling: 272 ± 108 AU, n = 3 slices; t-test, P > 0.05; Fig.1A). Resting somatic Ca23 concentrations were not signifi-cantly different (bulk-labeling: 94.2 3 47.6 nM, n 3 96 cells, n 3 4 slices; spin-labeling: 109.2 3 57.6 nM, n 3 70 cells, n 3 3 slices; t-test, P 3 0.05). Finally, Ca23 responses elicited by bath application of the selective group I mGluR agonist (S)-3,5-dihydroxyphenylglycine (DHPG, 50 μM, 90 s) and by KCl depolarization (60 mM, 30 s) were undistinguishable (Fig. 1B). These results suggest that spin-labeling, compared with conventional bulk-labeling, does not change the basic Ca23 response properties of LSO neurons.
FIG. 1.
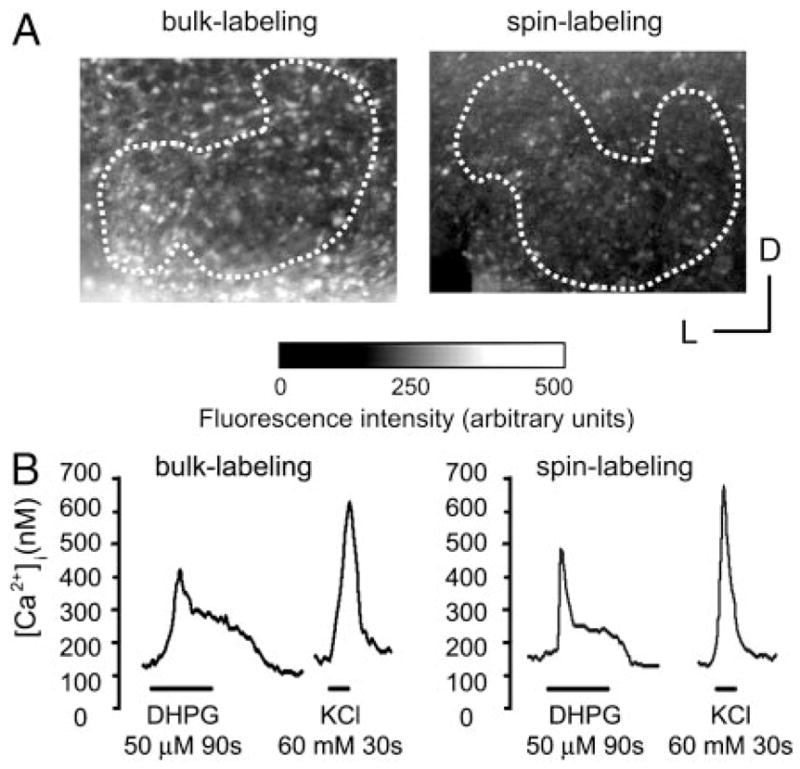
Comparisons between bulk-labeling and spin-labeling. A: examples of lateral superior olive (LSO) slices labeled using bulk (left) and spin-labeling (right) at postnatal day 0 (P0: the day of birth). Images show fluorescence at 360 nm (exposure time 20 ms, ×20 objective). Dotted lines outline the boundaries of the LSO. D, dorsal; L, lateral. B: example of changes in intracellular Ca2+ elicited by bath application of (S)-3,5-dihydroxyphenylglycine (DHPG, 50 μM, 90 s) and KCl (60 mM, 30 s). LSO neurons from a P3-old mouse.
Developmental changes of group I mGluR-mediated Ca2+ responses
In LSO neurons from neonatal mice (P0–P5), Ca2+ responses elicited by DHPG (50 μM, 90 s) consisted of an initial peak followed by a large plateau phase that always exceeded the duration of the agonist application (Fig. 2). During post-natal development, these Ca2+ responses gradually changed their profile, amplitude, and duration (Fig. 2 and Table 1). At P9–P12, around hearing onset (approximately P10–P12; Shnerson and Pujol 1981; Song et al. 2006), peak and plateau amplitudes decreased and response durations had become shorter. By the end of the third postnatal week, plateau phases were absent in almost all responses. Response amplitudes, durations, and areas were significantly larger in the P0–P5 group compared with all older age groups (Mann–Whitney test, P < 0.05) and were significantly smaller in the P17–P19 group compared with all younger age groups (Mann–Whitney test, P < 0.05; Fig. 2B and Table 1; see also Supplemental Fig. S2).1 Plateau amplitudes and response durations also signifi-cantly decreased from P9–P12 to P13–P16 (Mann–Whitney test, P 3 0.05; Fig. 2B and Table 1).
In the first postnatal week, all Ca2+ responses elicited by DHPG showed a prominent plateau and were categorized as pp responses (n = 222/222 cells, Fig. 2C). With increasing age, the frequency of pp responses decreased, from 100% at P0–P5 to 24% at P9–P12 (n = 46/178 cells), to 11% at P13–P16 (n = 15/132 cells) and to 7% at P17–P19 (n = 4/54 cells) (Fig. 2C). Responses with a peak and small plateau (psp responses) were found in all older age groups. The relative occurrence of responses that completely lacked a plateau (pnp responses) was age dependent as well, steadily increasing from 32% at P9–P12 (n = 59/178 cells), to 57% at P13–P16 (n = 75/132) and to 80% at P17–P19 (n = 43/54 cells).
In summary, during the first 3 week of postnatal development, group I mGluR-elicited Ca2+ responses gradually lose the sustained plateau phase that, in neonatal mice, is caused by an influx of extracellular Ca2+ through a channel not sensitive to voltage-gated calcium channel (VGCC) blockers (Ene et al. 2003). Next we used pharmacological tools to investigate the sources of [Ca2+]i triggered by group I mGluRs in developing LSO neurons in more detail.
Contribution of extracellular Ca2+ to group I mGluR-elicited responses decreases during development
To determine the age dependency of mGluR-elicited influx of extracellular Ca2+ and its contribution to the three types of responses (pp, psp, pnp) we compared mGluR-elicited Ca2+ responses in standard and nominally Ca2+-free ACSF (Fig. 3). Regardless of age, eliminating extracellular Ca2+ significantly reduced both the peak and the plateau phase of responses of the pp and psp type (P0–P5 pp response: peak reduced to 32 ± 2% and area to 13 ± 1%, n = 82; P9–P12 psp response: peak reduced to 23 ± 4% and area to 17 ± 4%, n = 14; P13–P16.psp response: Peak reduced to 44 ± 10% and area to 29 ± 6%, n 3 22). For pnp responses, however, removal of extracellular Ca2+ had no significant effect on response amplitudes or areas (P13–P16 pnp response: peak reduced to 63 ± 11% and area to 80.9 ± 12.7%, n = 19; P17–P18 pnp response: peak reduced to 71 ± 12% and area to 88 ± 14%, n = 16). Thus in pp and psp responses extracellular Ca2+ influx accounted for 60–85% of the Ca2+ responses, whereas in pnp responses, extracellular Ca2+ contributed little (10–25%, statistically not significant). Finally, the hypothesis that mGluRs activate a membrane ion channel is supported by electrophysiological whole cell patch-clamp recordings from neonatal LSO neurons, demonstrating that DHPG elicits long-lasting membrane depolarizations (see Supplemental material).
FIG. 3.
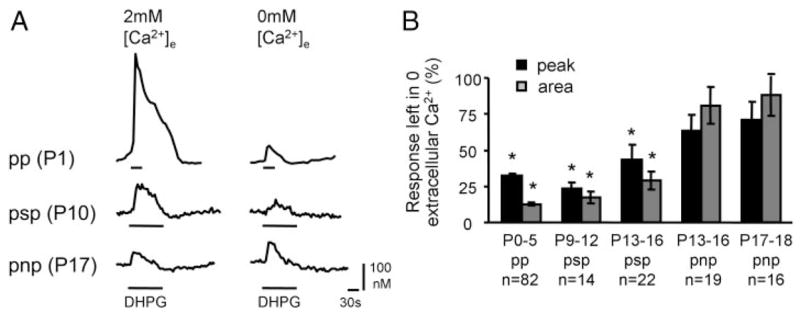
Developmental changes in the contribution of extracellular Ca2+ to group I mGluR-mediated Ca2+ responses. A: examples of individual responses to DHPG application in 2 mM extracellular Ca2+ (left traces) and in 0 mM Ca2+ (right traces). B: peak and area of Ca2+ responses after removal of extracellular Ca2+ as a function of age and response type. n, number of cells. Asterisks: P < 0.05 (paired t-test)
In summary, during LSO development, the contribution of external Ca2+ influx to group I mGluR-elicited responses diminished progressively until it was absent in 3-wk-old mice, whereas the contribution of Ca2+ release from internal stores was present throughout the investigated period. We next investigated the pharmacological characteristics of the channel that mediates mGluR-elicited influx of extracellular Ca2+.
mGluR-elicited extracellular Ca2+ influx is blocked by Ni2+
In neonatal animals, short application of Ni2+ (2 mM for 2 min) during the plateau phase transiently abolished the response, indicating that the plateau is mediated by a Ni2+-sensitive channel (Fig. 4Ai). Application of Ni2+ during the peak substantially decreased the peak amplitude to 14 ± 2% of its initial value (n = 10 cells, P5), indicating the contribution of Ni2+-sensitive Ca2+ channels to the peak as well (Fig. 4Aii). In cells with pp and psp responses, DHPG application in the presence of Ni2+ only elicited small peak responses without plateaus (Fig. 4A, iii–vi). In contrast, in cells with pnp responses, Ni2+ had no effect (pnp responses, P13–P16: n = 38; P17–P18: n = 23) (Fig. 4, Avii and Aviii). This is consistent with our finding that extracellular Ca2+ contributes little, if any, to pnp responses (Fig. 3B). As a positive control, in cells with pnp responses, Ni2+ significantly reduced subsequent Ca2+ responses elicited by KCl depolarization (60 mM, 30 s) (66 ± 7% reduction at P13–P16, n = 38, and 80 ± 2% reduction at P17–P19, n = 23; t-test, P < 0.05).
FIG. 4.
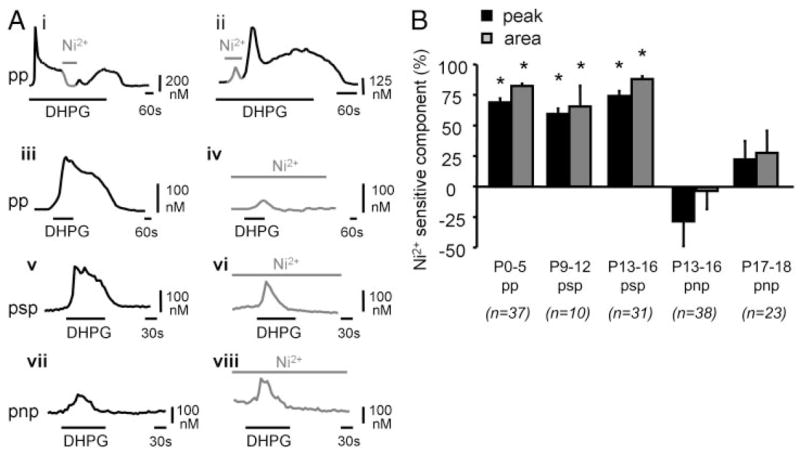
Effect of Ni2+ on mGluR-mediated Ca2+ responses. A: examples of DHPG-elicited (20–50 μM) Ca2+ responses from individual LSO neurons. Ai: short application of Ni2+ (2 mM, 2 min) during the plateau phase reduces the response to baseline. Aii: application of Ni2+ (2 mM, 2 min) together with DHPG reduces the amplitude of the initial peak response. Example is from a P5 neuron. Aiii–Avi: DHPG-elicited (50 μM, 90 s) responses of the pp and psp type (iii and v) are reduced by Ni2+ (2 mM). Plateau phase is completely abolished and the peak phase is substantially reduced. Examples are from a P2 (pp response) and a P10 mouse (psp response). Avii–Aviii: DHPG-elicited (50 μM, 90 s) responses of the pnp type are unaffected by Ni2+ (2 mM). Example is from a P15 mouse. B: summary of the effects of Ni2+ on DHPG-elicited Ca2+ responses. Asterisks: sig-nificantly different from control (P < 0.05, paired t-test).
In a small fraction of cells (P13–P16, n = 7 cells, n = 3 slices), Ni2+ potentiated the amplitude of pnp responses about fourfold (data not shown). It is possible that these cells represent a different population of LSO neurons (Ollo and Schwartz 1979), perhaps expressing different channel types.
DHPG-elicited Ca2+ responses are sensitive to 2-APB
In several neuron types, mGluRs can activate Ca2+-permeable TRPCs (Bengtson et al. 2004; Kim et al. 2003; Tozzi et al. 2003). Because in LSO neurons, DHPG-elicited extracellular Ca2+ influx is independent of voltage-gated Ca2+ channels (Ene et al. 2003) but is blocked by Ni2+, an unspecific blocker of TRPCs (Parekh et al. 1997), LSO neurons might express mGluR-activated TRPCs. To test this possibility, we used 2-aminoethoxydiphenyl borate (2-APB) that, in addition to its well-described function as a membrane-permeable inositol 1,4,5-triphosphate receptor (IP3R) inhibitor, can act from the extracellular site to block TRPCs (Bootman et al. 2002; Ma et al. 2001; Maruyama et al. 1997).
At concentrations of 2–5 μM, 2-APB increased DHPG-elicited Ca2+ responses in LSO neurons (Fig. 5), consistent with what was reported in other neuronal and nonneuronal cells (Bootman et al. 2002; Prakriya and Lewis 2001; Tozzi et al. 2003). At concentrations of 10–50 μM, 2-APB reduced both peak and plateau amplitudes with the effect being more pronounced on the plateau phase, consistent with the hypothesis that TRPCs contribute more to the plateau phase than to the peak. Finally, at concentrations >50 μM, 2-APB completely abolished the response, most likely by inhibiting intracellular IP3Rs and blocking Ca2+ release from intracellular stores, which is necessary to activate extracellular Ca2+ influx (Ene et al. 2003). Washout of 50 μM 2-APB for 30 min restored the peak component to 87 3 3%, whereas the plateau recovered to only 16 3 2% (n 3 65 cells). 2-APB did not reduce KCl-evoked Ca2+ responses (Fig. 5D), consistent with its reported lack of inhibiting VGCCs (Bootman et al. 2002; Maruyama et al. 1997) and further supporting our previous finding that mGluR-elicited influx of extracellular Ca2+ is independent of VGCCs (Ene et al. 2003).
FIG. 5.
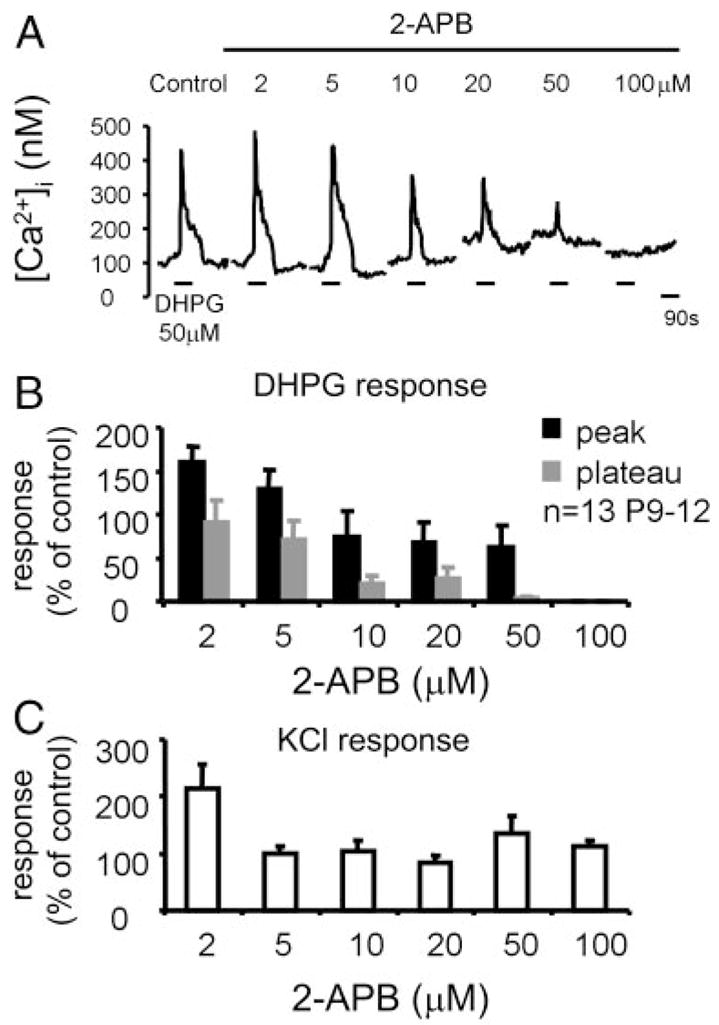
Effect of 2-aminoethoxydiphenylborane (2-APB) on group I mGluR-mediated Ca2+ responses. A: effect of increasing concentrations of 2-APB on DHPG-elicited Ca2+ responses in a P10 LSO neuron. B. summary data. C: effect of 2-APB on KCl-elicited (60 mM) Ca2+ responses. Data are from the same cells as in B.
Taken together, the sensitivity of DHPG-elicited Ca2+ response plateaus to Ni2+ and 2-APB and its insensitivity to specific VGCC blockers suggests that the plateau component is mediated by Ca2+ influx through a TRP-like channel.
DHPG responses are sensitive to La3+
Most members of the TRPC family are either inhibited (TRPC3, TRPC6, and TRPC7 (Inoue et al. 2001; Okada et al. 1999; Zhu et al. 1998) or potentiated (TRPC4 and TRPC5; Schaefer et al. 2000; Strubing et al. 2001) by micromolar concentrations of La3+. In neonatal LSO neurons (P0–P5), La3+ reduced DHPG responses in a dose-dependent manner and completely abolished them at 1 mM (Fig. 6A). La3+ at 1 mM also completely abolished KCl responses (n = 49 cells; data not shown), consistent with the known effect of La3+ on VGCCs (Ozawa et al. 1989). In P9–P12 animals, La3+-potentiated responses at 0.1 and 0.5 mM but had no effect at 1 mM (Fig. 6, B and C). Sensitivity of DHPG-evoked responses to La3+ provides additional support that mGluR-evoked Ca2+ responses involve the activation of TRPCs. The age-dependent transition from inhibition to potentiation might be a consequence of an age-dependent expression of different TRPCs in LSO neurons.
FIG. 6.
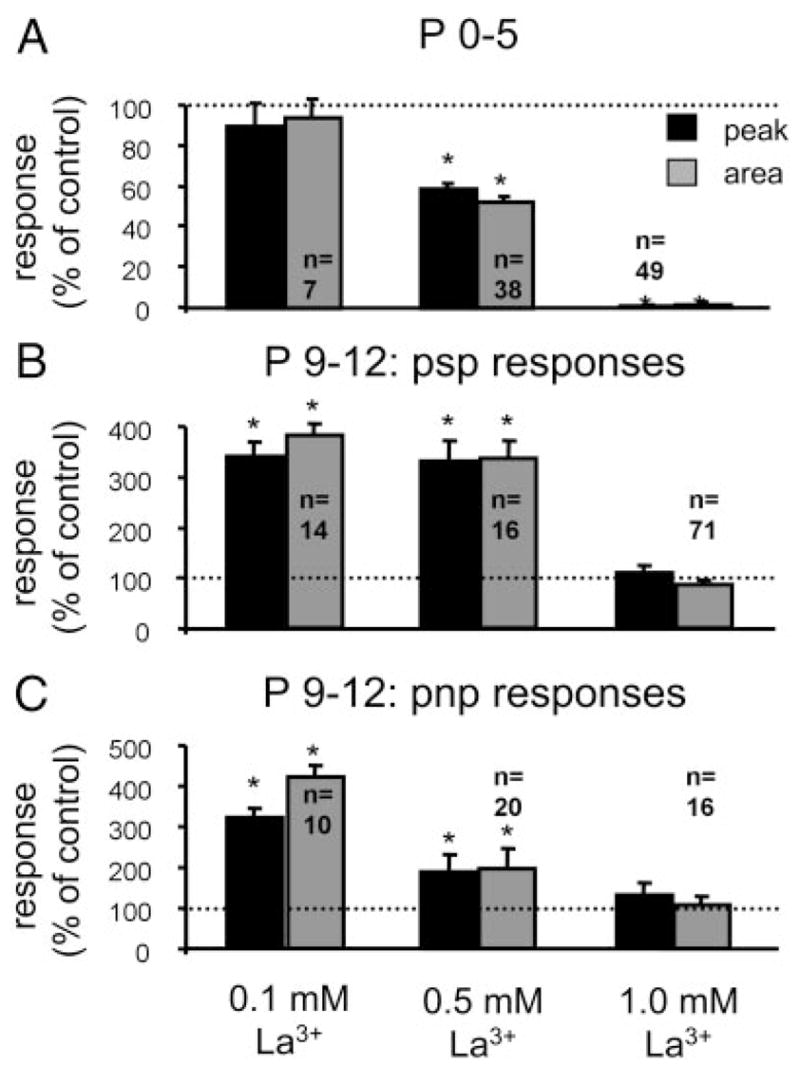
Effect of La3+ on group I mGluR-mediated Ca2+ responses. A: dose-dependent effect of La3+ on peak and area of DHPG-evoked (50 μM, 90 s) responses at P0–P5. B and C: in LSO neurons from P9–P12 mice (B: psp responses; C: pnp responses), La3+ potentiated responses at 0.1 and 0.5 mM, whereas it had no effect at 1.0 mM.
Developmental downregulation of mGluR-mediated Ca2+ responses is impaired in deaf animals
The downregulation of group I mGluR-elicited Ca2+ responses and the disappearance of the plateau phase after hearing onset raises the question of whether these changes depend on auditory-evoked neuronal activity. To explore this idea, we compared DHPG-elicited Ca2+ responses from hearing wild-type (wt) C57/Bl mice to responses from age-matched deaf waltzer mice. In these experiments, we assessed hearing ability by the presence of a startle response to hand clapping. All wt mice older than P11 but none of the waltzer mice showed a clear startle response. LSO neurons from deaf waltzer mice showed DHPG-elicited Ca2+ responses of the pp, psp, and pnp types (Fig. 7A). Waltzer LSO neurons also showed an age-dependent decrease in peak amplitudes, areas, and duration of responses (P11–P16: n = 107 cells, P17–P20: 58 cells; Mann–Whitney test, P < 0.05). However, compared with age-matched wt mice, these changes were significantly different in waltzer mice (Fig. 7B). In both the P11–P16 group and the P17–P20 group, peak amplitudes were larger in waltzer mice than in age-matched wt mice (P11–P16: waltzer n = 107, wt n = 68; P17–P20: waltzer n = 58, wt n = 72; KS test, P < 0.05). This difference may be the result of a developmental delay because peak amplitudes from P17–P20 waltzer mice were not significantly different from those from P11–P16 wt mice (KS test, P > 0.05; Fig. 7Bi). Similarly, response durations were significantly larger in waltzer mice compared with aged-matched wt-mice (KS test, P < 0.01, Fig. 7Bii). However, response durations in P17–P20 waltzer mice were shorter than those in P11–P16 wt mice (KS test, P < 0.01).
FIG. 7.
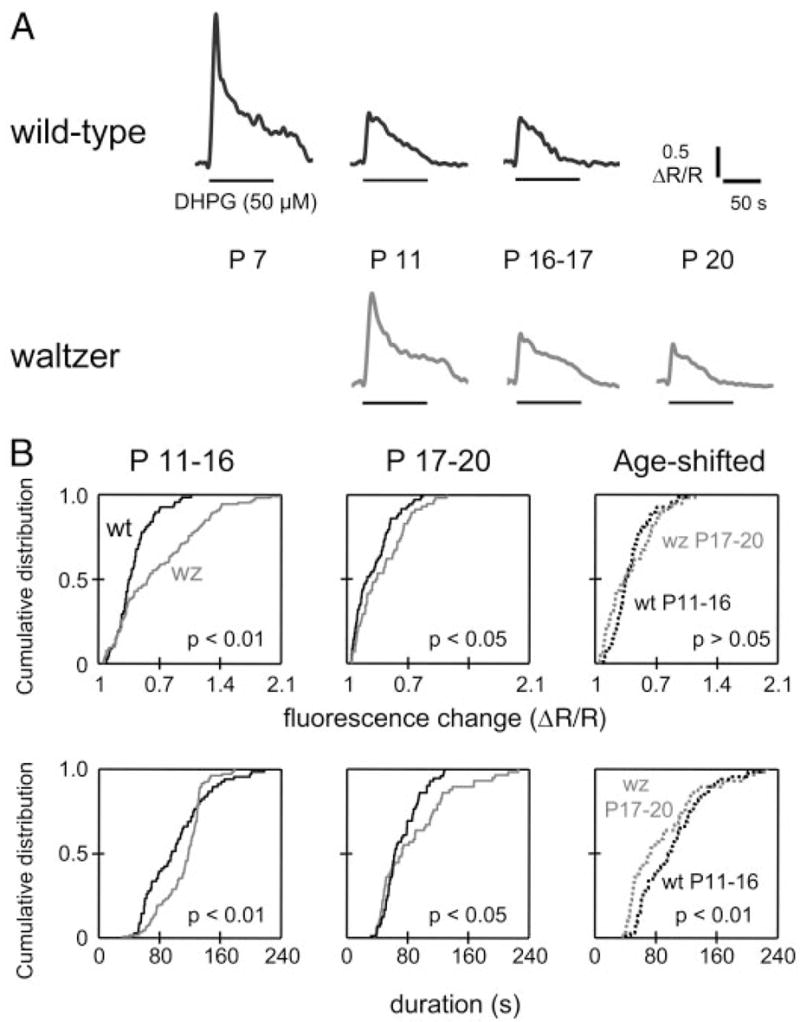
Group I mGluR-mediated Ca2+ responses in deaf waltzer mice. A: examples of DHPG-elicited Ca2+ responses in wild-type and waltzer mice. B: cumulative distribution plots of peak (i) and duration (ii) of DHPG-elicited responses of LSO neurons from wild-type (wt, black lines, P11–P16: n = 68 cells, P17–P20: n = 72 cells) and waltzer mice (wz, gray lines, P11–P16: n = 107 cells, P17–P20: n = 58 cells). P values for Kolmogorov–Smirnov (KS) test.
We also investigated DHPG-elicited Ca2+ responses in heterozygote waltzer mice, which can hear but have elevated compound action potential thresholds and are more prone to noise-induced hearing loss (Holme and Steel 2004). The duration of DHPG-elicited Ca2+ responses in LSO neurons from hearing (startle positive) heterozygote mice (n = 4 animals) was the same as in neurons from hearing wt mice (n = 11 animals) (P11–P16: het mice n = 44, wt n = 68; P17–P20: het mice n = 16, wt n = 72; KS test, P > 0.1) (Fig. 8, A and B). At P11–P16, responses were significantly shorter in heterozygote mice compared with homozygote walzer mice (n = 6 animals; n = 107 cells, KS test, P < 0.005). In heterozygote mice, response durations in startle-negative P12–P13 mice were significantly longer compared with startle-positive P14–P16 mice (Fig. 8, C and D).
FIG. 8.
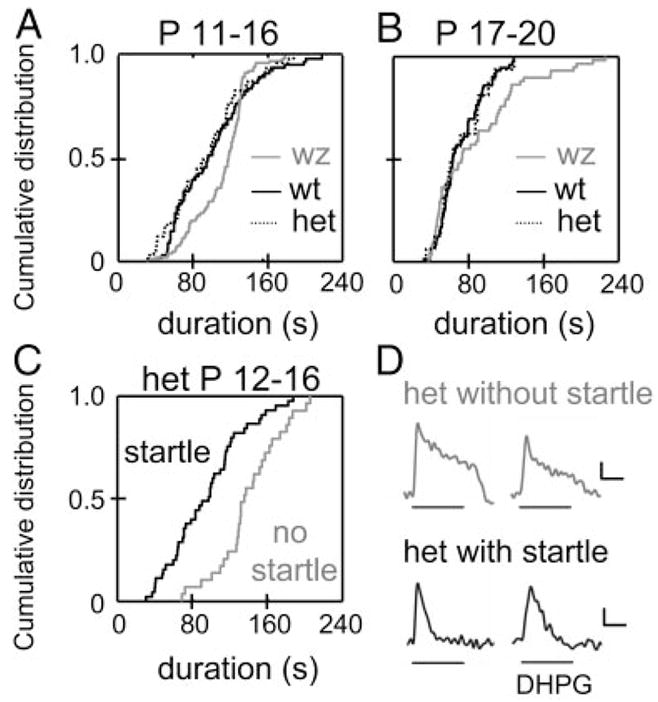
Durations of group I mGluR-mediated Ca2+responses in waltzer, heterozygote, and wild-type mice. A: cumulative distribution of DHPG-elicited Ca2+ response durations in P11 to P16 LSO neurons from homozygote waltzer (wz, gray line, n = 107 cells), hearing heterozygote (het, dotted line, n = 44 cells), and hearing wild-type mice (wt, black line, n = 68 cells). Durations in heterozygote mice were significantly shorter compared with homozygote mice (P < 0.005) and were not different from wild-type mice (P > 0.1). B: same as A but for postnatal days 17–20. Durations in heterozygote mice were not significantly different from wild-type or homozygote mice (wz: n = 58 cells; het: n = 16 cells; wt: n = 72 cells). C: response durations in heterozygote mice with a startle response (black line; P14–P16, n = 44 cells) and mice lacking a startle response (gray line; P12–P13, n = 29 cells). Durations in mice lacking a startle response were significantly longer than in mice with a startle response (P < 0.001). D: examples of DHGP-elicited Ca2+-responses from heterozygote mice with and without startle response. Scale bars, 0.02 ΔR/R (top traces), 0.01 ΔR/R (bottom traces), 50 s. P values for KS test.
Similar to wt mice, in waltzer mice we also observed a developmental decrease in the percentage of DHPG-responses of the pp and psp types and a developmental increase in the percentage of pnp responses (Fig. 9; Fisher’s exact test, P < 0.05). However, in waltzer mice, more cells showed pp and psp responses at P11–P16 (Fig. 9A; waltzer n = 80 cells, wt n = 32 cells; Fisher’s exact test, P < 0.05).
FIG. 9.
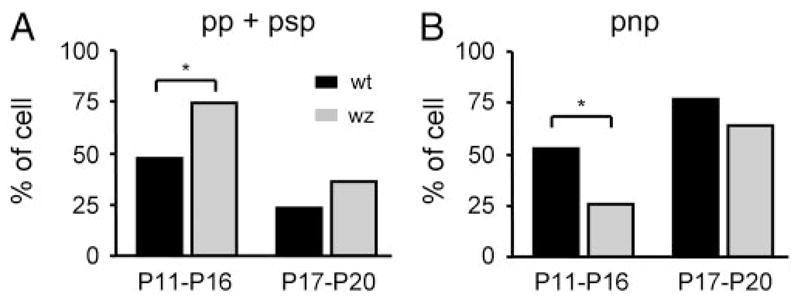
Developmental changes in response types in wild-type and waltzer mice. A: percentage of pp and psp responses is greater in wz than in wt mice for both age groups (P11–P16: wt = 32 cells, wz = 80 cells; Fisher’s exact test, P < 0.05; P17–P20: wt = 17 cells, wz = 21 cells; Fisher’s exact test, P > 0.05). B: percentage of pnp responses is smaller in wz than in wt mice (P11–P16: wt = 36 cells, wz = 27 cells; Fisher’s exact test, P < 0.05; P17–P20: wt = 55 cells, wz = 37 cells; Fisher’s exact test, P > 0.05)
Taken together, in deaf waltzer mice the age-dependent changes of mGluR-mediated Ca2+ responses were significantly smaller, suggesting that auditory experience is necessary for the normal time course of the functional downregulation of TRP-like channel(s). In support of this hypothesis, in P16 waltzer mice DHPG-elicited responses were strongly diminished by the TRPC antagonist Ni2+ (peak amplitude reduced by 80.5 ± 4.7%; area reduced by 82.4 ± 4.2%; n = 21 cells with responses in Ni2+).
DISCUSSION
This study provides a developmental picture of the Ca2+ entry pathways underlying group I mGluR-mediated Ca2+ responses in developing LSO neurons. In neonatal mice, these Ca2+ responses were characterized by a large transient peak followed by a sustained plateau. We identified two Ca2+ sources that contributed to these responses: release of Ca2+ from internal stores and influx of Ca2+ from the extracellular milieu. The extracellular component was sensitive to the TRPC blockers Ni2+, 2-APB, and La3+, suggesting that it was mediated by a TRP-like Ca2+ channel. With increasing age, the contribution of extracellular Ca2+ influx to group I mGluR-mediated Ca2+ responses diminished and was mostly absent after hearing onset, whereas the contribution of internal stores was unchanged. Finally, the developmental downregulation of mGluR-elicited extracellular Ca2+ influx was delayed in deaf waltzer mice, suggesting that an age-dependent decrease in the activation and/or expression of TRPCs is influenced by auditory experience.
Developing LSO neurons express functional TRP-like channels
Our results suggest that LSO neurons in prehearing mice express Ca2+-permeable, TRP-like channels that are activated by group I mGluRs. This is supported by the following observations. First, the typical biphasic peak-plateau profile of mGluR-elicited Ca2+ responses in LSO neurons resembles the response profiles that were observed in a number of other neuronal and nonneuronal cell types that express store-operated Ca2+ channels or TRPCs (Boulay et al. 1997; Hu et al. 1994; Moller et al. 1997; Ramsey et al. 2006; Sosa et al. 2002). Second, the plateau phase of mGluR-elicted Ca2+ responses was mediated entirely by influx of extracellular Ca2+ (Ene et al. 2003). Third, extracellular Ca2+ influx was insensitive to specific blockers of voltage-gated Ca2+ channels (Ene et al. 2003) but was sensitive to blockers of TRPCs such as Ni2+, 2-APB, and La3+ (Figs. 4–6). Fourth, activation of mGluRs by the specific agonist DHPG resulted in depolarizations (see Supplemental material) similar to the activation of TRPCs in other neuronal systems (Kim et al. 2003. Tempia et al. 2001).
TRPCs can be activated by a variety of intracellular messengers depending on the exact TRPC and cell type. In LSO neurons, Ca2+ release from intracellular Ca2+ stores is required for coupling mGluR activation to the opening of TRP-like channels—based on the observation that depletion of intracellular Ca2+ stores with thapsigargine abolished all mGluR-elicited Ca2+ responses (Ene et al. 2003). However, Ca2+ does not appear to be the only signal responsible for activation of TRP-like channels because influx of Ca2+ through VGCC while intracellular Ca2+ stores were depleted by thapsigargine did not trigger long-lasting Ca2+ plateaus. Further studies are needed to identify what additional components of the group I mGluR-activated intracellular cascade are the relevant stimuli that, together with Ca2+, activate the opening of TRP-like channels in LSO neurons.
Which TRPCs are responsible for group I mGluR-mediated Ca2+ responses in LSO neurons? All members of the TRPC family (TRPC1–TRPC7) are expressed in the brain (Minke and Cook 2002; Riccio et al. 2002), although their expression patterns have not been investigated specifically for the LSO. TRPCs are activated by an increase in [Ca2+]i and/or diacyl-glycerol (Boulay et al. 1997; Hofmann et al. 1999; Kim et al. 2003; Okada et al. 1998; Schaefer et al. 2000; Zitt et al. 1997) and it was previously suggested that the TRPC3 channel mediates group I mGluR-mediated Ca2+ responses in retinal amacrine cells (Sosa et al. 2002). TRPC3 also seems to be a good candidate for LSO neurons because TRPC3 is strongly expressed in the embryonic and early postnatal brain stem, including in auditory brain stem nuclei (Li et al. 1999), and because TRPC3 expression levels decrease after P10, which corresponds to the time when group I mGluR-mediated Ca2+ responses in LSO neurons also decrease. Finally, DHPG-evoked responses in neonatal LSO neurons were inhibited by millimolar concentrations of La3+ (Fig. 6), concentrations that are in the range of TRPC3 sensitivity to La3+ (Zhu et al. 1998).
Developmental decrease in the contribution of TRP-like channels to mGluR-mediated Ca2+ responses and the role of sensory-evoked activity
During the first three postnatal weeks, group I mGluR-mediated Ca2+ responses became progressively smaller in amplitude, shorter in duration, and the plateau component disappeared (Fig. 2). These changes are consistent with the idea that the decrease in mGluR-elicited Ca2+ responses resulted, in large part, from a developmental downregulation of TRP-like channels or their functional uncoupling from mGluR activation. In support of this, both Ni2+, which reduced the peak amplitude and abolished the Ca2+ plateau by blocking the influx of extracellular Ca2+ in neonatal LSO neurons, and removal of extracellular Ca2+ (Fig. 3) had no effect on responses during the third postnatal week, when most responses were small and short and were mediated primarily by Ca2+ release from internal stores (Fig. 4, A and B).
The contribution of extracellular Ca2+ influx to mGluR-mediated Ca2+ responses declined at, and shortly after, hearing onset (approximately P12; Song et al. 2006), raising the possibility that sensory activity plays a role in the functional downregulation of TRP-like channels. We tested this hypothesis by investigating mGluR-mediated Ca2+ responses in developing waltzer mice. Waltzer mice have spontaneous mutation in the gene for cadherin 23, which causes a disorganization of stereocilia bundles of cochlear hair cells resulting in deafness (Di Palma et al. 2001; Holme and Steel 2002). In waltzer mice, the changes in group I mGluR-mediated Ca2+ responses appeared to be significantly delayed compared with heterozygote and wild-type control mice (Figs. 7–9). Notably, more LSO neurons in waltzer mice than in age-matched control mice displayed responses with a long Ca2+ plateau and fewer neurons displayed peak-only responses. These results support the idea that auditory experience is important for the developmental downregulation of TRP-like channel expression or their developmental uncoupling from mGluR activation.
It should be noted that our results cannot exclude the possibility that spontaneous activity before hearing onset also contributes to the downregulation of TRP-like responses. Further studies that investigate mGluR-mediated Ca2+ responses in prehearing waltzer mice and characterize spontaneous activity patterns in vivo are necessary to conclusively address this possibility. Another issue to consider is that cadherin 23 is expressed not only in hair cells but also in the brain (Di Palma 2001; Rzadzinska et al. 2005), although it is currently unknown whether cadherin 23 is expressed in the LSO or in any other auditory nuclei. Anatomical studies investigating cadherin expression in developing LSO neurons are required to address the question whether impairment in the developmental decrease in TRP-like channels activation by mGluRs in waltzer mice is influenced by potential changes of cadherin 23 in the LSO. Nevertheless, our conclusion that auditory experience is an important contributing factor to the downregulation of TRP activation by mGluRs is supported by our results from heterozygote waltzer mice. Heterozygotes, after hearing onset, as indicated by the presence of an acoustic startle response, showed mGluR-mediated Ca2+ responses similar to age-matched wild-type mice and in P12–P16 heterozygotes response durations became significantly shorter right after hearing onset (Fig. 8, C and D).
Functional implications
Similar to other neuronal types, in neonatal LSO neurons mGluRs are activated preferentially by high-frequency stimulation of glutamatergic inputs (Ene et al. 2003). Spontaneous high-frequency bursts of neuronal activity exist in a variety of auditory nuclei before hearing onset (Durham et al. 1989; Gummer and Mark 1994; Jones et al. 2001; Kotak and Sanes 1995; Kros et al. 1998; Leao et al. 2006; Lippe 1994), making it likely that TRP-like channels in LSO neurons are activated by spontaneous burstlike activity in vivo. In addition to LSO neurons, opening of TRPCs by mGluR activation also seems to occur in other auditory brain stem neurons. For example, in neonatal rat MNTB neurons, DHPG elicits inward currents (Kushmerick et al. 2004) and in preliminary studies we observed long-lasting Ca2+ responses with typical peak-plateau profiles after DHPG application in the MNTB.
Spontaneous activity before hearing onset is important for numerous developmental processes including neuronal survival of auditory brain stem neurons (Sie and Rubel 1992; Zirpel and Rubel 1996) and various other aspects of auditory brain stem circuits (Gabriele et al. 2000; Kitzes et al. 1995; Kotak and Sanes 1997; Russell and Moore 1995; Sanes and Takacs 1993). Intracellular Ca2+ plays a central role in linking neuronal activity to the survival of auditory brain stem neurons (Lachica et al. 1995; Lohmann et al. 1998; Zirpel et al. 1995) and was previously implicated in activity-dependent refinement of LSO circuitry (Kotak and Sanes 2000). TRP-like channels, by greatly amplifying the initial mGluR-elicited Ca2+ response (Figs. 3 and 4), may provide an important function in translating burstlike synaptic activity into functional LSO circuitry, perhaps by influencing growth cone behavior (Li et al. 2005; Wang and Poo 2005), regulating neurite length (Greka et al. 2003), or by adjusting synaptic strength (Baba et al. 2003). In the LSO, TRP-like channels are activated preferentially before hearing onset, which is the period of major developmental changes that include dendritic and synaptic refinement (Kim and Kandler 2003; Rietzel and Friauf 1998; Sanes and Takacs 1993; Sanes et al. 1992), activity-dependent plasticity (Kotak and Sanes 2000), and a switch of neurotransmitter phenotype (Gillespie et al. 2005; Kotak et al. 1998; Nabekura et al. 2004).
It will be interesting to find out whether and to what degree mGluR activation and TRPCs are involved in mediating any or which of these events and thus contribute to the development of normal sound localization and hearing.
Although activation of TRPCs by mGluRs might play an important role in the cellular detection and encoding of spontaneous bursts of activity, auditory experience-dependent downregulation of TRPC activation might protect LSO neurons from reaching toxic levels of [Ca2+]i after hearing onset when glutamatergic inputs to LSO neurons fire at high rates and when LSO neurons express Ca2+-permeable μ-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Caicedo et al. 1998).
Finally, TRPCs might plausibly be the mechanism responsible for long-lasting depolarizations that occur in LSO neurons of neonatal gerbils after tetanic stimulation of glutama-tergic cochlea nucleus afferents (Kotak and Sanes 1995). These long-lasting depolarizations occur during the first two postnatal weeks, require mGluR receptors activation, and are sensitive to Ni2+, properties that parallel mGluR-mediated TRPC activation.
Supplementary Material
Acknowledgments
We thank K. Cihil for excellent technical support, D. Goldring for editorial help, NBK for encouragement, and Dr. Elias Aizenman and members of the Kandler laboratory for valuable discussion.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grants DC-04199 and DC-008938 to K. Kandler and A. Kalmbach, respectively, and by the Center for the Neural Basis of Cognition at Carnegie Mellon University and the University of Pittsburgh.
Footnotes
The online version of this article contains supplemental data.
References
- Baba A, Yasui T, Fujisawa S, Yamada RX, Yamada MK, Nishiyama N, Matsuki N, Ikegaya Y. Activity-evoked capacitative Ca2+ entry: implications in synaptic plasticity. J Neurosci. 2003;23:7737–7741. doi: 10.1523/JNEUROSCI.23-21-07737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtson CP, Tozzi A, Bernardi G, Mercuri NB. Transient receptor potential-like channels mediate metabotropic glutamate receptor EPSCs in rat dopamine neurones. J Physiol. 2004;555:323–330. doi: 10.1113/jphysiol.2003.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- Boudreau JC, Tsuchitani C. Binaural interaction in the cat superior olive S segment. J Neurophysiol. 1968;31:442–454. doi: 10.1152/jn.1968.31.3.442. [DOI] [PubMed] [Google Scholar]
- Boulay G, Zhu X, Peyton M, Jiang M, Hurst R, Stefani E, Birnbaumer L. Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. J Biol Chem. 1997;272:29672–29680. doi: 10.1074/jbc.272.47.29672. [DOI] [PubMed] [Google Scholar]
- Caicedo A, Kungel M, Pujol R, Friauf E. Glutamate-induced Co2+ uptake in rat auditory brainstem neurons reveals developmental changes in Ca2+ permeability of glutamate receptors. Eur J Neurosci. 1998;10:941–954. doi: 10.1046/j.1460-9568.1998.00104.x. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Di Palma F, Holme RH, Bryda EC, Belyantseva IA, Pellegrino R, Kachar B, Steel KP, Noben-Trauth K. Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in waltzer, the mouse model for Usher syndrome type 1D. Nat Genet. 2001;27:103–107. doi: 10.1038/83660. [DOI] [PubMed] [Google Scholar]
- Durham D, Rubel EW, Steel KP. Cochlear ablation in deafness mutant mice: 2-eoxyglucose analysis suggests no spontaneous activity of cochlear origin. Hear Res. 1989;43:39–46. doi: 10.1016/0378-5955(89)90057-9. [DOI] [PubMed] [Google Scholar]
- Ene FA, Kullmann PH, Gillespie DC, Kandler K. Glutamatergic calcium responses in the developing lateral superior olive: receptor types and their specific activation by synaptic activity patterns. J Neurophysiol. 2003;90:2581–2591. doi: 10.1152/jn.00238.2003. [DOI] [PubMed] [Google Scholar]
- Friauf E, Lohmann C. Development of auditory brainstem circuitry. Activity-dependent and activity-independent processes. Cell Tissue Res. 1999;297:187–195. doi: 10.1007/s004410051346. [DOI] [PubMed] [Google Scholar]
- Gabriele ML, Brunso-Bechtold JK, Henkel CK. Plasticity in the development of afferent patterns in the inferior colliculus of the rat after unilateral cochlear ablation. J Neurosci. 2000;20:6939–6949. doi: 10.1523/JNEUROSCI.20-18-06939.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee CE, Benquet P, Gerber U. Group I metabotropic glutamate receptors activate a calcium-sensitive transient receptor potential-like conductance in rat hippocampus. J Physiol. 2003;546:655–664. doi: 10.1113/jphysiol.2002.032961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie DC, Kim G, Kandler K. Inhibitory synapses in the developing auditory system are glutamatergic. Nat Neurosci. 2005;8:332–338. doi: 10.1038/nn1397. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea. Science. 2000;288:2366–2368. doi: 10.1126/science.288.5475.2366. [DOI] [PubMed] [Google Scholar]
- Greka A, Navarro B, Oancea E, Duggan A, Clapham DE. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6:837–845. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Gummer AW, Mark RF. Patterned neural activity in brain stem auditory areas of a prehearing mammal, the tammar wallaby (Macropus eugenii) Neuroreport. 1994;5:685–688. doi: 10.1097/00001756-199402000-00006. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Holme RH, Steel KP. Stereocilia defects in waltzer (Cdh23), shaker1 (Myo7a) and double waltzer/shaker1 mutant mice. Hear Res. 2002;169:13–23. doi: 10.1016/s0378-5955(02)00334-9. [DOI] [PubMed] [Google Scholar]
- Holme RH, Steel KP. Progressive hearing loss and increased susceptibility to noise-induced hearing loss in mice carrying a Cdh23 but not a Myo7a mutation. J Assoc Res Otolaryngol. 2004;5:66–79. doi: 10.1007/s10162-003-4021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Vaca L, Zhu X, Birnbaumer L, Kunze DL, Schilling WP. Appearance of a novel Ca2+ influx pathway in Sf9 insect cells following expression of the transient receptor potential-like (trpl) protein of Drosophila. Biochem Biophys Res Commun. 1994;201:1050–1056. doi: 10.1006/bbrc.1994.1808. [DOI] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca(2+)-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Jones TA, Jones SM, Paggett KC. Primordial rhythmic bursting in embryonic cochlear ganglion cells. J Neurosci. 2001;21:8129–8135. doi: 10.1523/JNEUROSCI.21-20-08129.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Gillespie DC. Developmental refinement of inhibitory sound-localization circuits. Trends Neurosci. 2005;28:290–296. doi: 10.1016/j.tins.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Kandler K. Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat Neurosci. 2003;6:282–290. doi: 10.1038/nn1015. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426:285–291. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- Kitzes LM, Kageyama GH, Semple MN, Kil J. Development of ectopic projections from the ventral cochlear nucleus to the superior olivary complex induced by neonatal ablation of the contralateral cochlea. J Comp Neurol. 1995;353:341–363. doi: 10.1002/cne.903530303. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Korada S, Schwartz IR, Sanes DH. A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J Neurosci. 1998;18:4646–4655. doi: 10.1523/JNEUROSCI.18-12-04646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH. Synaptically evoked prolonged depolarizations in the developing auditory system. J Neurophysiol. 1995;74:1611–1620. doi: 10.1152/jn.1995.74.4.1611. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH. Deafferentation weakens excitatory synapses in the developing central auditory system. Eur J Neurosci. 1997;9:2340–2347. doi: 10.1111/j.1460-9568.1997.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH. Long-lasting inhibitory synaptic depression is age- and calcium-dependent. J Neurosci. 2000;20:5820–5826. doi: 10.1523/JNEUROSCI.20-15-05820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros CJ, Ruppersberg JP, Rusch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998;394:281–284. doi: 10.1038/28401. [DOI] [PubMed] [Google Scholar]
- Kushmerick C, Price GD, Taschenberger H, Puente N, Renden R, Wadiche JI, Duvoisin RM, Grandes P, von Gersdorff H. Retroinhibition of presynaptic Ca2+ currents by endocannabinoids released via postsynaptic mGluR activation at a calyx synapse. J Neurosci. 2004;24:5955–5965. doi: 10.1523/JNEUROSCI.0768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachica EA, Rubsamen R, Zirpel L, Rubel EW. Glutamatergic inhibition of voltage-operated calcium channels in the avian cochlear nucleus. J Neurosci. 1995;15:1724–1734. doi: 10.1523/JNEUROSCI.15-03-01724.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao RN, Sun H, Svahn K, Berntson A, Youssoufian M, Paolini AG, Fyffe RE, Walmsley B. Topographic organization in the auditory brainstem of juvenile mice is disrupted in congenital deafness. J Physiol. 2006;571:563–578. doi: 10.1113/jphysiol.2005.098780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HS, Xu XZ, Montell C. Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron. 1999;24:261–273. doi: 10.1016/s0896-6273(00)80838-7. [DOI] [PubMed] [Google Scholar]
- Li Y, Jia YC, Cui K, Li N, Zheng ZY, Wang YZ, Yuan XB. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894–898. doi: 10.1038/nature03477. [DOI] [PubMed] [Google Scholar]
- Lippe WR. Rhythmic spontaneous activity in the developing avian auditory system. J Neurosci. 1994;14:1486–1495. doi: 10.1523/JNEUROSCI.14-03-01486.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann C, Ilic V, Friauf E. Development of a topographically organized auditory network in slice culture is calcium dependent. J Neurobiol. 1998;34:97–112. doi: 10.1002/(sici)1097-4695(19980205)34:2<97::aid-neu1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Ma HT, Venkatachalam K, Li HS, Montell C, Kurosaki T, Patterson RL, Gill DL. Assessment of the role of the inositol 1,4,5-trisphosphate receptor in the activation of transient receptor potential channels and store-operated Ca2+ entry channels. J Biol Chem. 2001;276:18888–18896. doi: 10.1074/jbc.M100944200. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem (Tokyo) 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- Moller T, Nolte C, Burger R, Verkhratsky A, Kettenmann H. Mechanisms of C5a and C3a complement fragment-induced [Ca2+]i signaling in mouse microglia. J Neurosci. 1997;17:615–624. doi: 10.1523/JNEUROSCI.17-02-00615.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. TRP channels in Drosophila photoreceptor cells. J Physiol. 2005;567:45–51. doi: 10.1113/jphysiol.2005.092551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabekura J, Katsurabayashi S, Kakazu Y, Shibata S, Matsubara A, Jinno S, Mizoguchi Y, Sasaki A, Ishibashi H. Developmental switch from GABA to glycine release in single central synaptic terminals. Nat Neurosci. 2004;7:17–23. doi: 10.1038/nn1170. [DOI] [PubMed] [Google Scholar]
- Oertel D. The role of timing in the brain stem auditory nuclei of vertebrates. Annu Rev Physiol. 1999;61:497–519. doi: 10.1146/annurev.physiol.61.1.497. [DOI] [PubMed] [Google Scholar]
- Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, Mori Y. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca(2+)-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J Biol Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- Okada T, Shimizu S, Wakamori M, Maeda A, Kurosaki T, Takada N, Imoto K, Mori Y. Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J Biol Chem. 1998;273:10279–10287. doi: 10.1074/jbc.273.17.10279. [DOI] [PubMed] [Google Scholar]
- Ollo C, Schwartz IR. The superior olivary complex in C57BL/6 mice. Am J Anat. 1979;155:349–374. doi: 10.1002/aja.1001550306. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Tsuzuki K, Iino M, Ogura A, Kudo Y. Three types of voltage-dependent calcium current in cultured rat hippocampal neurons. Brain Res. 1989;495:329–336. doi: 10.1016/0006-8993(89)90225-4. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Fleig A, Penner R. The store-operated calcium current I(CRAC): nonlinear activation by InsP3 and dissociation from calcium release. Cell. 1997;89:973–980. doi: 10.1016/s0092-8674(00)80282-2. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Potentiation and inhibition of Ca(2+) release-activated Ca(2+) channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP(3) receptors. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to trp channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Riccio A, Mattei C, Kelsell RE, Medhurst AD, Calver AR, Randall AD, Davis JB, Benham CD, Pangalos MN. Cloning and functional expression of human short TRP7, a candidate protein for store-operated Ca2+ influx. J Biol Chem. 2002;277:12302–12309. doi: 10.1074/jbc.M112313200. [DOI] [PubMed] [Google Scholar]
- Rietzel HJ, Friauf E. Neuron types in the rat lateral superior olive and developmental changes in the complexity of their dendritic arbors. J Comp Neurol. 1998;390:20–40. [PubMed] [Google Scholar]
- Romand R, Ehret G. Development of tonotopy in the inferior colliculus. I. Electrophysiological mapping in house mice. Dev Brain Res. 1990;54:221–234. doi: 10.1016/0165-3806(90)90145-o. [DOI] [PubMed] [Google Scholar]
- Russell FA, Moore DR. Afferent reorganisation within the superior olivary complex of the gerbil: development and induction by neonatal, unilateral cochlear removal. J Comp Neurol. 1995;352:607–625. doi: 10.1002/cne.903520409. [DOI] [PubMed] [Google Scholar]
- Rzadzinska AK, Derr A, Kachar B, Noben-Trauth K. Sustained cadherin 23 expression in young and adult cochlea of normal and hearing-impaired mice. Hear Res. 2005;208:114–121. doi: 10.1016/j.heares.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Rubel EW. The ontogeny of inhibition and excitation in the gerbil lateral superior olive. J Neurosci. 1988;8:682–700. doi: 10.1523/JNEUROSCI.08-02-00682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Siverls V. Development and specificity of inhibitory terminal arborizations in the central nervous system. J Neurobiol. 1991;8:837–854. doi: 10.1002/neu.480220805. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Song J, Tyson J. Refinement of dendritic arbors along the tonotopic axis of the gerbil lateral superior olive. Dev Brain Res. 1992;67:47–55. doi: 10.1016/0165-3806(92)90024-q. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Takacs C. Activity-dependent refinement of inhibitory connections. Eur J Neurosci. 1993;5:570–574. doi: 10.1111/j.1460-9568.1993.tb00522.x. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Plant TD, Obukhov AG, Hofmann T, Gudermann T, Schultz G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J Biol Chem. 2000;275:17517–17526. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]
- Scheenen WJ, Makings LR, Gross LR, Pozzan T, Tsien RY. Photodegra-dation of indo-1 and its effect on apparent Ca2+ concentrations. Chem Biol. 1996;3:765–774. doi: 10.1016/s1074-5521(96)90253-7. [DOI] [PubMed] [Google Scholar]
- Shnerson A, Pujol R. Age-related changes in the C57BL/6J mouse cochlea. I. Physiological findings. Brain Res. 1981;254:65–75. doi: 10.1016/0165-3806(81)90059-6. [DOI] [PubMed] [Google Scholar]
- Sie KC, Rubel EW. Rapid changes in protein synthesis and cell size in the cochlear nucleus following eighth nerve activity blockade or cochlea ablation. J Comp Neurol. 1992;320:501–508. doi: 10.1002/cne.903200407. [DOI] [PubMed] [Google Scholar]
- Song L, Mcgee J, Walsh EJ. Frequency- and level-dependent changes in auditory brainstem responses (ABRS) in developing mice. J Acoust Soc Am. 2006;119:2242–2257. doi: 10.1121/1.2180533. [DOI] [PubMed] [Google Scholar]
- Sosa R, Hoffpauir B, Rankin ML, Bruch RC, Gleason EL. Metabotropic glutamate receptor 5 and calcium signaling in retinal amacrine cells. J Neu-rochem. 2002;81:973–983. doi: 10.1046/j.1471-4159.2002.00883.x. [DOI] [PubMed] [Google Scholar]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Tempia F, Alojado ME, Strata P, Knopfel T. Characterization of the mGluR(1)-mediated electrical and calcium signaling in Purkinje cells of mouse cerebellar slices. J Neurophysiol. 2001;86:1389–1397. doi: 10.1152/jn.2001.86.3.1389. [DOI] [PubMed] [Google Scholar]
- Tollin DJ. The lateral superior olive: a functional role in sound source localization. Neuroscientist. 2003;9:127–143. doi: 10.1177/1073858403252228. [DOI] [PubMed] [Google Scholar]
- Tozzi A, Bengtson CP, Longone P, Carignani C, Fusco FR, Bernardi G, Mercuri NB. Involvement of transient receptor potential-like channels in responses to mGluR-I activation in midbrain dopamine neurons. Eur J Neu-rosci. 2003;18:2133–2145. doi: 10.1046/j.1460-9568.2003.02936.x. [DOI] [PubMed] [Google Scholar]
- Wada T, Wakabayashi Y, Takahashi S, Ushiki T, Kikkawa Y, Yonekawa H, Kominami R. A point mutation in a cadherin gene, Cdh23, causes deafness in a novel mutant, Waltzer mouse niigata. Biochem Biophys Res Commun. 2001;283:113–117. doi: 10.1006/bbrc.2001.4724. [DOI] [PubMed] [Google Scholar]
- Wang GX, Poo MM. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature. 2005;434:898–904. doi: 10.1038/nature03478. [DOI] [PubMed] [Google Scholar]
- Zhu X, Jiang M, Birnbaumer L. Receptor-activated Ca2+ influx via human Trp3 stably expressed in human embryonic kidney (HEK)293 cells. Evidence for a non-capacitative Ca2+ entry. J Biol Chem. 1998;273:133–142. doi: 10.1074/jbc.273.1.133. [DOI] [PubMed] [Google Scholar]
- Zirpel L, Lachica EA, Rubel EW. Activation of a metabotropic glutamate receptor increases intracellular calcium concentrations in neurons of the avian cochlear nucleus. J Neurosci. 1995;15:214–222. doi: 10.1523/JNEUROSCI.15-01-00214.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirpel L, Rubel EW. Eighth nerve activity regulates intracellular calcium concentration of avian cochlear nucleus neurons via a metabotropic glutamate receptor. J Neurophysiol. 1996;76:4127–4139. doi: 10.1152/jn.1996.76.6.4127. [DOI] [PubMed] [Google Scholar]
- Zitt C, Obukhov AG, Strubing C, Zobel A, Kalkbrenner F, Luckhoff A, Schultz G. Expression of TRPC3 in Chinese hamster ovary cells results in calcium-activated cation currents not related to store depletion. J Cell Biol. 1997;138:1333–1341. doi: 10.1083/jcb.138.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


