Abstract
The factors that influence the timing of puberty and the onset of adult fertility are poorly understood. While focus on the juvenile period has provided insights into how growth-related cues affect pubertal timing, growth velocity during infancy that is sustained into the juvenile period may be important. On the other hand, social factors, specifically exposure to psychosocial stressors, can delay sexual maturation, possibly by altering growth velocities during development. Using female rhesus monkeys, the present study used a prospective analysis to determine how neonatal growth hormone (GH) inhibition with a sandostatin analog or suppression of the pituitary – gonadal axis with a GnRH analog affected growth and sexual maturation. Secondly, a separate retrospective analysis was done assessing the effects of social dominance status during development on pubertal timing. Because a specific polymorphism in the gene encoding the serotonin (5HT) reuptake transporter increases vulnerability to psychosocial stressors, females were also genotyped and were then classified as socially dominant having both alleles for the long promoter variant or having at least one allele for the short promoter variant or classified as socially subordinate having the long variant or subordinate having the short variant. Neonatal treatments were not balanced for social status or genotype so analyses were performed separately. Although the neonatal treatments reduced GH secretion postnatally and through the juvenile period, neither growth nor sexual maturation were affected. In contrast, the retrospective analysis showed sexual maturation was delayed significantly in subordinate females carrying at least one allele of the short promoter variant in the gene encoding the serotonin reuptake transporter and this delay was associated with reduced GH and leptin secretion during the juvenile phase but not with differences in growth velocities from birth. These data suggest that decreased neonatal GH secretion does not adversely affect sexual maturation but that polymorphisms in the gene encoding the 5HT transporter modulate the adverse consequences of social subordination on the timing of puberty in female rhesus monkeys.
Keywords: neonate, puberty, social status, 5HTTLPR, Lupron, GH deficiency, rhesus monkey
Introduction
While the understanding of the common neural pathways [1–3] that drive the appropriate release of GnRH from the medial basal hypothalamus to initiate puberty are well accepted [4–7], the factors that influence the variation in pubertal timing are less clear. Prepubertal growth or factors that regulate growth have long been considered important for timing maturation of the reproductive axis. Indeed, changes in hormones that either stimulate skeletal or muscular growth [8–12] or increase in response to accumulation of body fat [13–15] explain some of the variation in puberty timing, suggesting these can act centrally to influence systems regulating developmental increases in GnRH [16, 17].
While focus on the juvenile period has provided insights into how growth-related cues affect pubertal timing, birth weight and postnatal growth may also be important. Clinical data indicate that low birth weight followed by rapid catch-up growth is associated with increases insulin-like growth factor (IGF) I secretion and early puberty [18, 19]. These children are taller, heavier, and have more fat than age match normal birth weight children [20]. Other epidemiological data indicate that girls heavier at birth have early puberty compared with normal birth weight girls showing similar patterns of growth during infancy [21]. In an analysis of birth weight and growth patterns of female monkeys, low birth weight is associated with blunted postnatal and juvenile growth and later age at fertility [22]. The key variable in these studies appears to be the growth velocity during infancy that is sustained into the juvenile period. Additional support for the notion that growth during infancy may be important is provided by studies showing the experimental manipulation of the neonatal hypothalamic – pituitary – gonadal (HPG) axis in male monkeys affects growth and puberty. Males treated with a GnRH analog during the neonatal period have blunted growth and delayed puberty [23, 24]. The mechanism by which GnRH analog treatment during the neonatal period reduces growth is not clear but could be due to altered gonadal steroid stimulation of the growth hormone (GH) – IGF1 axis.
For children, social factors can also influence growth and, thus, puberty timing. Again, epidemiological data on adolescents living in urbanized settings indicate girls in families with higher socio-economical status (SES) have earlier menarche preceded by accelerated growth [19, 25–27]. While access to better nutrition may explain the positive relationship between higher SES and early puberty [19], SES and caloric intake is complex. Indeed, other data indicate that in industrial societies, children from families of low SES may consume high caloric, inexpensive foods that accelerates weight gain and body fat accumulation [28–31], advancing the age of puberty [32]. In these cases, indices of body fat, such as BMI, are better predictors of puberty timing than SES [33]. However, adolescent girls who show an increased vulnerability to psychosocial stress have an increased incidence of anovulation [34]. Similarly, studies of socially-living rhesus monkeys, where diets are limited to standard low fat, high fiber meals, indicate social status is a robust predictor of puberty timing [35] and reproductive success [36, 37]. Male rhesus monkeys from socially subordinate matrilines have delayed puberty [23, 38] while females from subordinate matrilines show a significantly later age at first ovulation [35, 39–41]. Although weight at first ovulation is similar between the later maturing subordinates compared with the earlier maturing dominant females, it is not known whether these differences are predicted from growth during infancy.
Although social status accounts for a significant amount of the variance in puberty timing in rhesus monkey groups, there are nevertheless differences in the tempo of maturation between females of similar social dominance ranks. For example, 42% of dominant and 88% of subordinate females show a later age at first ovulation [35]. These data suggest that factors that modify an individual’s response to social status may further explain variance in pubertal timing. In this regard, the variable number tandem repeat polymorphisms in the length of the promoter region of the SLC6A4 gene that encodes the serotonin reuptake transporter could be important. The short (s) promoter length 5HTTLPR has diminished transcriptional activity compared to the long (l) promoter length variant [42], and when present in humans, increases the incidence of affective disorders in response to life stressors [43–46]. Homologous promoter length variations in the SLC6A4 gene with reduced transcriptional activity are also present in rhesus monkeys [42, 47]. The presence of the s 5HTTLPR is associated with greater emotional reactivity in juvenile monkeys [48–50] and potentiates the adverse consequence of social subordination on metabolic and anthropometric measures in adult females [51]. Previous studies of rhesus monkeys also indicate that females with the s variant 5HTTLPR show reduced ovulatory frequency, lower body weights, and reduced serum levels of leptin compared with those with homozygous for the long promoter variant [52] and the 5HTTLPR accounts for some variation in the timing of reproductive output in males [53]. Because the 5HTTLPR is associated with differences in reactivity to social environments, it is possible that these polymorphisms interact with social status to influence the timing of reproductive maturation.
Using a rhesus monkey model, the present analysis combines both a prospective study to determine how birth weight and growth during infancy predict the timing of pubertal events and a separate retrospective study to determine how social status and SCL6A4 polymorphisms influence pubertal timing. The neonatal period was manipulated in one of two ways compared to control females. One group was treated continuously with a somatostatin analog to decrease GH secretion while the other was treated continuously with a GnRH analog to block the neonatal activation of the pituitary-gonadal axis. Although not as robust as occurs in males, the HPG axis is active during the postnatal period in females [54, 55]. Because postnatal treatment with a GnRH analog blunts growth in male monkeys [23, 24], we tested the hypothesis that such treatment would slow growth in females in a manner similar to that as the inhibition of GH secretion. In addition, given the importance of social status as a predictor of age at first ovulation and the possibility that this may be influenced by 5HTTLPR polymorphisms, data were analyzed separately to determine if growth and puberty is slowed significantly in subordinate females with the s-variant 5HTTLPR genotype.
Methods
Female rhesus monkeys (Macaca mulatta) were subjects. All females were members of established social groups maintained at the Yerkes National Primate Research Center Field Station. These groups varied in size from 25 to 85 animals and each contained one to two adult males, multiple adult females and their juvenile or infant offspring. Animals were fed a standard monkey diet (Purina Mills Lab Diet #5037, containing 13% of calories from fat, 18% from protein, and 69% from carbohydrates) and received seasonal fruit and vegetables daily. The Emory University Animal Care and Use Committee in accordance with the Animal Welfare Act and the U.S. Department of Health and Human Services “Guide for Care and Use of Laboratory Animals” approved the protocol.
On the day of birth, animals were randomly assigned to one of three treatment groups: control (Con), depot Lupron treated (Lup), or Sandostatin LAR treated (GHx). Control animals (n = 6) were weighed and injected SC were saline (0.25 ml) that continued every 25 days until the last treatment at 225 days of age. Lup females (n = 6) were weighed and injected with 750 μg/kg SC Depot Lupron (Tap Pharmaceuticals) every 25 days until the last treatment at 225 days of age. This dose effectively blocks activation of the pituitary – gonadal axis in juvenile female monkeys [56, 57] and arrests precocious puberty in girls [58]. GHx females (n = 6) were weighed and injected with 625 μg/kg, IM of Sandostatin LAR (Sandoz Pharmaceuticals) every 25 days until the last treatment at 225 days of age. This dose produces GH deficiency in juvenile female monkeys [56]. Animals received their respective treatments every 25 days with the last treatment given at 225 days or 7.4 mo of age. Because both Depot Lupron and Sandostatin LAR sustain serum levels of the drug for 28 days, treatments effectively ended at 8.3 mo of age (252 days of age).
The relative dominance status of each female in her social group was defined by the outcome of dyadic interactions observed opportunistically throughout the study period [59]. Social status is determined by membership in a matriline, as infants acquire the rank of their mothers [59]. However, as the animals age and begin to engage in more agonistic interactions, their respective dominance positions become evident. Because the specific number of animals within a group varied amongst the subjects, it was not possible to assign individual numerical ranks to females. Consequently, females were classified as dominant or subordinate depending whether their position was in the top half or bottom half of the group. It was not possible to further categorize females as high, middle, or low ranking as the number of animals in some cells in the social status by genotype matrix would have had one subject, precluding any meaningful analysis.
5HTTLPR polymorphisms were determined as described previously for rhesus monkeys [42]. Specifically, DNA was extracted from whole blood using the Pure Gene Blood Kit (Gentra, D-4000). Polymorphisms in the promoter region of the SCL6A4 gene were identified following amplification of the relevant gene segments by polymerase chain reaction using the oligonucleotide primers fwd (cag ggg aga tcc tgg gag gga) and rev (ggc gtt gcc gct ctg aat gc) based on the protocol described previously. The “s” amplicon (398bps) and the “l” amplicon (419bps) were separated on an agarose gel containing ethidium bromide and identified by direct visualization. Because the l/s genotype produces a similar phenotype as the s/s genotype on most measures [47, 49], females carrying at least one s allele were categorized as having an “s-variant” genotype. Because subjects were not selected a priori on the basis of social status and 5HTTLPR polymorphisms, social status classification and 5HTTLPR were not balanced across neonatal treatments and the effects of each were analyzed separately (see below). A description of the subjects is contained in Table 1.
Table 1.
Samples sizes for the prospective analysis of neonatal treatments and the retrospective analysis of social status – genotype effects on pubertal timing.
| Social Status X 5HTTLPR | |||||
|---|---|---|---|---|---|
| Dominant | Subordinate | ||||
| Neonatal Treatment | Sample Size | l/l | s-variant | l/l | s-variant |
| Control | 6 | 1 | 0 | 3 | 2 |
| Lupron-treated | 6 | 1 | 2 | 0 | 3 |
| Sandostatin Treated | 6 | 2 | 1 | 1 | 2 |
| Sample sizes | 18 | 4 | 3 | 4 | 7 |
Females were studied from birth through first ovulation
The primary outcome measures were reproductive and anthropometric. Puberty was characterized in several ways. In addition to menarche, assessed from daily observations, the age at the initial perineal swelling and coloration was recorded. For rhesus monkeys, this typically proceeds menarche and is an external indication estradiol secretion has commenced [60]. First ovulation was determined in all females and the interval from menarche to first ovulation was considered as the tempo of puberty [61]. Body weights were obtained at birth and monthly thereafter. Heights were obtained every 90 days while the females were anesthetized (10 mg/kg ketamine hydrochloride). Females were placed in a supine position with the legs straightened on a long piece of paper and a mark was made at the top of the head and at bottom of the heel. Two individuals measured the distance using MHC vernier calipers and the mean of the two measurements was calculated for an animal’s height. Body mass index was calculated as body weight in kg divided height in m2.
Serum samples were obtained by femoral venipuncture of infants following removal from her mother. By 4 mo of age, females had become habituated to allow venipuncture of the saphenous vein. Conscious venipuncture of captive acclimated rhesus monkeys does not adversely affect limbic – HP – adrenal (LHPA) arousal, puberty, or reproductive performance [62–64]. Morning, non-fasted samples were obtained once a month from birth through 18 months of age, once weekly until menarche, and twice weekly until first ovulation was confirmed. Samples obtained at 6 and 7 mo were assayed for GH to determine whether either of the neonatal treatments affected GH secretion. Nocturnal samples, collected at 18 and 24 mo of age on each subjected were also assayed for GH. To better estimate GH secretion at these ages, samples were obtained at 2200 ans 2230 hr and the average GH value between these two time points was used for analysis. Other hormone analyses were focused on the interval from 24 through ~32 mo of age, as this represents the age range from pre-menarche through the earliest age of first ovulation in outdoor housed rhesus monkeys at our facility [64]. Daytime samples obtained from 24 through ~32 mo of age were also assayed for leptin and cortisol. Following menarche, all samples were assayed for progesterone. First ovulation was inferred from a sustained rise in serum progesterone (>1 ng/ml for >5 days). Short luteal phase cycles were identified by a blunted rise in progesterone (1 to 3 ng/ml) for 4 to 6 days followed by the appearance of menstruation [8].
All assays were performed in the YNPRC Biomarkers Core Lab. Progesterone was measured using a previously described radioimmunoassay (RIA) that employs a commercially available kit from Diagnostic Products Corporation (Los Angeles CA) [52]. The assay has a sensitivity of 0.10 ng/ml with an inter- and intra-assay CV of 8.14% and 7.73%, respectively. Serum GH was determined by a commercially available ELISA (Diagnostic Systems Laboratory (DSL), Webster TX) having a sensitivity of 0.10 ng/ml using 20 μl of serum with an inter and intra assay CV of 9.13% and 6.33%, respectively. Serum leptin were measured by RIA using a commercially available kit validated for nonhuman primates (Linco, St. Louis MO). Assaying 100 μl, the assay has a range of 0.5 to 100 ng/ml. Intra-assay CVs were 5.5% and inter-assay were 8.8%. Serum levels of cortisol were measured by RIA with a kit from DSL. The assay has a sensitivity of 0.10 ng/ml with an inter- and intra-assay coefficient of variation (CV) of 6.3% and 9.1%, respectively. Data were summarized as the mean ± sem. As noted above, neither social status nor 5HTTLPR genotype was balanced across the neonatal treatment groups (Con, Lup, or GHx) so data were analyzed separately. The first analysis tested the hypothesis that neonatal treatments with Lurpon or Sandostatin were significantly slow growth and delay puberty. The separate retrospective analysis tested the hypothesis that social subordination, exacerbated by the presence of the s-variant allele in the SCL6A4 gene would significantly slow growth and delay puberty. Data were analyzed with ANOVA models. Tests having a p < 0.05 were considered significant. A Bonferroni adjustment (p = .05/n) was used for n pair wise comparisons if interaction terms are significant. Data were transformed, as needed, to produce homogeneity of variance. Because sample size for the social status by 5HTTLPR analyses was small, we also calculated effects size that measures the magnitude of a treatment effect independent of sample size. Effect sizes >0.8 are considered large, with percentage of overlap in the distribution of measures between two populations becoming smaller as effect size increases [65].
Results
Consequences of neonatal treatments
As shown in Table 2, neonatal concentrations of GH were significantly affected by the treatments (F2, 15 = 3.85, p = 0.04). Serum levels of morning GH were significantly higher in Con compared to GHx females, with concentrations in Lup females being intermediate. While birth weight did not vary significantly between treatment groups (F 2, 15 = 3.15, p = 0.06), weight gain during the 8 mo treatment interval was significantly greater in Con compared to Lup females, with GHx females being intermediated (F 1, 15 = p = 0.03). In contrast, neither height at birth (F2, 15 = 0.62, p = 0.53) nor the increase in height during the 8-month treatment period (F2, 15 = 0.49, p = 0.62) varied significantly between groups.
Table 2.
Developmental parameters (mean ± sem) for females treated neonatally with saline (Con), depot Lupron (Lup), or Sandostatin LAR (GHx). For a given measure, groups with different letters are significantly different from one another (p < 0.05).
| Parameter | Con n = 6 | Lup n = 6 | GHx n = 6 |
|---|---|---|---|
| Birth weight (kg) | 0.45 ± 0.02 | 0.43 ± 0.03 | 0.40 ± 0.01 |
| Birth Height (cm) | 29.9 ± 0.5 | 30.1 ± 0.4 | 29.0 ± 0.4 |
| Weight gain | |||
| Birth – 8 mo | 1.12 ± 0.052 | 0.89 ± 0.07b | 1.05 ± 0.06a, b |
| 8 – 30 mo | 2.57 ± 0.10 | 2.49 ± 0.15 | 2.58 ± 0.10 |
| Height Gain | |||
| Birth – 8 mo | 18.5 ± 0.4 | 17.5 ± 0.5 | 18.4 ± 1.1 |
| 8 – 30 mo | 46.6 ± 0.5 | 47.1 ± 1.2 | 47.4 ± 0.6 |
| GH (ng/ml): birth – 8 mo | 3.43 ± 1.06a | 1.25 ± 0.42a, b | 0.67 ± 0.23b |
| Nocturnal GH (ng/ml) | |||
| 18 mo | 4.42 ± 1.01a | 1.30 ± 0.40c | 1.89 ± 0.49b |
| 24 mo | 8.16 ± 1.19a | 5.82 ± 1.51a, b | 4.38 ± 0.41b |
| Cortisol (μg/dl): birth – 8 mo | 25.1 ± 3.6 | 29.3 ± 3.89 | 24.6 ± 3.84 |
| Puberty | |||
| Age at swelling (mo) | 30.42 ± 1.10 | 29.34 ± 0.49 | 29.64 ± 1.55 |
| Menarche age (mo) | 32.81 ± 0.81 | 33.04 ± 2.22 | 31.01 ± 1.85 |
| First ovulation age (mo) | 35.54 ± 2.14 | 37.62 ± 2.57 | 36.71 ± 2.13 |
| Tempo (mo) | 5.12 ± 1.78 | 8.27 ± 2.26 | 7.07 ± 2.05 |
As can be seen in Table 2, the neonatal treatments had no effect on any of these same parameters of puberty. Age at the appearance of secondary sexual characteristics, measured by perineal swelling and coloration, did not vary among the three groups (F2, 15 = 0.18, p = 0.84). In addition, neither age at menarche (F2, 15 = 0.68, p = 0.68) nor was age at first ovulation (F2, 15 = 0.21, p = 0.82) differed significantly as a function of the neonatal treatments. Consequently, the tempo of maturation, defined as the interval from menarche to first ovulation, was also not affected by the neonatal treatments (F2, 15 = 0.61, p = 56).
Body growth from the end of treatment at 8 mo through 30 mo of age was not affected by neonatal treatments, as the increase in body weight (F2, 15 = 0.18, p = 0.84) and height (F2, 15 = 0.25, p = 0.78) was not significantly different among the groups. In contrast, a significant neonatal treatment age interaction was observed for serum GH (F2, 15 = 8.36, p < 0.01). At 18 mo of age, nocturnal GH levels were significantly higher in Con compared with GHx females that, in turn, were significantly higher than Lup-treated females. At 24 mo of age, nocturnal GH concentrations were still significantly higher in Con compared with GHx females, with animals treated neonatally with Lupron being intermediate. Serum leptin in the months prior to and following menarche (25 through 30 mo of age) also did not vary significantly between the three neonatally treated groups (F2, 15 = 0.43, p = 0.66, data not shown). Finally, serum levels of cortisol did not vary significantly between the three groups during the neonatal treatment period (Table 2; F 2, 15 = 0.52, p = 0.61). Serum levels did increase significantly in all females during this period (F 5, 75 = 7.58, p < 0.05), from average concentrations at birth of 18.66 ± 2.22 μg/dl to 29.46 ± 2.63 μg/dl at the end of the neonatal treatment period.
Importance of social status – 5HTTLPR
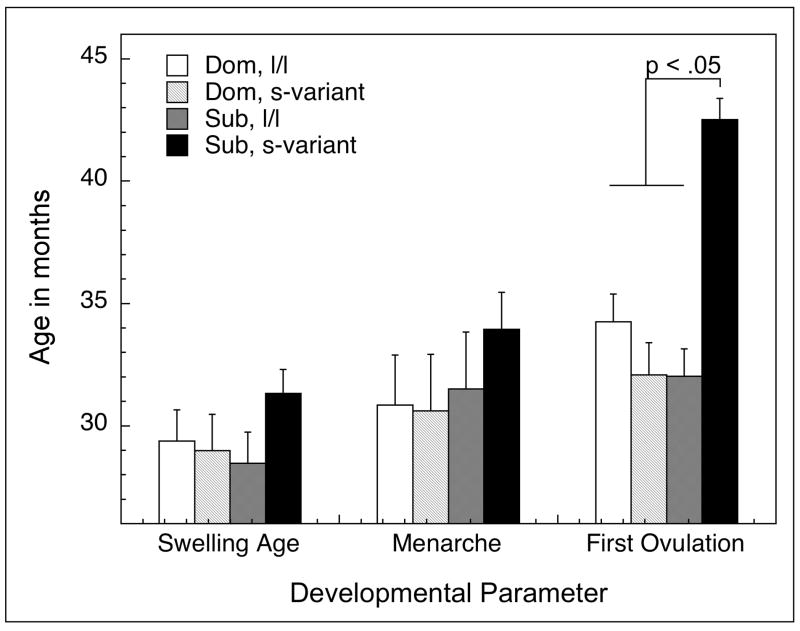
Reproductive maturation was significantly influenced by the interaction of social status and 5HTTLPR genotype (Figure 1). The age at the onset of perineal swelling was not influenced by a female’s social status during development (F1, 14 = 0.32, p = 0.58), genotype (F1, 14 = 0.96, p = 0.35), nor the interaction of status and genotype (F1, 14 = 1.67, p = 0.22). Similarly, age at menarche was not influenced by social status (F1, 14 = 1.02, p = 0.33), genotype (F1, 14 = 0.30, p = 0.59), or a status by genotype interaction (F1, 14 = 0.46, p = 0.51). In contrast, age at first ovulation occurred significantly earlier in dominant females (F1, 14 = 13.40, p < 0.01) and those with an l/l genotype (F1, 14 = 13.82, p < 0.01). However, the variance in the age at first ovulation was best explained by a significant status by genotype interaction (F1, 14 = 32.04, p < 0.01), as subordinate females with the s-variant genotype had first ovulation at a significantly older age than all other females. The computation of effect size for age at first ovulation for these subordinate females compared to all other females was 1.81. Consequently, the tempo of puberty was significantly longer (F1, 14 = 7.72, p = 0.02) in subordinate s-variant females (11.2 ± 1.3 mo) compared subordinate females with an l/l genotype (3.6 ± 1.8 mo), or dominant l/l (5.2 ± 1.8 mo) or s-variant females (3.1 ± 2.0 mo). The effect size for the tempo of maturation between subordinate, s-variant females and all other females was also large (1.47).
Figure 1.
Distribution of the mean ± SEM age of initial perineal swelling, menarche, and first ovulation in females categorized as dominant (dom) or subordinate (sub) being homogenous for the long promoter length variant or (l/l) or having at least one short promoter length allele (s-variant) polymorphism in the SCL6A4 gene.
Further analyses revealed that hormonal but not anthropometric measures were also affected by status and 5HRRLPR. As illustrated in Table 3, birth weights and heights were not significantly influenced by status (weight: F1, 14 = 0.46, p = 0.51; height: F1, 14 = 0.40, p = 0.54), genotype (weight: F1, 14 = 1.79, p = 0.20; height: F1, 14 = 0.29, p = 0.60), or status by genotype interaction (weight: F1, 14 = 0.10, p = 0.99; height: F1, 14 = 3.36, p = 0.09). Similarly, neither body weight gain nor accumulation of height through 30 mo of age was influenced by status (weight: F1, 14 = 0.04, p = 0.84; height: F1, 14 = 1.34, p = 0.27), genotype (weight: F1, 14 = 0.08, p = 0.78; height: F1, 14 = 0.90, p = 0.36), or by a status - genotype interaction (weight: F1, 14 = 1.49, p = 0.24; height: F1, 14 = 1.03, p = 0.33). Effect sizes comparing subordinate, s-variant females to all other females was also small for the gain in both weight (0.21) and height (0.34).
Table 3.
Mean ± sem anthropometric measures of females classified as dominant or subordinate having either the l/l or s-variant SCL6A4 genotype. For a given measure, groups with different letters are significantly different from one another (p < 0.05).
| Parameter | Dominant | Subordinate | ||
|---|---|---|---|---|
| l/l (n = 4) | s-variant (n = 3) | l/l (n = 4) | s-variant (n = 7) | |
| Birth weight (kg) | 0.43 ± 0.02 | 0.40 ± 0.02 | 0.44 ± 0.02 | 0.41 ± 0.01 |
| Birth Height (cm) | 29.2 ± 0.5 | 29.8 ± 0.6 | 30.5 ± 0.5 | 29.2 ± 0.4 |
| Weight gain | ||||
| Birth – 30 mo | 3.49 ± 0.20 | 3.67 ± 0.23 | 3.76 ± 0.20 | 3.47 ± 0.15 |
| Height Gain | ||||
| Birth – 30 mo | 35.5 ± 0.9 | 37.1 ± 0.9 | 35.3 ± 0.9 | 35.3 ± 0.6 |
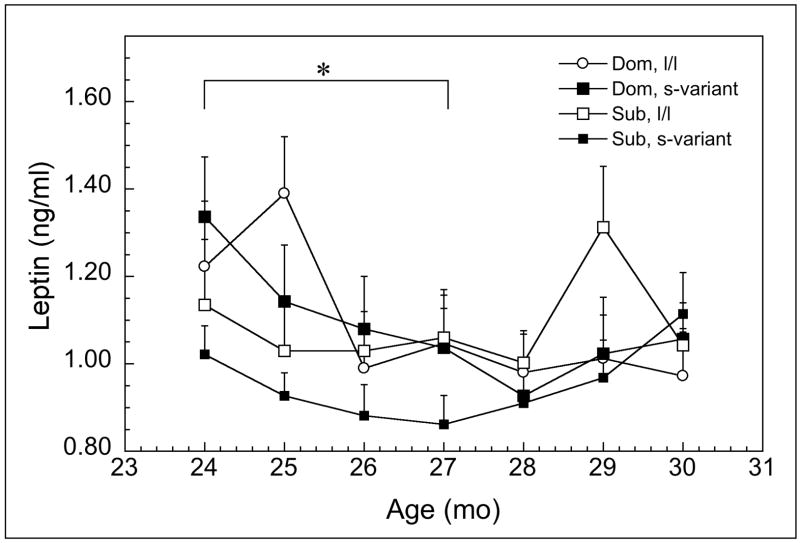
In contrast to the anthropometric data, nocturnal concentrations of GH (Figure 2) were significantly influenced a status by 5HTTLPR interaction (F1, 14 = 9.32, p = 0.01) that did not vary with advancing age (F1, 14 = 0.16, p = 0.70). As can be seen, the main effect of genotype is significant (F1, 14 = 33.31, p < 0.01), with levels higher in females homozygous for the l-allele. However, GH is significantly lower in subordinate s-variant females compared to all other females and this was reflected in a large effect size of 1.25 for these females compared to all others. Serum leptin also varied significantly between dominant and subordinate females between 24 and 30 months of age (Figure 3; F6, 84 = 2.69, p = 0.02). Post hoc analyses revealed that serum concentrations were significantly lower in subordinate, s-variant females between 24 and 27 mo of age compared with all other females, again reflected in an effect size of 0.98.
Figure 2.
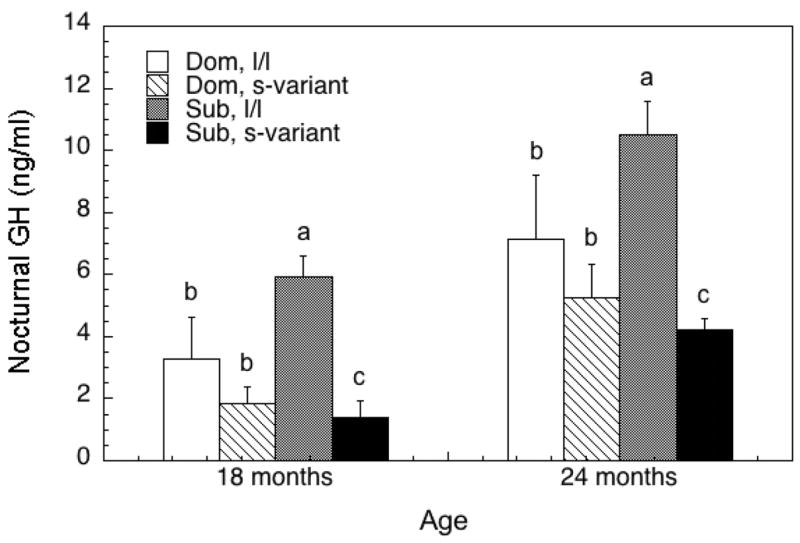
Mean ± SEM serum concentrations of nocturnal GH (ng/ml) at 18 and 24 months of age for dominant (dom) or subordinate (sub) being homogenous for the long promoter length variant or (l/l) or having at least one short promoter length allele (s-variant) polymorphism in the SCL6A4 gene. Different letters at each age indicate groups are significantly different (p < 0.05).
Figure 3.
Mean ± SEM serum concentrations of morning leptin (ng/ml) from 24 through 30 months af age for dominant (dom) or subordinate (sub) being homogenous for the long promoter length variant or (l/l) or having at least one short promoter length allele (s-variant) polymorphism in the SCL6A4 gene. Asterisks indicate subordinate, s-variant females had significantly lower concentrations than other groups (p < 0.05).
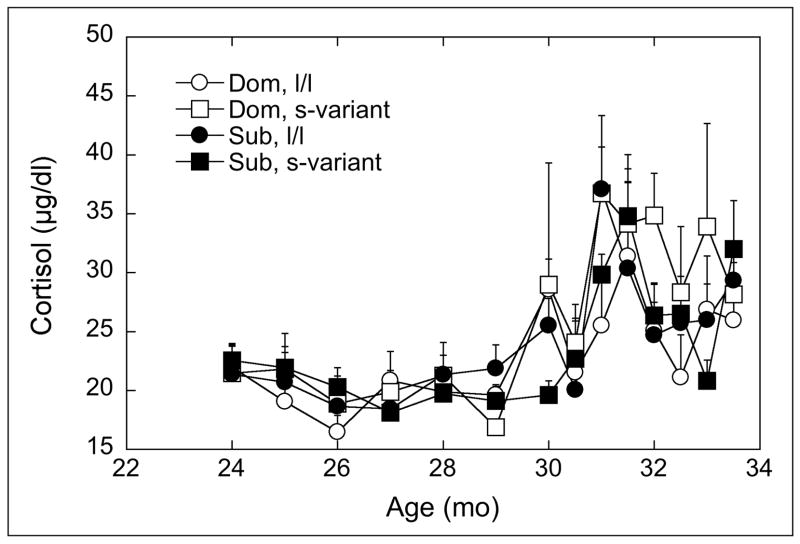
Analysis of developmental patterns of morning cortisol concentrations indicated that the significantly age dependent increase during the adolescent period (Figure 4, F 13, 182 = 11.90, p < 0.05) was not affect by social status (F2, 15 = 1.00, p = 0.34) or 5HTTLPR (F2, 15 = 0.34, p = 0.57) nor their interaction with age (F13, 182 = 0.98, p = 0.48).
Figure 4.
Mean ± SEM serum concentrations of morning cortisol (μg/dl) from 24 through 33.5 months of age for dominant (dom) or subordinate (sub) being homogenous for the long promoter length variant or (l/l) or having at least one short promoter length allele (s-variant) polymorphism in the SCL6A4 gene. Different letters at each age indicate groups are significantly different (p < 0.05).
Because the neonatal treatment groups were not formed on the basis of social status and 5HTTLPR, the interactive effects of these treatments with social status and genotype cannot be statistically evaluated. However, it appeared that neonatal treatments would have had little additive effect. Of the seven subordinate females with the s-variant genotype who ovulated significantly later than all other females, two were control, three were Lupron treated, and two were Sandostatin-treated.
Discussion
The prospective analysis revealed the neonatal suppression of the HPG or GH axis decreased the developmental secretion of GH but had no adverse effects on growth or the timing of puberty. However, reanalyzing the data to determine whether social status interacted with polymorphisms in the gene encoding the 5HT transporter influenced development indicated the socially subordinate females carrying the s-variant allele in the SCL6A4 gene have delayed puberty and this is associated with reduced concentrations of GH and leptin during the prepubertal period. While these analyses do not support the hypothesis that perturbations in neonatal GH secretion influence pubertal timing, they do show how social factors and genetic polymorphisms can interact to influence the occurrence of first ovulation in female rhesus monkeys and this effect is associated with significant differences in growth-related signals.. The rationale for manipulating the HPG and GH axes neonatally was based on data from children [20, 21] and monkeys [22] that postnatal growth velocities appeared to influence the timing of puberty possibly, due to changes in GH and IGF1 [18, 19]. Furthermore, suppression of the neonatal HPG axis in male monkeys slowed growth and delayed puberty [23, 24]. Thus, our intent was to disrupt postnatal growth in females by suppressing the either the HPG or the GH axis. However, we saw no effect of either the GnRH analog or somatostatin analog treatments during the neonatal interval on the eventual parameters of growth or puberty in female monkeys. While GH secretion was lowered significantly neonatally and during prepuberty by both treatments compared to saline, no other effects were evident. Given the sex differences in the neonatal activation of the HPG axis in rhesus monkeys [54, 55], perhaps it is not surprising that the neonatal administration of Lupron had no effect on eventual puberty as the axis shows much greater activity in males compared with females. What the data do indicate that the lower serum GH concentrations throughout development produced by Lupron and Sandostatin postnatal do not adversely affect growth or eventual puberty. Although significantly lower than controls, these GH concentrations were not suppressed to the degree of those produced in other monkey models of juvenile GH deficiency that does result in a slowing of growth and a delay in first ovulation [10, 56]. Taken together, these data suggest there may be a critical lower limit below of GH secretion below which normal growth and puberty is compromised. Nevertheless, the data from the present study clearly indicate that perturbations of the GH and HPG axes during the neonatal period have no adverse effects on maturation in female monkeys.
In contrast, the retrospective analyses not only confirms previous reports on male [23, 38] and female monkeys [35, 39–41] that social subordination delays puberty but extends these findings to indicate that this effect of subordination occurs predominantly in those subordinate females having at least on s-allele in the encoding 5HT transporter. These data are consistent with a recent report of an increased incidence of functional hypothalamic amenorrhea is girls experienced an increased incidence of stress-induced psychopathology [34]. In macaque groups, social subordination is largely maintained through continual harassment and the threat of aggression from dominant towards subordinate animals [59]. As a result, socially subordinate adult females are characteristically hypercortisolemic secondary to a dysregulation of the LHPA axis [66–68]. Because the presence of the s allele is associated with the increased response to psychosocial stressors in rhesus monkeys [47–50, 69], we expected that morning cortisol concentrations would be highest in subordinate females with the s-variant genotype. Such a finding would have supported that hypothesis that activation of the LHPA axis accounted for the delayed puberty in this cohort [70, 71]. However, morning cortisol during the juvenile period did not vary significantly between the groups. Recognizing that this measure is an inadequate marker of LHPA activity, other assessments such as a dexamethasone suppression test to determine glucocorticoid negative feedback, may have better differentiated the groups [66]. Although anthropometric measures also did not differ as a function of social status and 5HTTLPR, morning GH and leptin concentrations were significantly lower in these slowly maturing females. It is possible that increased psychosocial stress accounted for these differences. CSF concentrations of somatostatin are higher [72] and the GH response to clonidine blunted [73] in macaques raised in an unpredictable environment, possibly due to a CRH increasing somatostatin [74–76]. CRH inhibits pulsatile GH release [77] and GHRH-induced GH secretion in rats [78, 79] while a CRH antagonist increases GH secretion and hypothalamic GHRH mRNA [80]. While the lower concentrations of serum leptin could be the result of stress-induced attenuation of food intake, lowering body fat stores [81], no differences in body weight or BMI scores were observed among the animals. Given similarities in body weight, it would seem differences in food intake and adequate nutrition would not explain the delayed puberty in the subordinate, s-variant females. However, the present analysis also cannot determine whether the lower levels of GH and leptin during in juvenile subordinates with the s-variant genotype played any role in the delayed occurrence of first ovulation. As noted above, experimentally induced deficits in juvenile concentrations of serum GH account for variation in pubertal timing [10, 56] and leptin administration advances puberty in female [82] but not male monkeys [83]. Furthermore, leptin administration restores LH secretion and ovulatory function in some women with functional hypothalamic anovulation [84]. Obviously further studies are needed to identify what signals mediate the slowing of puberty in these subordinate monkeys.
This retrospective analysis must be interpreted cautiously as it was severely under-powered. Furthermore, we must emphasize that our sample size is not adequate to determine the genetic contributions to behavior and physiology and it is entirely likely that the reproductive phenotypes examined in this study are influenced by many genes [85]. Indeed, recent analyses have begun to show variations in puberty timing are explained by polymorphisms in genes whose protein products affect GnRH secretion, including the insulin receptor subtype 1 (IRS1) [86], the gene encoding leptin (LEP) [87], as well the gene encoding kisspeptin (KISS1) [88] and its receptor (KISS1R) [89]. Furthermore, a polymorphism in the SHBG gene (SHBG), that may regulate bioavailability of estradiol, is associated with the timing of menarche [90]. In contrast, attempts to find associations between puberty timing and polymorphisms in the gene encoding the GnRH receptor (GnRHR) have not been successful [91]. The data from the present study show that a gene polymorphism that affects individual reactivity to socio-environmental stressors can also affect puberty, presumably through activation of the LHPA axis. It is likely that this polymorphism could act independently or synergistically with other gene polymorphisms to influence the timing of puberty by acting at multiple levels of the HPG axis. These present data can best serve as the foundation for broader linkage and association analyses to understand how social context and genes interact to time the onset of puberty [92]. Nevertheless, the data confirm the critical importance of the psychosocial environment for development and illustrate the potential of using socially housed macaques to understand gene by environment effects on behavior and physiology [93].
Acknowledgments
We appreciate the expert technical assistance of Jeff Fisher, Kathy Chikazawa, and Juliet Brown. This work was supported by HD35183, HD46501, and, in part, RR00165. The YNPRC is fully accredited by the American Association for Laboratory Animal Care International.
References
- 1.Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- 2.El Majdoubi M, Sahu A, Ramaswamy S, Plant TM. Neuropeptide Y: A hypothalamic brake restraining the onset of puberty in primates. Proc Natl Acad Sci U S A. 2000;97:6179–6184. doi: 10.1073/pnas.090099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasuya E, Nyberg CL, Mogi K, Terasawa E. A role of gamma-amino butyric acid (GABA) and glutamate in control of puberty in female rhesus monkeys: effect of an antisense oligodeoxynucleotide for GAD67 messenger ribonucleic acid and MK801 on luteinizing hormone-releasing hormone release. Endocrinology. 1999;140:705–712. doi: 10.1210/endo.140.2.6574. [DOI] [PubMed] [Google Scholar]
- 4.Wildt L, Marshall G, Knobil E. Experimental induction of puberty in the infantile female rhesus monkey. Science. 1980;207:1373–1375. doi: 10.1126/science.6986658. [DOI] [PubMed] [Google Scholar]
- 5.Terasawa E, Luchansky LL, Kasuya E, Nyberg CL. An increase in glutamate release follows a decrease in gamma aminobutyric acid and the pubertal increase in luteinizing hormone releasing hormone release in the female rhesus monkeys. J Neuroendocrinol. 1999;11:275–282. doi: 10.1046/j.1365-2826.1999.00325.x. [DOI] [PubMed] [Google Scholar]
- 6.Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- 7.Plant TM, Shahab M. Neuroendocrine mechanisms that delay and initiate puberty in higher primates. Physiol Behav. 2002;77:717–722. doi: 10.1016/s0031-9384(02)00924-1. [DOI] [PubMed] [Google Scholar]
- 8.Wilson ME. Premature elevation in serum insulin-like growth factor-I advances first ovulation in rhesus monkeys. J Endocrinol. 1998;158:247–257. doi: 10.1677/joe.0.1580247. [DOI] [PubMed] [Google Scholar]
- 9.Wilson ME, Chikazawa K, Fisher J, Mook D, Gould KG. Reduced growth hormone (GH) secretion prolongs puberty but does not delay the developmental increase in luteinizing hormone (LH) in the absence of gonadal negative feedback. Biology of Reproduction. 2004:71. doi: 10.1095/biolreprod.104.027656. [DOI] [PubMed] [Google Scholar]
- 10.Wilson ME, Tanner JM. Somatostatin analog treatment slows growth and the tempo of reproductive maturation in female rhesus monkeys. J Clin Endocrinol Metab. 1994;79:495–501. doi: 10.1210/jcem.79.2.7519192. [DOI] [PubMed] [Google Scholar]
- 11.Hiney JK, Srivastava V, Nyberg CL, Ojeda SR, Dees WL. Insulin-like growth factor I of peripheral origin acts centrally to accelerate the initiation of female puberty. Endocrinology. 1996;137:3717–3728. doi: 10.1210/endo.137.9.8756538. [DOI] [PubMed] [Google Scholar]
- 12.Danilovich N, Wernsing D, Coschigano KT, Kopchick JJ, Bartke A. Deficits in female reproductive function in GH-R-KO mice; role of IGF1. Endocrinology. 1999;140:2637–2640. doi: 10.1210/endo.140.6.6992. [DOI] [PubMed] [Google Scholar]
- 13.Wilson ME, Fisher J, Chikazawa K, Yoda R, Legendre A, Mook D, Gould KG. Leptin administration increases nocturnal concentrations of luteinizing hormone and growth hormone in juvenile female rhesus monkeys. J Clin Endocrinol Metab. 2003;88:4874–4883. doi: 10.1210/jc.2003-030782. [DOI] [PubMed] [Google Scholar]
- 14.Foster DL, Jackson LM. Integration of leptin with other signals regulating the timing of puberty. In: Henson MC, Castracane VD, editors. Leptin and Reproduction. New York: Plenum Publishers; 2003. pp. 169–186. [Google Scholar]
- 15.Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology. 1997;138:855–858. doi: 10.1210/endo.138.2.5054. [DOI] [PubMed] [Google Scholar]
- 16.Daftary SS, Gore AC. The hypothalamic insulin-like growth factor-1 receptor and its relationship to gonadotropin-releasing hormones neurones during postnatal development. J Neuroendocrinol. 2004;16:160–169. doi: 10.1111/j.0953-8194.2004.01149.x. [DOI] [PubMed] [Google Scholar]
- 17.Foster DL, Nagatani S. Physiological perspectives on leptin as a regulator of reproduction: role in timing puberty. Biol Reprod. 1999;60:205–215. doi: 10.1095/biolreprod60.2.205. [DOI] [PubMed] [Google Scholar]
- 18.Dunger DB, Ahmed ML, Ong KK. Early and late weight gain and the timing of puberty. Mol Cell Endocrinol. 2006;254–255:140–145. doi: 10.1016/j.mce.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Adair LS. Size at birth predicts age at menarche. Pediatrics. 2001;107:E59. doi: 10.1542/peds.107.4.e59. [DOI] [PubMed] [Google Scholar]
- 20.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. Bmj. 2000;320:967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.dos Santos Silva I, De Stavola BL, Mann V, Kuh D, Hardy R, Wadsworth ME. Prenatal factors, childhood growth trajectories and age at menarche. Int J Epidemiol. 2002;31:405–412. doi: 10.1093/ije/31.2.405. [DOI] [PubMed] [Google Scholar]
- 22.Coe CL, Shirtcliff EA. Growth trajectory evident at birth affects age of first delivery in female monkeys. Pediatr Res. 2004;55:914–920. doi: 10.1203/01.PDR.0000125259.45025.4D. [DOI] [PubMed] [Google Scholar]
- 23.Mann DR, Akinbami MA, Gould KG, Paul K, Wallen K. Sexual maturation in male rhesus monkeys: importance of neonatal testosterone exposure and social rank. J Endocrinol. 1998;156:493–501. doi: 10.1677/joe.0.1560493. [DOI] [PubMed] [Google Scholar]
- 24.Mann DR, Akinbami MA, Gould KG, Tanner JM, Wallen K. Neonatal treatment of male monkeys with a gonadotropin-releasing hormone agonist alters differentiation of central nervous system centers that regulate sexual and skeletal development. J Clin Endocrinol Metab. 1993;76:1319–1324. doi: 10.1210/jcem.76.5.8496324. [DOI] [PubMed] [Google Scholar]
- 25.Qamra SR, Mehta S, Deodhar SD. A mixed-longitudinal study on the pattern of pubertal growth: relationship to socioeconomic status and caloric-intake--IV. Indian Pediatr. 1991;28:147–156. [PubMed] [Google Scholar]
- 26.Henneberg M, Louw GJ. Average menarcheal age of higher socioeconomic status urban Cape coloured girls assessed by means of status quo and recall methods. Am J Phys Anthropol. 1995;96:1–5. doi: 10.1002/ajpa.1330960102. [DOI] [PubMed] [Google Scholar]
- 27.Khan AD, Schroeder DG, Martorell R, Rivera JA. Age at menarche and nutritional supplementation. J Nutr. 1995;125:1090S–1096S. doi: 10.1093/jn/125.suppl_4.1090S. [DOI] [PubMed] [Google Scholar]
- 28.Gnavi R, Spagnoli TD, Galotto C, Pugliese E, Carta A, Cesari L. Socioeconomic status, overweight and obesity in prepuberal children: a study in an area of Northern Italy. Eur J Epidemiol. 2000;16:797–803. doi: 10.1023/a:1007645703292. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Zhang Q. Are American children and adolescents of low socioeconomic status at increased risk of obesity? Changes in the association between overweight and family income between 1971 and 2002. Am J Clin Nutr. 2006;84:707–716. doi: 10.1093/ajcn/84.4.707. [DOI] [PubMed] [Google Scholar]
- 30.Oliver LN, Hayes MV. Neighbourhood socio-economic status and the prevalence of overweight Canadian children and youth. Can J Public Health. 2005;96:415–420. doi: 10.1007/BF03405180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodman E, Slap GB, Huang B. The public health impact of socioeconomic status on adolescent depression and obesity. Am J Public Health. 2003;93:1844–1850. doi: 10.2105/ajph.93.11.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics. 2002;110:903–910. doi: 10.1542/peds.110.5.903. [DOI] [PubMed] [Google Scholar]
- 33.Rapkin AJ, Tsao JC, Turk N, Anderson M, Zeltzer LK. Relationships among self-rated tanner staging, hormones, and psychosocial factors in healthy female adolescents. J Pediatr Adolesc Gynecol. 2006;19:181–187. doi: 10.1016/j.jpag.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Bomba M, Gambera A, Bonini L, Peroni M, Neri F, Scagliola P, Nacinovich R. Endocrine profiles and neuropsychologic correlates of functional hypothalamic amenorrhea in adolescents. Fertil Steril. 2007;87:876–885. doi: 10.1016/j.fertnstert.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Wilson ME. Factors determining the onset of puberty. In: Gerrall AA, Moltz H, Ward IL, editors. Handbook of behavioral neurobiology: sexual differentiation. Vol. 11. New York: Plenum Press; 1992. pp. 275–312. [Google Scholar]
- 36.Wilson ME, Gordon TP, Bernstein IS. Timing of births and reproductive success in rhesus monkey social groups. J Med Primatol. 1978;7:202–212. doi: 10.1159/000459880. [DOI] [PubMed] [Google Scholar]
- 37.Johnson SE. Life history and the competitive environment: trajectories of growth, maturation, and reproductive output among chacma baboons. Am J Phys Anthropol. 2003;120:83–98. doi: 10.1002/ajpa.10139. [DOI] [PubMed] [Google Scholar]
- 38.Rose RM, Bernstein IS, Gordon TP, Lindsley JG. Changes in testosterone and behavior during adolescence in the male rhesus monkey. Psychosom Med. 1978;40:60–70. doi: 10.1097/00006842-197802000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz SM, Wilson ME, Walker ML, Collins DC. Social and growth correlates of the onset of puberty in female rhesus monkeys. Nutrition and Behavior. 1985;2:225 – 232. [Google Scholar]
- 40.Wilson ME, Walker ML, Gordon TP. Consequences of first pregnancy in rhesus monkeys. Am J Phys Anthropol. 1983;61:103–110. doi: 10.1002/ajpa.1330610111. [DOI] [PubMed] [Google Scholar]
- 41.Zehr JL, Van Meter PE, Wallen K. Factors Regulating the Timing of Puberty Onset in Female Rhesus Monkeys (Macaca mulatta): Role of Prenatal Androgens, Social Rank, and Adolescent Body Weight. Biol Reprod. 2004;72:1087–1094. doi: 10.1095/biolreprod.104.027755. [DOI] [PubMed] [Google Scholar]
- 42.Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poustka F, Bengel D, Mossner R, Riederer P, Heils A. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. Rapid communication. J Neural Transm. 1997;104:1259–1266. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- 43.Veenstra-VanderWeele J, Anderson GM, Cook EH., Jr Pharmacogenetics and the serotonin system: initial studies and future directions. Eur J Pharmacol. 2000;410:165–181. doi: 10.1016/s0014-2999(00)00814-1. [DOI] [PubMed] [Google Scholar]
- 44.Melke J, Landen M, Baghei F, Rosmond R, Holm G, Bjorntorp P, Westberg L, Hellstrand M, Eriksson E. Serotonin transporter gene polymorphisms are associated with anxiety-related personality traits in women. Am J Med Genet. 2001;105:458–463. doi: 10.1002/ajmg.1434. [DOI] [PubMed] [Google Scholar]
- 45.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 46.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 47.Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- 48.Bethea CL, Streicher JM, Coleman K, Pau FK, Moessner R, Cameron JL. Anxious Behavior and Fenfluramine-Induced Prolactin Secretion in Young Rhesus Macaques with Different Alleles of the Serotonin Reuptake Transporter Polymorphism (5HTTLPR) Behav Genet. 2004;34:295–307. doi: 10.1023/B:BEGE.0000017873.61607.be. [DOI] [PubMed] [Google Scholar]
- 49.Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- 50.Barr CS, Newman TK, Schwandt M, Shannon C, Dvoskin RL, Lindell SG, Taubman J, Thompson B, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci U S A. 2004;101:12358–12363. doi: 10.1073/pnas.0403763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jarrell H, Hoffman JB, Kaplan JR, Berga SL, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in females rhesus monkeys. Physiol Behav. 2008 doi: 10.1016/j.physbeh.2007.11.042. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoffman JB, Kaplan JR, Kinkead B, Berga SL, Wilson ME. Metabolic and reproductive consequences of the serotonin transporter promoter polymorphism (5-HTTLPR) in the adult female rhesus macaque (Macaca mulatta) Endocrine. 2007;31:202–211. doi: 10.1007/s12020-007-0017-8. [DOI] [PubMed] [Google Scholar]
- 53.Krawczak M, Trefilov A, Berard J, Bercovitch F, Kessler M, Sauermann U, Croucher P, Nurnberg P, Widdig A, Schmidtke J. Male reproductive timing in Rhesus macaques is influenced by the 5HTTLPR promoter polymorphism of the serotonin transporter gene. Biol Reprod. 2005;72:1109–1113. doi: 10.1095/biolreprod.104.038059. [DOI] [PubMed] [Google Scholar]
- 54.Pohl CR, deRidder CM, Plant TM. Gonadal and nongonadal mechanisms contribute to the prepubertal hiatus in gonadotropin secretion in the female rhesus monkey (Macaca mulatta) J Clin Endocrinol Metab. 1995;80:2094–2101. doi: 10.1210/jcem.80.7.7608261. [DOI] [PubMed] [Google Scholar]
- 55.Plant TM. A striking sex difference in the gonadotropin response to gonadectomy during infantile development in the rhesus monkey (Macaca mulatta) Endocrinology. 1986;119:539–545. doi: 10.1210/endo-119-2-539. [DOI] [PubMed] [Google Scholar]
- 56.Wilson ME, Chikazawa K, Fisher J, Mook D, Gould KG. Reduced growth hormone secretion prolongs puberty but does not delay the developmental increase in luteinizing hormone in the absence of gonadal negative feedback. Biol Reprod. 2004;71:588–597. doi: 10.1095/biolreprod.104.027656. [DOI] [PubMed] [Google Scholar]
- 57.Golub MS, Styne DM, Wheeler MD, Keen CL, Hendrickx AG, Moran F, Gershwin ME. Growth retardation in premenarchial female rhesus monkeys during chronic administration of a GnRH agonist (leuprolode acetate) Journal of Medical Primatology. 1997;26:248–256. doi: 10.1111/j.1600-0684.1997.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 58.Clemons RD, Kappy MS, Stuart TE, Perelman AH, Hoekstra FT. Long-term effectiveness of depot gonadotropin-releasing hormone analogue in the treatment of children with central precocious puberty. Am J Dis Child. 1993;147:653–657. doi: 10.1001/archpedi.1993.02160300059023. [DOI] [PubMed] [Google Scholar]
- 59.Bernstein IS. Dominance, aggression and reproduction in primate societies. J Theor Biol. 1976;60:459–472. doi: 10.1016/0022-5193(76)90072-2. [DOI] [PubMed] [Google Scholar]
- 60.Wilson ME, Gordon TP, Collins DC. Ontogeny of luteinizing hormone secretion and first ovulation in seasonal breeding rhesus monkeys. Endocrinology. 1986;118:293–301. doi: 10.1210/endo-118-1-293. [DOI] [PubMed] [Google Scholar]
- 61.Hartman CG. Reproduction. Annu Rev Physiol. 1952;14:499–518. doi: 10.1146/annurev.ph.14.030152.002435. [DOI] [PubMed] [Google Scholar]
- 62.Blank MS, Gordon TP, Wilson ME. Effects of capture and venipuncture on serum levels of prolactin, growth hormone and cortisol in outdoor compound-housed female rhesus monkeys (Macaca mulatta) Acta Endocrinol (Copenh) 1983;102:190–195. doi: 10.1530/acta.0.1020190. [DOI] [PubMed] [Google Scholar]
- 63.Walker ML, Gordon TP, Wilson ME. Reproductive performance in capture-acclimated female rhesus monkeys (Macaca mulatta) J Med Primatol. 1982;11:291–302. [PubMed] [Google Scholar]
- 64.Wilson ME, Gordon TP, Rudman CG, Tanner JM. Effects of a natural versus artificial environment on the tempo of maturation in female rhesus monkeys. Endocrinology. 1988;123:2653–2661. doi: 10.1210/endo-123-6-2653. [DOI] [PubMed] [Google Scholar]
- 65.Cohen JD. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 66.Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Sapolsky RM. Are subordinates always stressed? a comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- 67.Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biological Psychiatry. 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- 68.Wilson ME, Legendre A, Pazol K, Fisher J, Chikazawa K. Gonadal steroid modulation of the limbic-hypothalamic- pituitary-adrenal (LHPA) axis is influenced by social status in female rhesus monkeys. Endocrine. 2005;26:89–97. doi: 10.1385/ENDO:26:2:089. [DOI] [PubMed] [Google Scholar]
- 69.Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 70.Breen KM, Karsch FJ. Does cortisol inhibit pulsatile luteinizing hormone secretion at the hypothalamic or pituitary level? Endocrinology. 2004;145:692–698. doi: 10.1210/en.2003-1114. [DOI] [PubMed] [Google Scholar]
- 71.Ferin M. Clinical review 105: Stress and the reproductive cycle. J Clin Endocrinol Metab. 1999;84:1768–1774. doi: 10.1210/jcem.84.6.5367. [DOI] [PubMed] [Google Scholar]
- 72.Coplan JD, Trost RC, Owens MJ, Cooper TB, Gorman JM, Nemeroff CB, Rosenblum LA. Cerebrospinal fluid concentrations of somatostatin and biogenic amines in grown primates reared by mothers exposed to manipulated foraging conditions. Arch Gen Psychiatry. 1998;55:473–477. doi: 10.1001/archpsyc.55.5.473. [DOI] [PubMed] [Google Scholar]
- 73.Coplan JD, Smith EL, Trost RC, Scharf BA, Altemus M, Bjornson L, Owens MJ, Gorman JM, Nemeroff CB, Rosenblum LA. Growth hormone response to clonidine in adversely reared young adult primates: relationship to serial cerebrospinal fluid corticotropin-releasing factor concentrations. Psychiatry Res. 2000;95:93–102. doi: 10.1016/s0165-1781(00)00173-6. [DOI] [PubMed] [Google Scholar]
- 74.Mitsugi N, Arita J, Kimura F. Effects of intracerebroventricular administration of growth hormone-releasing factor and corticotropin-releasing factor on somatostatin secretion into rat hypophysial portal blood. Neuroendocrinology. 1990;51:93–96. doi: 10.1159/000125322. [DOI] [PubMed] [Google Scholar]
- 75.Katakami H, Arimura A, Frohman LA. Involvement of hypothalamic somatostatin in the suppression of growth hormone secretion by central corticotropin-releasing factor in conscious male rats. Neuroendocrinology. 1985;41:390–393. doi: 10.1159/000124207. [DOI] [PubMed] [Google Scholar]
- 76.Hisano S, Daikoku S. Existence of mutual synaptic relations between corticotropin-releasing factor-containing and somatostatin-containing neurons in the rat hypothalamus. Brain Res. 1991;545:265–275. doi: 10.1016/0006-8993(91)91295-c. [DOI] [PubMed] [Google Scholar]
- 77.Rivier C, Vale W. Corticotropin-releasing factor (CRF) acts centrally to inhibit growth hormone secretion in the rat. Endocrinology. 1984;114:2409–2411. doi: 10.1210/endo-114-6-2409. [DOI] [PubMed] [Google Scholar]
- 78.Frias J, Ruiz E, Ortega E. Effect of corticotropin releasing factor injected into the median eminence on growth hormone secretion in male rats. Neurochem Res. 1999;24:715–718. doi: 10.1023/a:1020719227235. [DOI] [PubMed] [Google Scholar]
- 79.Puertas A, Frias J, Ruiz E, Ortega E. Effect of CRF injected into the median eminence on GH secretion in female rats under different steroid status. Neurochem Res. 1996;21:897–901. doi: 10.1007/BF02532338. [DOI] [PubMed] [Google Scholar]
- 80.Mounier F, Pellegrini E, Kordon C, Epelbaum J, Bluet-Pajot MT. Continuous intracerebroventricular administration of a corticotropin releasing hormone antagonist amplifies spontaneous growth hormone pulses in the rat. J Endocrinol. 1997;152:431–436. doi: 10.1677/joe.0.1520431. [DOI] [PubMed] [Google Scholar]
- 81.Glowa JR, Gold PW. Corticotropin releasing hormone produces profound anorexigenic effects in the rhesus monkey. Neuropeptides. 1991;18:55–61. doi: 10.1016/0143-4179(91)90164-e. [DOI] [PubMed] [Google Scholar]
- 82.Wilson ME, Fisher J, Brown J. Chronic subcutaneous leptin infusion diminishes the responsiveness of the hypothalamic - pituitary - adrenal (HPA) axis in female rhesus monkeys. Physiology & Behavior. 2005;84:449 – 458. doi: 10.1016/j.physbeh.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 83.Barker-Gibb ML, Sahu A, Pohl CR, Plant TM. Elevating circulating leptin in prepubertal male rhesus monkeys (Macaca mulatta) does not elicit precocious gonadotropin-releasing hormone release, assessed indirectly. J Clin Endocrinol Metab. 2002;87:4976–4983. doi: 10.1210/jc.2002-020784. [DOI] [PubMed] [Google Scholar]
- 84.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 85.Rogers J, Martin LJ, Comuzzie AG, Mann JJ, Manuck SB, Leland M, Kaplan JR. Genetics of monoamine metabolites in baboons: overlapping sets of genes influence levels of 5-hydroxyindolacetic acid, 3-hydroxy-4-methoxyphenylglycol, and homovanillic acid. Biol Psychiatry. 2004;55:739–744. doi: 10.1016/j.biopsych.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 86.Xin X, Xiao J, Luan X, Zhou Y, Lu D, Wei D, Yang S. Association study of six activity SNPS in adrenal steroid hormone metabolism and IBM related genes with precocious puberty in Chinese girls. Neuro Endocrinol Lett. 2006;27:219–224. [PubMed] [Google Scholar]
- 87.Banerjee I, Trueman JA, Hall CM, Price DA, Patel L, Whatmore AJ, Hirschhorn JN, Read AP, Palmert MR, Clayton PE. Phenotypic variation in constitutional delay of growth and puberty: relationship to specific leptin and leptin receptor gene polymorphisms. Eur J Endocrinol. 2006;155:121–126. doi: 10.1530/eje.1.02184. [DOI] [PubMed] [Google Scholar]
- 88.Luan X, Zhou Y, Wang W, Yu H, Li P, Gan X, Wei D, Xiao J. Association study of the polymorphisms in the KISS1 gene with central precocious puberty in Chinese girls. Eur J Endocrinol. 2007;157:113–118. doi: 10.1530/EJE-07-0061. [DOI] [PubMed] [Google Scholar]
- 89.Luan X, Yu H, Wei X, Zhou Y, Wang W, Li P, Gan X, Wei D, Xiao J. GPR54 polymorphisms in Chinese girls with central precocious puberty. Neuroendocrinology. 2007;86:77–83. doi: 10.1159/000107511. [DOI] [PubMed] [Google Scholar]
- 90.Xita N, Tsatsoulis A, Stavrou I, Georgiou I. Association of SHBG gene polymorphism with menarche. Mol Hum Reprod. 2005;11:459–462. doi: 10.1093/molehr/gah178. [DOI] [PubMed] [Google Scholar]
- 91.Sedlmeyer IL, Pearce CL, Trueman JA, Butler JL, Bersaglieri T, Read AP, Clayton PE, Kolonel LN, Henderson BE, Hirschhorn JN, Palmert MR. Determination of sequence variation and haplotype structure for the gonadotropin-releasing hormone (GnRH) and GnRH receptor genes: investigation of role in pubertal timing. J Clin Endocrinol Metab. 2005;90:1091–1099. doi: 10.1210/jc.2004-0649. [DOI] [PubMed] [Google Scholar]
- 92.Rogers J, Garcia R, Shelledy W, Kaplan J, Arya A, Johnson Z, Bergstrom M, Novakowski L, Nair P, Vinson A, Newman D, Heckman G, Cameron J. An initial genetic linkage map of the rhesus macaque (Macaca mulatta) genome using human microsatellite loci. Genomics. 2006;87:30–38. doi: 10.1016/j.ygeno.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 93.Suomi SJ. Gene-environment interactions and the neurobiology of social conflict. Ann N Y Acad Sci. 2003;1008:132–139. doi: 10.1196/annals.1301.014. [DOI] [PubMed] [Google Scholar]