Abstract
In mammalian oocytes, the maintenance of meiotic prophase I arrest prior to the surge of luteinizing hormone (LH) that stimulates meiotic maturation depends on a high level of cAMP within the oocyte. In mouse and rat, the cAMP is generated in the oocyte, and this requires the activity of a constitutively active, Gs –linked receptor, GPR3 or GPR12, respectively. To examine if human oocyte meiotic arrest depends on a similar pathway, we looked at whether human oocytes express the same components for maintaining arrest as rodent oocytes using RT-PCR and Western blotting. RNA encoding GPR3, but not GPR12, was expressed. RNA encoding adenylate cyclase type 3 (AC3), which is the major adenylate cyclase required for maintaining meiotic arrest in the mouse oocyte, was also expressed, as was Galphas protein. To determine if this pathway is functional in the human oocyte, we examined the effect of injecting a function blocking antibody against Galphas on meiotic resumption. This antibody stimulated meiotic resumption of human oocytes that were maintained at the prophase I stage using a phosphodiesterase inhibitor. These results demonstrate that human oocytes maintain meiotic arrest prior to the LH surge using a similar signaling pathway as rodent oocytes.
Introduction
Mammalian oocytes are stored in the ovary arrested at prophase I of meiosis. Throughout the reproductive period of the female, ovarian follicles grow in response to stimulation by the pituitary gonadotropin follicle stimulating hormone (FSH). Oocyte growth occurs concomitantly with follicle growth, but the oocyte remains arrested at prophase I until a preovulatory surge of luteinizing hormone (LH) from the pituitary stimulates meiotic resumption. The prophase I-arrested oocyte acquires the ability to resume meiosis as it approaches its full size. In response to LH, the oocyte resumes meiosis and progresses to metaphase II, at which point it becomes arrested again and is at the appropriate stage to be fertilized. The progression from prophase I to metaphase II is termed “oocyte maturation,” and is a process that includes nuclear as well as cytoplasmic changes that allow the mature egg to be fertilized. The LH surge that initiates meiotic resumption also stimulates ovulation, and these two events are coordinated such that by the time the oocyte is ovulated, it has completed the maturation processes necessary to produce a fertilizable egg.
Meiotic arrest in fully grown, meiotically competent oocytes is dependent on high levels of cAMP within the oocyte [1, 2]. In rodent oocytes, cAMP is generated in the oocyte through the activity of a G-protein coupled receptor, GPR3 (mouse) or GPR12 (rat), that activates a Gs G-protein, stimulating the activity of adenylate cyclase and the production of cAMP [3-7]. If the activity of any of these proteins is inhibited, the follicle-enclosed oocyte is no longer able to maintain meiotic arrest.
The mechanisms that regulate meiotic arrest and resumption in the human oocyte are not as well understood due to the limited availability of material for study. However, the widespread use of in vitro fertilization (IVF) has provided an opportunity to obtain human oocytes for study. Results from the limited number of studies that have been done to date suggest that meiotic arrest may be regulated by a similar pathway as in rodents. For example, prophase I-stage human oocytes released from their follicles mature spontaneously in culture [8-10], and this can be reversibly inhibited by incubating oocytes in the presence of phosphodiesterase inhibitors [11, 12], demonstrating that cAMP is likely to have an important role in meiotic regulation. In addition, human oocytes contain the same cell cycle regulatory proteins that regulate meiosis in a diverse array of species [13, 14]. However, one important difference between humans and rodents is the length of their cycle. In humans, oocytes acquire meiotic competence and attain their full size during the menstrual cycle, which generally lasts ∼28 days, whereas rodent oocytes grow and acquire meiotic competence during the much shorter estrous cycle (typically ∼4-5 days). The increased time during which meiotically competent oocytes must remain arrested in human oocytes compared to rodents could require additional mechanisms to keep oocytes arrested in prophase until the LH surge occurs. It is therefore important to examine if human oocyte meiotic arrest and resumption are regulated by similar mechanisms as in rodents.
In this study, we addressed the question of how meiotic arrest is maintained in human oocytes, using similar approaches to those used previously for studies of rodent oocytes. In particular, we examined whether human oocytes contain the same components of the signaling pathways leading to the production of cAMP, as well as the requirement for Gs activity in the maintenance of meiotic arrest. Our results demonstrate that human oocytes maintain meiotic arrest prior to the LH surge using a similar signaling pathway as rodent oocytes.
Materials and Methods
Source of human and mouse oocytes
This study was approved by the Institutional Review Board at the University of Connecticut Health Center (IRB #06-125). Prior to participation in the study, all patients gave informed consent to donate immature oocytes. Immature oocytes were retrieved from ovaries of women ages 21-44 who were undergoing standard in vitro fertilization procedures using intracytoplasmic sperm injection (ICSI). All patients underwent pituitary suppression with either a GnRH agonist or antagonist. Controlled ovarian stimulation was achieved with daily subcutaneous injections of 150-450 IU recombinant follicle-stimulating hormone with or without 75-150 IU human menopausal gonadotropin. Doses were adjusted based on follicular response as evidenced by serial transvaginal ultrasounds and serum estradiol levels. A subcutaneous injection of 3,300-10,000 IU human chorionic gonadotropin was administered when 3 or more follicles reached a mean diameter of 18 mm. Transvaginal ultrasound-guided oocyte retrieval was performed 35 hours after hCG injection.
Oocytes were aspirated from ovarian follicles ∼14-22 mm in diameter. Most oocytes retrieved from such ovaries are at the metaphase II stage (mature eggs). Approximately 10-15% of oocytes are in prophase I (immature); these oocytes are identified by the presence of the nucleus, or germinal vesicle (GV). As these immature oocytes are not clinically utilized at our center, they are routinely discarded. Cumulus-oocyte complexes were aspirated into culture medium (see below) and incubated in an environment of 5% CO2 and 95% air at 37°C for 3-5 hours. Cumulus cells were stripped enzymatically using hyaluronidase type VIII from bovine testes (cat. #H-3757; Sigma-Aldrich; St. Louis, MO) and mechanically by pipetting them up and down through a small-bore pipet. Following cumulus removal, oocytes that had a GV were placed into medium containing 10 μM cilostamide (Calbiochem, La Jolla, CA), a phosphodiesterase 3A-specific inhibitor that prevents spontaneous meiotic resumption. Oocytes ranged in diameter from 105 to 115 μm. Oocytes retrieved from all patients on a given day were pooled.
Fully grown, GV-stage mouse oocytes were obtained from the ovaries of 22-25 day old B6SJLF1 mice (The Jackson Laboratory, Bar Harbor, ME) that had been primed with 5 IU eCG 40-46 hrs prior to collection. Cumulus cells were removed by repeated aspiration through a small-bore pipet. The medium for oocyte collection was HEPES-buffered MEM [15] supplemented with 250 μM dbcAMP to prevent spontaneous maturation during the collection process. All experiments were done with prior approval of the Animal Care and Use Committee at the University of Connecticut Health Center.
RT-PCR and Western blotting
GV-stage human oocytes were placed in microcentrifuge tubes shortly after isolation from their cumulus complexes and were pelleted gently in small microfuge tubes. Most of the culture medium was removed, and oocytes were washed in phosphate buffered saline (PBS) to remove serum, pelleted gently, frozen in liquid nitrogen, and stored at -80°C until use. For RT-PCR, mRNA was purified from 9-32 oocytes using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Any contaminating DNA was removed using the TURBO DNA-free Kit (Ambion; #AM1907). Complementary DNA was prepared using the Superscript III Reverse Transcriptase kit (Invitrogen) according to the manufacturer's instructions and using oligo-dT as the primer.
Following reverse transcription, 3.0-6.5 oocyte equivalents were used for PCR to amplify GPR3, GPR12, and type 3 adenylate cyclase (AC3) using the following primer sequences: GPR3: 5′-CTCCACGGTTCCAGAATGTT-3′ and 5′-GGGAGAAGGCTCTGGTTTCT-3′ (485 base pair product); GPR12: 5′-GACGTACCCATTCGGAGAGGA-3′ and 5′-TGACAGGGTTGATGATGGAA -3′ (461 base pair product); AC3: 5′-GCTGGAATTGGACTGGTGTT-3′ and 5′-GCGCACAGGTAGAGGAAGAC-3′ (376 base pair product). GPR6 was amplified using the primer sequences 5′-TCCCATCATCTATGCCTTCC -3′ and 5′-TTTTGGCAGTTCAGCAGCTA-3′ (496 base pair product). Human ovary and brain cDNA were purchased from Ambion (Austin, TX). The cycling parameters were an initial denaturation of 2 min, 94EC, followed by 40 cycles of 94E for 30 sec, 55E for 45 sec, 72E for 45 sec, and a final extension of 72E for 7 min. PCR products were electrophoresed on 1.5% agarose gels, visualized by staining the gel with SYBRGold nucleic acid stain (Molecular Probes, Eugene, OR; cat #S11494), and photographed using Polaroid film. The identity of GPR3 was confirmed by TA cloning (TA cloning kit, Invitrogen, cat. #45-0046) and sequencing, using T7 as the primer, and the resulting sequence compared with the human sequence published in GenBank.
Western blotting was performed as previously described [16]. Briefly, frozen oocytes were solubilized in Laemmli sample buffer and proteins separated on 10% acrylamide gels. Gαs was detected using an affinity purified antibody generated in rabbit against the C-terminal decapeptide of human Gαs (RM; [17]); these 10 amino acids are identical in human and mouse. This antibody, which was also used for microinjection (see below), was generously provided by Teresa Jones (NIH).
Oocyte Culture
To determine what percentage of immature human oocytes undergo spontaneous maturation under our culture conditions, GV-stage oocytes were cultured in either Quinn's Advantage Cleavage Medium (cat. #ART-1027, SAGE BioPharma, Bedminster, N.J.) or in Quinn's Advantage Blastocyst Medium (cat. #ART-1029, SAGE), supplemented with Quinn's SPS Serum Protein Substitute (cat. #ART-3011, SAGE) and incubated in a humidified environment of 5% CO2 and 95% air at 37°C. These media differ slightly in composition, with the major difference being that Blastocyst Medium contains several more amino acids than Cleavage Medium. Both types of media yielded the same results and herein will be referred to as “culture medium.”
To determine whether spontaneous maturation of GV-stage oocytes could be inhibited reversibly under our culture conditions, GV-stage oocytes were placed in culture medium containing 10 μM cilostamide immediately after the surrounding cumulus cells were removed with enzymatic and mechanical stripping. Following a 24-48 hr culture period in a 200 μl drop of medium under light paraffin oil (Fisher Scientific, Pittsburgh, PA), GV-intact oocytes were washed into fresh medium without cilostamide and were observed using a Wild stereomicroscope for GVBD and polar body formation.
Oocyte Microinjection
For microinjection experiments, GV-stage oocytes were placed in culture medium containing 10 μM cilostamide immediately after the surrounding cumulus cells were removed with hyaluronidase. Oocytes were cultured overnight, and oocytes containing intact GVs the following morning were used for microinjection. Microinjection was carried out as previously described [18]. Briefly, oocytes were quantitatively microinjected using a pressure system and pipets backfilled with mercury [18, 19]. The antibody was spin-dialyzed and concentrated in PBS as described previously [20]. The non-immune rabbit IgG used for controls was purchased from Santa Cruz Biotechnology and was concentrated in the same way. Both the Gαs antibody and IgG stocks were diluted to 6 mg/ml and 20-30 pl injected to give a final intracellular concentration of 170-260 μg/ml in the oocyte. Both Gαs antibody- and IgG-injected oocytes were scored for germinal vesicle breakdown (GVBD) periodically. Final concentrations in the oocytes were calculated based on an oocyte volume of 700 pl, which was calculated by averaging the volume of 105 μm and 115 μm diameter oocytes. Following microinjection, oocytes were placed in 200 μl drops of medium containing cilostamide, under oil, and were cultured in a humidified atmosphere at 37EC with 5% CO2 and 95% air. Oocytes were scored periodically for the presence or absence of a GV.
Results
Expression of GPR3 and adenylate cyclase RNAs in human oocytes
In mouse oocytes, GPR3 is the receptor required for the oocyte to maintain meiotic arrest [3-5, 21], and the related receptor, GPR12, maintains meiotic arrest in the rat oocyte [5]. Due to the limited availability of human oocytes, we initially examined which of these receptors, as well as the closely related receptor, GPR6 [22], are expressed in the human ovary using RT-PCR. Bands of the expected sizes were amplified for both GPR3 and GPR12 (Fig. 1). GPR6 was not expressed, although the primers used detected a specific band in human brain.
Figure 1.
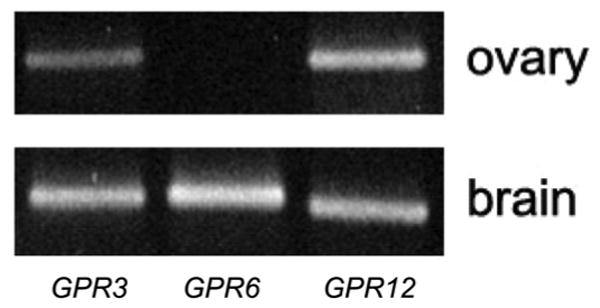
The Gs-coupled receptors, GPR3 and GPR12, but not GPR6, are expressed in human ovary. Upper panel: RT-PCR demonstrating the presence of GPR3 and GPR12 RNAs in human ovary. GPR6 is not detectable, though it was amplified from human brain cDNA, demonstrating that the primers were functional (lower panel). 1 ng of each cDNA was used in these experiments, which were performed once (ovary) and twice (brain).
Therefore, we examined if GPR3 and/or GPR12 were expressed in GV-stage human oocytes. A band of the expected size for GPR3 was amplified from cDNA derived from oocytes (Fig. 2), and its identity was confirmed by cloning and sequencing the PCR product. GPR12 was not detectable using the same cycling parameters as for GPR3 (Fig. 2 and legend), demonstrating that GPR3 is the only member of the GPR3/GPR6/GPR12 family detectable in the human oocyte. No band was detected when PCR was performed on RNA that had not undergone reverse transcription using the GPR3 primer set, demonstrating that there was no significant genomic DNA contamination (Fig. 2). In addition, the absence of a band for GPR12, despite a functional primer set, shows that there was no genomic DNA contamination.
Figure 2.
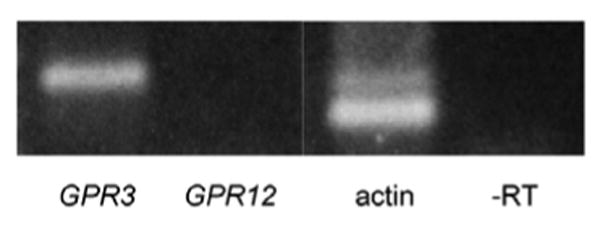
GPR3 RNA is expressed in human oocytes. RT-PCR using cDNA derived from 6.5 human oocyte equivalents amplified bands for GPR3, while GPR12 was not detected. All of the RT-PCRs shown above were performed on the same cDNA and run on the same gel. The presence of GPR3, as well as actin, demonstrate the quality of the cDNA. Possible genomic contamination was ruled out by performing PCR on the same batch of RNA that had not undergone reverse transcription. We estimate the amount of cDNA used for these PCR reactions corresponded to ∼20 ng total RNA. This figure is based on the amount of RNA in a mouse oocyte (∼1 ng; [3]), and taking into account that human oocytes contain 3X the volume of mouse oocytes. Each experiment was performed 7 times or more, using at least 6 different batches of cDNA.
In addition to GPR3, GPR6, and GPR12, we examined whether adenylate cyclase type 3 (AC3), which is necessary for the maintenance of meiotic arrest in mouse oocytes [23], is expressed in human oocytes. A band of the expected size for AC3 was amplified from cDNA derived from human ovary, as well as in GV-stage oocytes (Fig. 3).
Figure 3.
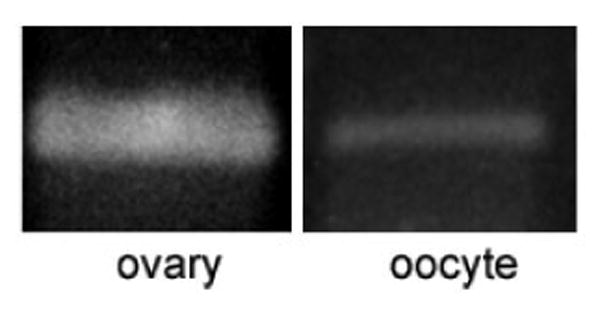
Expression of AC3 RNA in human ovary and oocytes. RT-PCR using cDNA derived from 5 human oocytes (∼15 ng RNA) and 1 ng cDNA (ovary). Experiments were performed once using ovary and twice using oocytes.
Expression of Gαs protein in human oocytes
We also examined the presence of the Gs G-protein in GV-stage human oocytes, using Western blot analysis. Mouse oocytes contain two forms of Gαs, a long form and a short form [6], and this protein is expressed in a high abundance, such that it can be detected using a small number of oocytes (see Fig. 4B). Likewise, both forms of Gαs were specifically detected in human oocytes (Fig. 4). Comparing the intensity of the bands in mouse and human oocytes, the amount of Gαs appears to be similar (see Fig. 4B and legend). Taken together, the expression of GPR3 and AC3 mRNA, as well as abundant Gαs protein present in human oocytes, suggest that meiotic arrest could be regulated by the same pathway as in the mouse.
Figure 4.

Human oocytes contain the Gαs G-protein. A) Western blot showing specific labelling of both the long and short forms of Gαs protein in lysates from 13 human oocytes (∼1.5 μg). B) The amount of Gαs protein is comparable in mouse and human oocytes. Since human oocytes have 3X the volume of mouse oocytes, and since the intensities of the Gαs bands in 5 mouse oocytes and 2 human oocytes are comparable, the concentration of Gαs protein in human and mouse oocytes is similar. Number of oocytes is indicated above each lane. Human oocyte blots were performed 5 times.
Establishing culture conditions to control meiotic arrest and resumption in human oocytes
Previous studies have shown that GV-stage human oocytes mature spontaneously in culture when removed from the follicle [8, 24-26](Fig. 5), and this spontaneous maturation can be reversibly inhibited by phosphodiesterase inhibitors [11, 12]. However, the timing of GVBD following phosphodiesterase inhibitor removal has not been carefully characterized. As a prelude to experiments to examine whether the Gs signaling pathway is involved in maintaining meiotic arrest in human oocytes, we determined the percentage of GV-stage oocytes that matured spontaneously under our culture conditions, as well as the timing of GVBD following removal of the phosphodiesterase inhibitor, cilostamide.
Fig. 5.
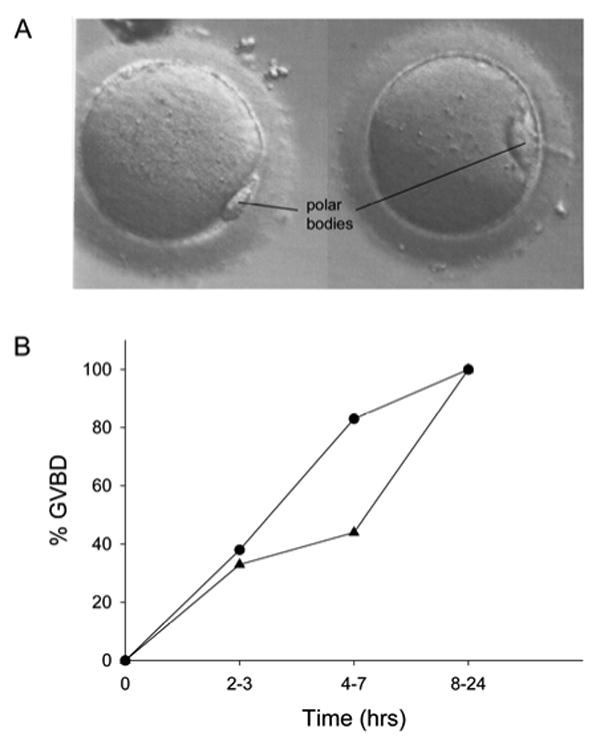
In vitro maturation of human oocytes. A) GV-stage oocytes retrieved from a patient undergoing IVF matured spontaneously in culture, undergoing GVBD and first polar body formation. B) Time course of spontaneous meiotic resumption. GV-stage oocytes were maintained in meiotic arrest by incubation overnight in medium containing 10 μM cilostamide. Oocytes were scored for GVBD after washing out the cilostamide. Circles: time course of GVBD in 24 uninjected oocytes; triangles: time course of GVBD in oocytes that resumed meiosis in response to injection of a Gs antibody in the presence of 10 μM cilostamide (see Fig. 6).
Fig. 5A shows two human oocytes that were GV-intact at the time of retrieval and matured in culture to the metaphase II stage, undergoing GVBD and first polar body extrusion within 24 hrs after oocyte retrieval. The PDE3A-specific inhibitor, cilostamide (10 μM), prevented spontaneous meiotic resumption in 94% of oocytes that had readily discernible GVs (examined using a stereomicroscope) at the time that they were placed in culture (n=36); meiotic arrest was maintained in these oocytes during overnight culture. Seventy-one percent of these GV-stage oocytes underwent GVBD within 24 hrs after they were subsequently transferred to culture medium without cilostamide, demonstrating that these oocytes were competent and that the meiotic arrest was reversible. The time from cilostamide removal to GVBD was variable, ranging from 2 to 48 hrs (Fig. 5B); for these studies, oocytes that underwent GVBD within 24 hrs were considered to be competent. These results demonstrate that with our culture conditions, we can maintain meiotic arrest in human oocytes, and control the onset of meiotic resumption. They also provide a basis for examining the requirement for the Gs signaling pathway in maintaining meiotic arrest.
Activity of the Gs G-protein is required for maintenance of meiotic arrest in human oocytes
To examine if Gs activity is necessary to maintain oocytes in prophase arrest, we determined if injection of an antibody against Gαs could overcome the meiotic inhibition of oocytes cultured in the presence of 10 μM cilostamide. The affinity purified antibody we used has previously been shown to inhibit Gs activity [17, 20], and causes GVBD when injected into frog and mouse oocytes [6, 20]. The specificity of this antibody for human Gαs, shown in Fig. 4, establishes that it is an appropriate antibody to use for these microinjection experiments. We injected a total of 22 oocytes that contained well-defined GVs following a 19-25 hr culture in medium containing cilostamide with 170-260 μg/ml of the Gs antibody or non-immune IgG. Following microinjection, oocytes were cultured in the presence of cilostamide and 5% CO2, 95% air, and examined periodically for the presence or absence of the GV.
Ninety percent of the oocytes injected with the Gs antibody underwent GVBD within 2-19 hrs following antibody injection (n = 10; Fig. 5B, 6B and legend). Of these, 70% formed first polar bodies after further culture, suggesting that the resumption of meiosis was physiological and that the injected oocytes were healthy. Eighty-nine percent of oocytes injected with non-immune IgG and maintained in the presence of cilostamide (n=9) did not undergo GVBD during the culture period, but underwent GVBD after washing the oocytes out of cilostamide (Fig. 6B).
Fig. 6.
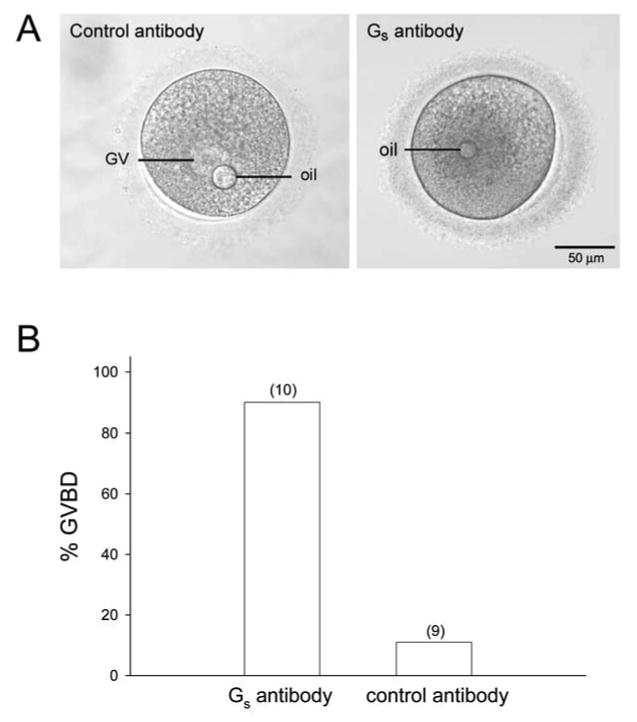
Inhibiting the activity of Gs causes meiotic resumption in human oocytes. A) Oocytes microinjected with 170-260 μg/ml Gs antibody or non-immune IgG in the presence of, and following an overnight culture in, 10 μM cilostamide. Oocytes injected with the Gs antibody (right) underwent GVBD, whereas control IgG-injected oocytes(left) remained arrested at the GV stage. The oil drops were introduced into the oocytes as a consequence of the microinjection procedure. B) Percentages of oocytes that underwent GVBD following injection of the Gs antibody or control IgG. The numbers in parentheses are total number of oocytes injected. If injected oocytes did not undergo GVBD within 24 hrs after injection, their meiotic competence was confirmed by washing them into culture medium that did not contain cilostamide. Oocytes that underwent GVBD within 24 hrs after washing out the cilostamide were considered to be competent. Oocytes that did not undergo GVBD within 24 hrs after washing out the cilostamide were considered to be incompetent and were not included in the analysis. For these experiments, we found that 83% (10/12) of the oocytes injected with the Gs antibody were competent, and 90% (9/10) of the oocytes injected with IgG were competent. These percentages were similar to those we obtained from uninjected oocytes that were washed out of cilostamide following overnight culture (see Results). Oocytes injected with control IgG were cultured for either 6 (n=2) or 19-21 hrs (n=8) hrs prior to washing out the cilostamide; these oocytes were pooled for this analysis (n=10 total).
Discussion
Recent results from several studies have shown that the Gs signal transduction pathway is necessary to maintain meiotic arrest in rodent oocytes, prior to the surge of LH that stimulates meiotic resumption. This signaling pathway is initiated by a constitutively active G-protein coupled receptor, GPR3 (mice: [3-6, 21]), or GPR12 (rats: [5]) in the oocyte. In the present study, we show that the Gs signaling pathway is also required to maintain meiotic arrest in the human oocyte. We found that human oocytes express RNAs encoding GPR3 and AC3, and express Gαs protein. Due to the difficulty of detecting native levels of G-protein coupled receptor protein using antibodies [27], as well as the inability to detect native levels of GPR3 in mouse oocytes [21], we were unable to examine if GPR3 protein is also present in the human oocyte.
The related receptors, GPR12 and GPR6, are not expressed in human oocytes (Figs. 1 and 2). Previous studies by others have shown that GV-stage human oocytes remain arrested at prophase I when cultured with PDE3 inhibitors [11, 12], indicating further that cAMP in the human oocyte is important for regulating meiosis. Here, we have shown that inhibiting Gs activity using a function blocking antibody caused isolated oocytes to mature spontaneously when cultured in the presence of the PDE3A-specific inhibitor cilostamide. These results indicate that in human oocytes, like their rodent counterparts, cAMP levels regulate meiotic arrest, and that the Gs signaling pathway is necessary for maintenance of meiotic arrest.
The somatic cells surrounding the mammalian oocyte are also necessary for the maintenance of meiotic arrest prior to the LH surge (see [13] for review). It is not yet known what role the somatic cells have in this process. In mice, as well as in larger mammals such as cows and pigs, the cumulus cells alone are not sufficient to hold the oocyte in prophase I arrest, whereas meiotic arrest is maintained in cumulus-enclosed oocytes that are physically connected to the surrounding mural granulosa cells [28-32]. Both isolated as well as cumulus-enclosed mouse oocytes resume meiosis soon after a fall in cAMP levels following isolation from the follicle [28, 33] in the absence of PDE inhibitor, although there is some evidence that the presence of the cumulus cells delays meiotic resumption slightly [28].
In contrast, we found that meiotic prophase I arrest could be maintained in cumulus-enclosed human oocytes for several hours prior to placing isolated oocytes in cilostamide. This difference could be due to a slower degradation of cAMP in human oocytes compared to rodents or to another inhibitory factor from the cumulus cells. It is interesting to note that GPR12 is expressed in the human ovary, and could conceivably provide a source of cAMP to the oocyte through gap junctions. A role for the cumulus cells in maintaining human meiotic arrest needs to be investigated further. Nevertheless, the finding that human oocyte meiotic arrest is regulated by a Gs pathway suggests that rodent models can be useful for studies of human oocyte maturation. In addition, the methods used here demonstrate that we can inhibit meiotic resumption in GV-stage human oocytes, and that we can experimentally stimulate meiotic resumption by targeting components of the cell signaling pathways responsible for meiotic resumption.
A limitation of this study is that the GV-stage oocytes available to us were retrieved from hormonally stimulated women, and the follicles they were retrieved from may have been too small to express a sufficient number of LH receptors to respond to the LH surge. However, for several reasons, these oocytes are not necessarily inappropriate for this study. In mice, the GPR3/Gs signaling pathway is in place and functional prior to the acquisition of meiotic competence [7]. In humans, meiotic competence is acquired when follicles are between 5-7 mm in diameter [25], while the GV-stage oocytes used in this study were retrieved from follicles at least 14 mm diameter. In addition, these oocytes underwent GVBD and formed polar bodies in culture, demonstrating that they were competent. Furthermore, some in vitro matured oocytes that were obtained in this way have been fertilized and produced live offspring [26], demonstrating that they can be physiologically normal. The developmental competence of oocytes obtained during this stage is low, however, even in oocytes retrieved from women who have not been hormonally stimulated [34-36]. Further studies are needed to identify the mechanisms by which human meiosis is regulated in oocytes, as well as the normal intracellular events that occur during human oocyte maturation in order to accurately assess the quality of oocytes matured in vitro.
A more complete understanding of the cellular processes involved in human oocyte maturation could potentially lead to improved culture conditions for maturing human oocytes in vitro (in vitro maturation; IVM). IVM has become a topic of intense research because the ability to mature human oocytes in vitro could significantly improve methods for infertility treatment. Currently, standard in vitro fertilization (IVF) protocols require hormone injections, the inconvenience of frequent monitoring of follicular response, unpleasant side effects, and the potential risk of developing ovarian hyperstimulation syndrome (OHSS), an often dangerous medical condition which may necessitate cycle cancellation and hospitalization in severe cases [37, 38]. With IVM, women could potentially undergo IVF with a significant reduction in these risks. In addition, IVM could provide some women the opportunity to preserve their fertility, particularly female cancer patients who are at risk of becoming infertile as a result of treatment [39-41]. Despite improving success rates, IVM is still in its infancy, and only ∼400-500 babies have been born worldwide from this procedure [10]. Results from the limited number of studies examining the basic science of human IVM have shown that several aspects of nuclear and cytoplasmic maturation are abnormal in these oocytes [11, 14, 42-44]. All of these factors are likely to contribute to the low developmental potential of fertilized, in vitro matured oocytes.
With an improved understanding of the mechanisms that regulate meiotic competence, arrest, and resumption, human oocytes could potentially be cultured under conditions that more closely simulate their physiological environment, and this, in turn, could improve the quality of mature oocytes retrieved following IVM. For example, immature oocytes that are cultured in the presence of the cumulus cells that normally surround the oocyte, as well as those cultured in a gel matrix that more accurately simulates the 3-dimensional environment of the oocyte-cumulus cell complex, have improved cytoplasmic maturation rates compared with oocytes separated from the cumulus cells [14, 45]. By identifying the importance of the GPR3/Gs signaling pathway in human oocytes, we add another component to our understanding of an important stage of meiotic regulation. In combination with a better understanding of the role for the cumulus cells in this process, methods for culturing cumulus-enclosed oocytes could potentially be improved such that these oocytes could be cultured under conditions that more accurately simulate their native environment. More studies are needed to clarify the physiological events that occur during normal nuclear and cytoplasmic maturation, as well as to compare the quality of oocytes matured from small follicles of unstimulated women with those obtained during standard, hormonally stimulated IVF cycles.
In summary, we report new aspects of the signaling pathway in the human oocyte that is necessary to maintain meiotic arrest prior to the LH surge. We have also shown that human meiotic maturation can be studied in a controlled way. This can be expanded to future studies examining the mechanisms of meiotic maintenance and resumption in human oocytes.
Acknowledgments
We would like to thank the attending physicians at UCHC, Drs. John Nulsen, Claudio Benediva, and David Schmidt, for supporting this work. We also thank all of the embryologists at the Center for Advanced Reproductive Services at the University of Connecticut Health Center for their excellent cooperation and willingness to prepare oocytes for this study: Pamela Daniels, Jean Jennings, Scott Kratka, Sally Kuslis, Jane Kwieraga, Cynthia McAllister, Melissa Palen, and Krista Traynor. We are grateful to Dr. Teresa Jones (NIH) for providing the Gs antibody and for useful comments and suggestions on the manuscipt; Dr. A. F. Parlow and the National Hormone and Peptide Pituitary Program for providing equine chorionic gonadotropin (eCG); and to Laurinda Jaffe for thoughtful advice throughout this work, as well as providing useful comments on the manuscript. This work was supported by grants HD056366 and the Center for Interdisciplinary Research in Women's Health at the University of Connecticut Health Center to L.M. Mehlmann, and DK073499 to L.A. Jaffe.
References
- 1.Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005a;130:791–799. doi: 10.1530/rep.1.00793. [DOI] [PubMed] [Google Scholar]
- 2.Conti M, Andersen CB, Richard F, Mehats C, Chun SY, Horner K, Jin C, Tsafriri A. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol. 2002;187:153–159. doi: 10.1016/s0303-7207(01)00686-4. [DOI] [PubMed] [Google Scholar]
- 3.Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306:1947–1950. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- 4.Ledent C, Demeestere I, Blum D, Petermans J, Hamalainen T, Smits G, Vassart G. Premature ovarian aging in mice deficient for Gpr3. Proc Natl Acad Sci U S A. 2005;102:8922–8926. doi: 10.1073/pnas.0503840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinckley M, Vaccari S, Horner K, Chen R, Conti M. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol. 2005;287:249–261. doi: 10.1016/j.ydbio.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Mehlmann LM, Jones TL, Jaffe LA. Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science. 2002;297:1343–1345. doi: 10.1126/science.1073978. [DOI] [PubMed] [Google Scholar]
- 7.Freudzon L, Norris RP, Hand AR, Tanaka S, Saeki Y, Jones TL, Rasenick MM, Berlot CH, Mehlmann LM, Jaffe LA. Regulation of meiotic prophase arrest in mouse oocytes by GPR3, a constitutive activator of the Gs G protein. J Cell Biol. 2005;171:255–265. doi: 10.1083/jcb.200506194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards RG. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature. 1965;208:349–351. doi: 10.1038/208349a0. [DOI] [PubMed] [Google Scholar]
- 9.Törnell J, Hillensjö T. Effect of cyclic AMP on the isolated human oocyte--cumulus complex. Hum Reprod. 1993;8:737–739. doi: 10.1093/oxfordjournals.humrep.a138131. [DOI] [PubMed] [Google Scholar]
- 10.Jurema MW, Nogueira D. In vitro maturation of human oocytes for assisted reproduction. Fertil Steril. 2006;86:1277–1291. doi: 10.1016/j.fertnstert.2006.02.126. [DOI] [PubMed] [Google Scholar]
- 11.Vanhoutte L, De Sutter P, Nogueira D, Gerris J, Dhont M, Van der Elst J. Nuclear and cytoplasmic maturation of in vitro matured human oocytes after temporary nuclear arrest by phosphodiesterase 3-inhibitor. Hum Reprod. 2007;22:1239–1246. doi: 10.1093/humrep/dem007. [DOI] [PubMed] [Google Scholar]
- 12.Nogueira D, Albano C, Adriaenssens T, Cortvrindt R, Bourgain C, Devroey P, Smitz J. Human oocytes reversibly arrested in prophase I by phosphodiesterase type 3 inhibitor in vitro. Biol Reprod. 2003;69:1042–1052. doi: 10.1095/biolreprod.103.015982. [DOI] [PubMed] [Google Scholar]
- 13.Eppig JJ, Vivieros MM, Marin-Bivens C, De La Fuente R. Regulation of mammalian oocyte maturation. In: Leung PCK, Adashi EY, editors. The Ovary. 2nd. Amsterdam: Academic Press/Elsevier; 2004. pp. 113–129. [Google Scholar]
- 14.Combelles CM, Fissore RA, Albertini DF, Racowsky C. In vitro maturation of human oocytes and cumulus cells using a co-culture three-dimensional collagen gel system. Hum Reprod. 2005;20:1349–1358. doi: 10.1093/humrep/deh750. [DOI] [PubMed] [Google Scholar]
- 15.Mehlmann LM, Kline D. Regulation of intracellular calcium in the mouse egg: calcium release in response to sperm or inositol trisphosphate is enhanced after meiotic maturation. Biol Reprod. 1994;51:1088–1098. doi: 10.1095/biolreprod51.6.1088. [DOI] [PubMed] [Google Scholar]
- 16.Mehlmann LM, Carpenter G, Rhee SG, Jaffe LA. SH2 domain-mediated activation of phospholipase Cγ is not required to initiate Ca2+ release at fertilization of mouse eggs. Dev Biol. 1998;203:221–232. doi: 10.1006/dbio.1998.9051. [DOI] [PubMed] [Google Scholar]
- 17.Simonds WF, Goldsmith PK, Woodard CJ, Unson CG, Spiegel AM. Receptor and effector interactions of Gs. Functional studies with antibodies to the alpha s carboxyl-terminal decapeptide. FEBS Lett. 1989;249:189–194. doi: 10.1016/0014-5793(89)80622-2. [DOI] [PubMed] [Google Scholar]
- 18.Kline D. Quantitative microinjection of mouse oocytes and eggs. Methods in Molecular Biology. 2007 doi: 10.1007/978-1-59745-202-1_11. In Press. [DOI] [PubMed] [Google Scholar]
- 19.Jaffe LA, Terasaki M. Quantitative microinjection of oocytes, eggs, and embryos. Methods Cell Biol. 2004;74:219–242. doi: 10.1016/s0091-679x(04)74010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallo CJ, Hand AR, Jones TL, Jaffe LA. Stimulation of Xenopus oocyte maturation by inhibition of the G-protein as subunit, a component of the plasma membrane and yolk platelet membranes. J Cell Biol. 1995;130:275–284. doi: 10.1083/jcb.130.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehlmann LM. Oocyte-specific expression of Gpr3 is required for the maintenance of meiotic arrest in mouse oocytes. Dev Biol. 2005b;288:397–404. doi: 10.1016/j.ydbio.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ignatov A, Lintzel J, Hermans-Borgmeyer I, Kreienkamp HJ, Joost P, Thomsen S, Methner A, Schaller HC. Role of the G-protein-coupled receptor GPR12 as high-affinity receptor for sphingosylphosphorylcholine and its expression and function in brain development. J Neurosci. 2003;23:907–914. doi: 10.1523/JNEUROSCI.23-03-00907.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horner K, Livera G, Hinckley M, Trinh K, Storm D, Conti M. Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev Biol. 2003;258:385–396. doi: 10.1016/s0012-1606(03)00134-9. [DOI] [PubMed] [Google Scholar]
- 24.Cha KY, Chian RC. Maturation in vitro of immature human oocytes for clinical use. Hum Reprod Update. 1998;4:103–120. doi: 10.1093/humupd/4.2.103. [DOI] [PubMed] [Google Scholar]
- 25.Trounson A, Anderiesz C, Jones G. Maturation of human oocytes in vitro and their developmental competence. Reproduction. 2001;121:51–75. doi: 10.1530/rep.0.1210051. [DOI] [PubMed] [Google Scholar]
- 26.Chian RC, Lim JH, Tan SL. State of the art in in-vitro oocyte maturation. Curr Opin Obstet Gynecol. 2004;16:211–219. doi: 10.1097/00001703-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Daly CJ, McGrath JC. Fluorescent ligands, antibodies, and proteins for the study of receptors. Pharmacol Ther. 2003;100:101–118. doi: 10.1016/j.pharmthera.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Eppig JJ, Ward-Bailey PF, Coleman DL. Hypoxanthine and adenosine in murine ovarian follicular fluid: Concentrations and activity in maintaining oocyte meiotic arrest. Biol Reprod. 1985;33:1041–1049. doi: 10.1095/biolreprod33.5.1041. [DOI] [PubMed] [Google Scholar]
- 29.Motlik J, Nagai T, Kikuchi K. Resumption of meiosis in pig oocytes cultured with cumulus and parietal granulosa cells: the effect of protein synthesis inhibition. J Exp Zool. 1991;259:386–391. doi: 10.1002/jez.1402590314. [DOI] [PubMed] [Google Scholar]
- 30.De Loos FA, Zeinstra E, Bevers MM. Follicular wall maintains meiotic arrest in bovine oocytes cultured in vitro. Mol Reprod Dev. 1994;39:162–165. doi: 10.1002/mrd.1080390207. [DOI] [PubMed] [Google Scholar]
- 31.Racowsky C, Baldwin KV. In vitro and in vivo studies reveal that hamster oocyte meiotic arrest is maintained only transiently by follicular fluid, but persistently by membrana/cumulus granulosa cell contact. Dev Biol. 1989;134:297–306. doi: 10.1016/0012-1606(89)90102-4. [DOI] [PubMed] [Google Scholar]
- 32.van Tol HT, van Eijk MJ, Mummery CL, van den Hurk R, Bevers MM. Influence of FSH and hCG on the resumption of meiosis of bovine oocytes surrounded by cumulus cells connected to membrana granulosa. Mol Reprod Dev. 1996;45:218–224. doi: 10.1002/(SICI)1098-2795(199610)45:2<218::AID-MRD15>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 33.Schultz RM, Montgomery RR, Belanoff JR. Regulation of mouse oocyte meiotic maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol. 1983;97:264–273. doi: 10.1016/0012-1606(83)90085-4. [DOI] [PubMed] [Google Scholar]
- 34.Chian RC, Buckett WM, Tulandi T, Tan SL. Prospective randomized study of human chorionic gonadotrophin priming before immature oocyte retrieval from unstimulated women with polycystic ovarian syndrome. Hum Reprod. 2000;15:165–170. doi: 10.1093/humrep/15.1.165. [DOI] [PubMed] [Google Scholar]
- 35.Söderstrom-Anttila V, Mäkinen S, Tuuri T, Suikkari AM. Favourable pregnancy results with insemination of in vitro matured oocytes from unstimulated patients. Hum Reprod. 2005;20:1534–1540. doi: 10.1093/humrep/deh768. [DOI] [PubMed] [Google Scholar]
- 36.Le Du A, Kadoch IJ, Bourcigaux N, Doumerc S, Bourrier MC, Chevalier N, Fanchin R, Chian RC, Tachdjian G, Frydman R, Frydman N. In vitro oocyte maturation for the treatment of infertility associated with polycystic ovarian syndrome: the French experience. Hum Reprod. 2005;20:420–424. doi: 10.1093/humrep/deh603. [DOI] [PubMed] [Google Scholar]
- 37.Beerendonk CC, van Dop PA, Braat DD, Merkus JM. Ovarian hyperstimulation syndrome: facts and fallacies. Obstet Gynecol Surv. 1998;53:439–449. doi: 10.1097/00006254-199807000-00024. [DOI] [PubMed] [Google Scholar]
- 38.MacDougall MJ, Tan SL, Balen A, Jacobs HS. A controlled study comparing patients with and without polycystic ovaries undergoing in-vitro fertilization. Hum Reprod. 1993;8:233–237. doi: 10.1093/oxfordjournals.humrep.a138029. [DOI] [PubMed] [Google Scholar]
- 39.Nieman CL, Kazer R, Brannigan RE, Zoloth LS, Chase-Lansdale PL, Kinahan K, Dilley KJ, Roberts D, Shea LD, Woodruff TK. Cancer survivors and infertility: a review of a new problem and novel answers. J Support Oncol. 2006;4:171–178. [PubMed] [Google Scholar]
- 40.Lobo RA. Potential options for preservation of fertility in women. N Engl J Med. 2005;353:64–73. doi: 10.1056/NEJMra043475. [DOI] [PubMed] [Google Scholar]
- 41.Seli E, Tangir J. Fertility preservation options for female patients with malignancies. Curr Opin Obstet Gynecol. 2005;17:299–308. doi: 10.1097/01.gco.0000169108.15623.34. [DOI] [PubMed] [Google Scholar]
- 42.Nogueira D, Staessen C, Van de Velde H, Van Steirteghem A. Nuclear status and cytogenetics of embryos derived from in vitro-matured oocytes. Fertil Steril. 2000;74:295–298. doi: 10.1016/s0015-0282(00)00642-7. [DOI] [PubMed] [Google Scholar]
- 43.Combelles CM, Cekleniak NA, Racowsky C, Albertini DF. Assessment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum Reprod. 2002;17:1006–1016. doi: 10.1093/humrep/17.4.1006. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Feng HL, Cao YJ, Zheng GJ, Yang Y, Mullen S, Critser JK, Chen ZJ. Confocal microscopic analysis of the spindle and chromosome configurations of human oocytes matured in vitro. Fertil Steril. 2006;85:827–832. doi: 10.1016/j.fertnstert.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 45.Torre ML, Munari E, Albani E, Levi-Setti PE, Villani S, Faustini M, Conte U, Vigo D. In vitro maturation of human oocytes in a follicle-mimicking three-dimensional coculture. Fertil Steril. 2006;86:572–576. doi: 10.1016/j.fertnstert.2006.02.090. [DOI] [PubMed] [Google Scholar]


