Abstract
In urethane-chloralose anesthetized, neuromuscularly blocked, ventilated rats, microinjection of NMDA (12 pmol) into the right fourth thoracic segment (T4) spinal intermediolateral nucleus (IML) immediately increased ipsilateral brown adipose tissue (BAT) sympathetic nerve activity (SNA; peak +492% of control), expired CO2 (+0.1%) heart rate (+48 beats min−1) and arterial pressure (+8 mmHg). The increase in BAT SNA evoked by T4 IML microinjection of NMDA was potentiated when it was administered immediately following a T4 IML microinjection of 5-hydroxytryptamine (5-HT, 100 pmol) or the 5-HT1A/5-HT7 receptor agonist, 8-OH-DPAT (600 pmol), (area under the curve: 184%, and 259% of the NMDA-only response, respectively). In contrast, T4 IML microinjection of the 5-HT2 receptor agonist, DOI (28 pmol) did not potentiate the NMDA-evoked increase in BAT SNA (101% of NMDA-only response). Microinjection into the T4 IML of the selective 5-HT1A antagonist, WAY-100635 (500 pmol), plus the 5-HT7 antagonist, SB-269970 (500 pmol), prevented the 5-HT-induced potentiation of the NMDA-evoked increase in BAT SNA. When administered separately, WAY-100635 (800 pmol) and SB-269970 (800 pmol) attenuated the 8-OH-DPAT-induced potentiation of the NMDA-evoked increase in BAT SNA through effects on the amplitude and duration of the response, respectively. The selective 5-HT2 receptor antagonist, ketanserin (100 pmol), did not attenuate the potentiations of the NMDA-evoked increase in BAT SNA induced by either 5-HT or 8-OH-DPAT. These results demonstrate that activation of 5-HT1A/5-HT7 receptors can act synergistically with NMDA receptor activation within the IML to markedly increase BAT SNA.
Keywords: 5-HT receptor, thermoregulation, sympathetic preganglionic neurons
Introduction
5-hydroxytryptamine (5-HT) receptor agonists produce changes in body temperature (Lipton and Clark, 1986) that depend on the receptor specificity of the agonist (Gudelsky et al., 1986) and on the route of administration. For example, systemic administration of 5-HT results in hypothermia whereas spinal administration of 5-HT results in hyperthermia (Clark and Lipton, 1986; Lopachin and Rudy, 1982). To elucidate the physiology and pharmacology underlying the changes in body temperature evoked by spinal administration of 5-HT receptor agonists, the current study sought to determine the specific pharmacology of the serotonergic potentiation of glutamate agonist-evoked increases in the sympathetic outflow to brown adipose tissue (BAT), the principle effector of thermogenesis in small mammals (Cannon and Nedergaard, 2004).
Many serotonergic neurons of the rostral raphe pallidus area provide input to the intermediolateral cell column (IML) (Allen and Cechetto, 1994; Bowker et al., 1981; Helke et al., 1997) and are retrogradely labelled by injection of trans-synaptic viral tracers into BAT (Cano et al., 2003). We have recently demonstrated that activation of 5-HT receptors within the IML of the spinal cord can activate the sympathetic outflow to BAT and that concomitant activation of 5-HT and N-methyl-D-aspartate (NMDA) receptors within the IML elicits a robust activation of the sympathetic outflow to BAT and an enhanced BAT thermogenesis arising from a 5-HT-mediated potentiation of the BAT response to glutamate receptor stimulation in the IML (Madden and Morrison, 2006). However, several subtypes of 5-HT receptors, including 5-HT1A/5-HT7, 5-HT2, and 5-HT5A are located within the IML (Cornea-Hebert et al., 1999; Doly et al., 2004; Maeshima et al., 1998; Marlier et al., 1991; Thor et al., 1993) and the detailed neuropharmacology within the IML responsible for the powerful potentiating effect of 5-HT on BAT sympathetic nerve activity (SNA) is not clear. Therefore, in the present study we sought to determine the specific serotonin receptor subtype(s) involved in enhancing the spinally-evoked increase in BAT SNA.
Materials and Methods
All procedures conform to the regulations detailed in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Oregon Health & Science University. Male Sprague-Dawley rats (Charles River, Indianapolis, IN, n=32) weighing 250–450g were given ad libitum access to standard rat chow and water in a colony room maintained at 22–23 °C and kept on a 12:12-hour light-dark cycle. All efforts were made to minimize animal suffering, and to limit the number of animals used. Rats were anesthetized with isoflurane (2–3% in oxygen) and instrumented with femoral arterial and venous catheters. Isoflurane anesthesia was gradually terminated over a ten minute period while urethane and chloralose anesthesia (750mg/kg and 60mg/kg iv, respectively) was administered. All physiological variables were digitized (Micro 1401 MKII; Cambridge Electronic Design (CED), Cambridge, UK) and recorded onto a computer hard drive. Arterial blood pressure was recorded from the arterial catheter attached to a pressure transducer and heart rate (HR) was derived from the arterial pressure signal. The trachea was cannulated, and the animals were ventilated (tidal volume: ~1ml per 100g body weight, ~60 cycles per minute) with 100% oxygen. End-expiratory CO2 was monitored using a capnometer (model 2200; Dynatech Electro-optics, Saline, MI, USA) and ventilation rate was adjusted to maintain resting end-expiratory CO2 in the range of 3.5–5.0%. Neuromuscular blockade was achieved by administration of d-tubocurarine (0.5 mg iv, supplemented with 0.1 mg as needed to suppress spontaneous contractions of the diaphragm). Adequacy of anesthesia was verified prior to initial and supplemental neuromuscular blockade by absence of a withdrawal reflex or pressor response to foot pinch as well as by absence of a corneal reflex. Colonic (core) temperature was monitored using a copper-constantan thermocouple inserted 6 cm into the rectum and was maintained between 36.5–37.5° C with a water perfused heating/cooling blanket and a heat lamp. Animals were placed in a stereotaxic instrument with the incisor bar positioned 4 mm below the interaural line. To stabilize the spinal cord a clamp was placed on the caudal thoracic spinal vertebrae. The third thoracic vertebra was removed to provide access to the underlying fourth thoracic spinal segment (T4), see (Gelderd and Chopin, 1977).
Recording BAT SNA and temperature
The BAT temperature was monitored using a thermocouple meter (TC-1000, Sable Systems International, Las Vegas, NV, USA) with a copper-constantan thermocouple (Physitemp, Clifton, NJ, USA) inserted into the intact, left interscapular fat pad. Postganglionic BAT SNA was recorded under mineral oil with a bipolar hook electrode from the central cut end of a small diameter (~100μm) nerve bundle isolated from the ventral surface of the right interscapular fat pad after dividing it along the midline and reflecting it laterally. Nerve activity was filtered (1–300 Hz) and amplified (10,000–50,000×) with a Cyberamp 380 (Axon Instruments, Union City, CA). Spike 2 software (CED) was used to obtain a continuous measure (4 s bins) of BAT SNA amplitude by calculating the root mean square (rms) amplitude of the BAT SNA (square root of the total power in the 0.1 to 20 Hz band) from the autospectra of sequential 4-s segments of BAT SNA. Control values of BAT SNA were the averages of the BAT SNA amplitudes during the 32-s periods immediately prior to treatments. Peak BAT SNA was defined as the average value during the 32-s period of maximal change in BAT SNA evoked by a treatment. The onset of the evoked response was defined as the time point at which the measured variable first exceeded the maximal value recorded during the control period and remained elevated for at least 12 seconds. The offset was defined as the time point at which the measured variable returned to the maximal value recorded during the control period. To provide a measure of the BAT SNA response that included both its amplitude and duration, the area under the curve (AUC) was assessed using the area function of Spike 2 analysis software (CED) and is defined as the sum of the BAT SNA amplitudes (rms, 4s bins) between the onset and offset of the response. For all paired comparisons between a control response and a test response following a treatment, the AUC for each response was calculated using the longer of the two response durations.
Microinjections into the spinal cord
Microinjection coordinates for the IML were 0.4–0.6 mm lateral to the midline (just medial to the area at which the dorsal roots enter the spinal cord), and 0.8 mm ventral to the dorsal surface of the spinal cord; and for the dorsal horn: 0.5 mm lateral to the midline and 0.15 mm ventral to the dorsal surface of the spinal cord. Glass micropipettes (outer tip diameter, 20–30 μm) were used for all microinjections which were given over a 5–20 second period using a pressure injection system (model IIe, Toohey Company, Fairfield, NJ, USA). The volume of the microinjection was determined using a reticule to watch the movement of the meniscus in the micropipette. To make multiple microinjections into the same IML coordinates the micropipette was retracted vertically, emptied, rinsed with distilled water or saline, refilled and then repositioned into the IML.
The IML and dorsal horn microinjection sites were marked by pressure microinjection of fluorescent polystyrene microspheres (FluoSpheres, F8801 or F8803, Molecular Probes, Eugene, OR, USA) either included in the injectate (1:200 dilution of FluoSpheres in the injectate) or microinjected at the appropriate coordinates at the conclusion of the experiment. Rats were perfused (10% paraformaldehyde) transcardially, spinal cords were removed, post fixed (12–24 hours) and sectioned (60μm coronal sections) on a vibratome. Spinal cord sections were mounted on slides and microinjection sites were localized and photographed, as described previously (Madden and Morrison, 2005).
Drugs and Solutions
All drugs were obtained from Sigma (St. Louis, MO, USA) except isoflurane, which was obtained from Abbott Laboratories (North Chicago, IL, USA) and SB-269970 hydrochloride which was obtained from Tocris Bioscience (Ellisville, MO, USA). All drugs were dissolved in saline, except for 5-HT which was dissolved in saline containing 0.1% ascorbic acid. The dose of 5-HT was chosen as the minimum dose capable of producing a significant potentiation of the NMDA-evoked increase in BAT SNA, based on our previous work (Madden and Morrison, 2006). Since 8-hydroxy-2-(di-n-propyl-amino)tetralin hydrobromide (8-OH-DPAT) has a binding affinity for the 5-HT1A and 5-HT7 receptors that is approximately equal to that of 5-HT (Stowe and Barnes, 1998; Zifa and Fillion, 1992), the dose of 8-OH-DPAT was chosen to be within the range of effective doses of 5-HT based on our previous work (Madden and Morrison, 2006). Since R(−)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI) has a binding affinity for the 5-HT2 receptor that is approximately five times that of 5-HT (Zifa and Fillion, 1992), the dose of DOI was chosen to be approximately one-fifth of the effective doses of 5-HT based on our previous work (Madden and Morrison, 2006). Doses of antagonists were chosen to exceed the agonist dose by at least five-fold according to the binding affinity at the receptor for which the antagonist has the highest affinity (Sharif et al., 2004; Stowe and Barnes, 1998; Zifa and Fillion, 1992; Tocris material data sheet).
Statistics
All statistics were performed using Systat software (version 10, Systat Software Inc., Richmond, CA, USA). Data are expressed as mean ± SE. Statistical significance was assessed using an ANOVA for repeated measures. Following a significant F value, post hoc testing was performed using layered Bonferroni analysis. Significance level was p<0.05.
Protocols
The test for a potentiation of the spinal NMDA-evoked response of BAT SNA consisted of a protocol with three microinjections into the T4 IML: an initial (control) microinjection of NMDA, microinjection of a test compound, followed by a microinjection of NMDA. All rats received control microinjections of NMDA (12 pmol in 60 nl) into the right T4 IML (ipsilateral to the nerve recording). After the increased BAT SNA following the first microinjection of NMDA returned to control levels, one of the following drugs was microinjected into the same IML site: 5-HT (100 pmol in 60nl, n=10); the 5-HT1A/5-HT7 receptor agonist, 8-OH-DPAT (600 pmol in 60 nl, n=14); the 5-HT2 receptor agonist, DOI (28 pmol in 60 nl, n=5) or the 5-HT1A receptor antagonist, WAY-100635 (800 pmol in 80 nl, n=6). Within 5 minutes of the microinjection of one of the preceding compounds a second microinjection of NMDA (12 pmol in 60 nl) was made at the same coordinates within the T4 IML.
To determine the 5-HT receptor subtype responsible for the potentiating effect of 5-HT on the NMDA-evoked response, the potentiation protocol was repeated, but immediately following the microinjection of 5-HT (100 pmol in 60 nl) a microinjection of either (1) the selective 5-HT1A receptor antagonist, WAY-100635 (500 pmol) plus the selective 5-HT7 antagonist, (2R)-1-[(3-Hydroxyphenyl)sulfonyl]-2-[2-(4-methyl-1-piperidinyl)ethyl] pyrrolidine hydrochloride (SB-269970; 500 pmol; (Thomas et al., 2000) in 100 nl, (n=5); or (2) the selective 5-HT2 receptor antagonist, ketanserin tartrate (100 pmol in 100 nl, n=5), was made into the T4 IML, followed by a subsequent microinjection of NMDA (12 pmol in 60 nl) into the same site. A similar protocol was used to evaluate the 5-HT receptor subtype responsible for the potentiating effect of 8-OH-DPAT with the exception that the three selective 5-HT receptor antagonists were administered in separate trials: WAY-100635 (800 pmol in 80 nl, n=5), SB-269970 (800 pmol in 80 nl, n=5), or ketanserin tartrate (80 pmol in 80 nl, n=5) and the 5-HT receptor antagonists were administered prior to the microinjection of 8-OH-DPAT (600 pmol in 60 nl), followed by a subsequent microinjection of NMDA (12 pmol in 60 nl) into the same site.
To control for the possibility that the responses evoked by microinjection of serotonergic agents into the IML were mediated by diffusion to sites outside of the IML a subset of rats (n=2) also received a microinjection of NMDA into the IML followed by a microinjection of 8-OH-DPAT into the dorsal horn and a subsequent microinjection of NMDA into the IML. In addition, we have previously demonstrated that microinjection of NMDA into the dorsal horn does not increase BAT SNA and that microinjection of serotonin into the dorsal horn does not affect the NMDA-evoked increase in BAT SNA (Madden and Morrison, 2006).
Results
NMDA-evoked BAT SNA
Consistent with our previous report (Madden and Morrison, 2006), in untreated rats whose core temperature was maintained at 37.0 ± 0.5 °C, right BAT SNA exhibited only low-amplitude discharge and microinjection of NMDA into the right T4 IML (ipsilateral to the BAT sympathetic nerve recording) immediately increased BAT SNA, expired CO2, arterial pressure and HR (Table 1), but not left BAT temperature which was recorded contralateral to the microinjections into the right T4 IML.
Table 1.
Effects on cardiovascular, metabolic and thermogenic variables elicited by microinjection of NMDA into the intermediolateral cell column at the fourth thoracic segment.
| Control | NMDA into T4 IML | |
|---|---|---|
| BAT SNA (% control) | 100 | +492 ± 52 * |
| Expired CO2 (%) | 4.6 ± 0.1 | +0.1 ± 0.0 * |
| HR (beats min−1) | 391 ± 9 | +48 ± 5 * |
| MAP (mmHg) | 102 ± 4 | +8 ± 1 * |
Values are mean ± SE (n=32) for physiological variables in the control condition and the peak changes within 10 minutes after the microinjection of NMDA into the intermediolateral cell column at the fourth thoracic segment on the right side (ipsilateral to the recorded nerve).
indicates p<0.05, compared to the control condition.
5-HT potentiation of the NMDA-evoked BAT SNA is reversed by 5-HT1A/5-HT7 receptor antagonism but not by 5-HT2 receptor antagonism
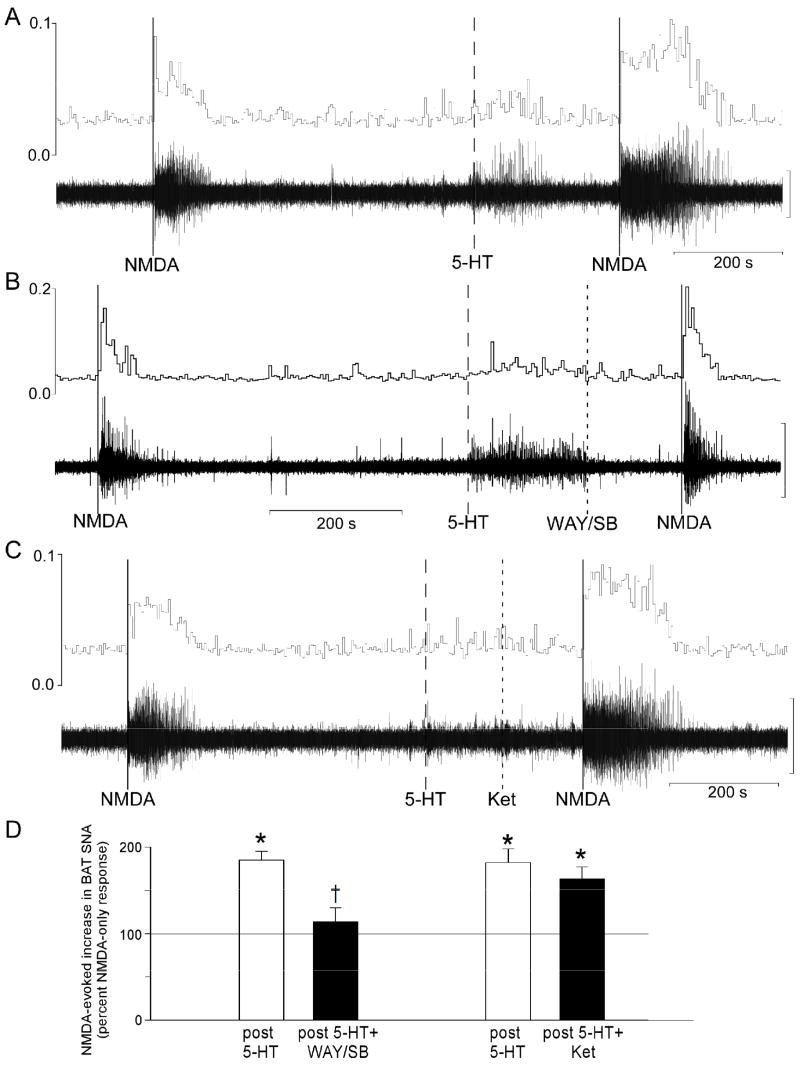
Microinjection of 5-HT into the T4 IML did not increase the basal level of BAT SNA (post-5-HT: 113 ± 7% of activity prior to 5-HT, n=10), though there was an occasional small amplitude, brief increase in BAT SNA that returned to basal levels prior to any subsequent injections. The NMDA-evoked increase in BAT SNA was significantly potentiated following microinjection of 5-HT into the T4 IML (Fig. 1A and 1D). Following 5-HT, the area under the curve (AUC) of the BAT SNA response evoked by microinjection of NMDA into the T4 IML was nearly doubled to 184 ± 10% of the control NMDA response. The 5-HT-evoked potentiation of the increase in BAT SNA evoked by NMDA was manifested as a significant increase in the peak amplitude of the BAT SNA (NMDA-evoked peak increase in BAT SNA prior to 5-HT was +484 ± 75% of control compared to +561 ± 66% of control following 5-HT) and a significant lengthening of the duration of the response (NMDA-only: 109 ± 8 seconds compared to NMDA after 5-HT: 238 ± 23 seconds) (Fig. 1A). In six of these ten cases, a microinjection of a mixture of the 5-HT1A receptor antagonist, WAY-100635 (500 pmol) and the 5-HT7 antagonist, SB269970 (500 pmol), following the microinjection of 5-HT (100 pmol), prevented the potentiation of the NMDA response (Fig. 1B and 1D). In contrast, in the other four cases, a microinjection of the 5-HT2 receptor antagonist, Ket (80 pmol) following the microinjection of 5-HT (100 pmol), did not attenuate the potentiation of the NMDA response (Fig. 1C and 1D).
Figure 1.
Representative examples of BAT SNA during microinjections of NMDA (solid lines) into the intermediolateral nucleus at the fourth thoracic segment (T4 IML). (A) The NMDA-evoked increase in BAT SNA was potentiated by prior microinjection of 5-hydroxytryptamine (5-HT) into the T4 IML (dashed line). (B) This potentiation was prevented by microinjection of the 5-HT1A receptor antagonist, WAY-100635 plus the 5-HT7 receptor antagonist, SB-269970 into the T4 IML (dotted line). (C) Microinjection of the 5-HT2A receptor antagonist, ketanserin into the T4 IML (dotted line) did not attenuate the 5-HT-evoked potentiation of the NMDA-evoked increase in BAT SNA. Vertical scale bar for BAT SNA represents 100μV in panels A, B, and C. (D) Magnitudes of the BAT SNA responses to NMDA were determined as the area under the curve (AUC) of the root mean square (rms) values of BAT SNA. For each treatment, the bar height represents the mean magnitude of the NMDA-evoked increases in BAT SNA expressed as a percent of the corresponding control response elicited by microinjection of NMDA alone (i.e., prior to 5-HT). Pre-treatment groups are: 5-HT (open bar, n=6), 5-HT followed by WAY-100635 and SB-269970 (5-HT+WAY/SB, filled bar, n=6), 5-HT (open bar, n=4) and 5-HT followed by ketanserin (5-HT+Ket, filled bar, n=4).
* p<0.05 repeated measure comparison to the NMDA-only response.
† p<0.05 repeated measure comparison to the NMDA after 5-HT response.
5-HT1A/5-HT7 but not 5-HT2 receptor activation potentiates NMDA-evoked BAT SNA
The NMDA-evoked increase in BAT SNA was also potentiated following microinjection of the selective 5-HT1A/5-HT7 receptor agonist, 8-OH-DPAT, into the T4 IML (Fig. 2A and 2C), but not following microinjection of 8-OH-DPAT into the dorsal horn (data not shown). The example in Fig. 2A shows that the 8-OH-DPAT potentiation of the NMDA-evoked response in BAT SNA was manifested as increases in both the peak amplitude and the duration of the response. The peak increase in BAT SNA evoked by NMDA following 8-OH-DPAT was +1815% of control compared to +1017% of control prior to 8-OH-DPAT, representing a +71% increase in amplitude (group mean, n=14: +88 ± 19% increase in amplitude compared to NMDA-evoked responses prior to 8-OH-DPAT). In the example in Fig 2A the response duration was 236 s following 8-OH-DPAT compared to 131 s prior to 8-OH-DPAT (for group data see Table 2). In contrast to the effect of 8-OH-DPAT, the NMDA-evoked increase in BAT SNA following microinjection of the selective 5-HT2 agonist, DOI into the T4 IML (Fig. 2B and 2C) did not differ from the increase evoked by NMDA alone (the AUC of the response following DOI was 101 ± 14% of the NMDA-only response; peak increase in BAT SNA: +494 ± 107% of control for NMDA alone versus +461 ± 148% of control following DOI; response duration: 161 ± 28 s for NMDA alone versus 170 ± 26 s following DOI; n=5).
Figure 2.
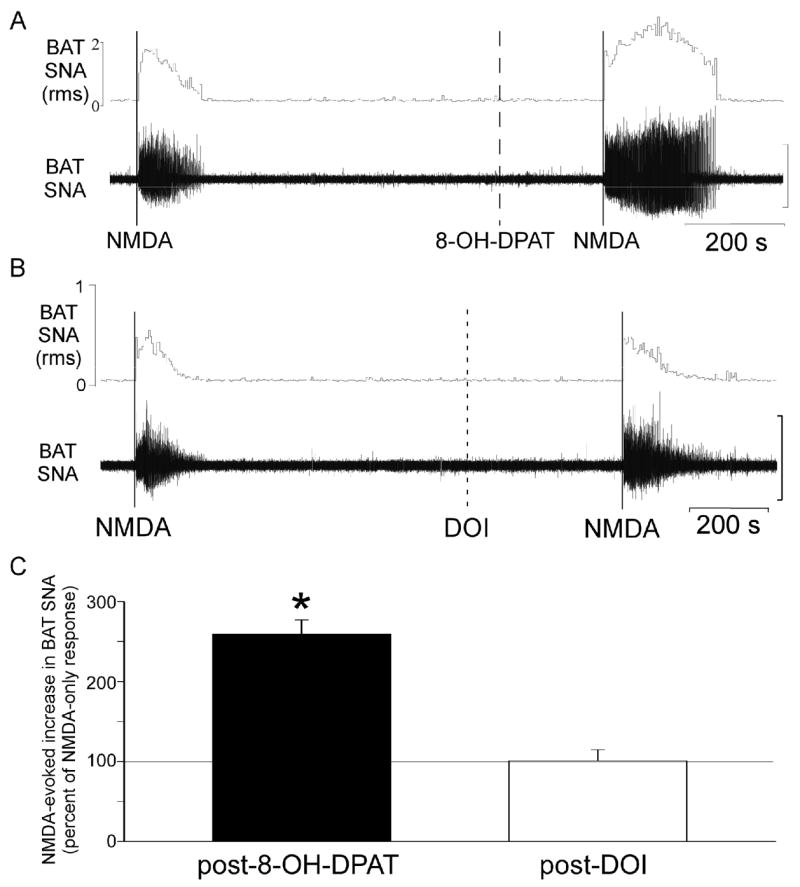
The NMDA-evoked increase in BAT SNA is potentiated by activation of 5-HT1A/5-HT7 receptors but not by activation of 5-HT2 receptors within the IML. (A) Representative example of BAT SNA during microinjections of NMDA (solid lines) into the intermediolateral nucleus at the fourth thoracic segment (T4 IML) before and after microinjection of the 5-HT1A/5-HT7 receptor agonist, 8-OH-DPAT into the T4 IML (dashed line). In this individual example, the NMDA response following 8-OH-DPAT was 278% of the NMDA response prior to 8-OH-DPAT. (B) Representative example of BAT SNA during microinjections of NMDA (solid lines) into the T4 IML before and after microinjection of the 5-HT2A receptor agonist, DOI, into the T4 IML (dashed line). In this individual example, the NMDA response following DOI was 109% of the NMDA response prior to microinjection of DOI into the same site. (C) Group data for the NMDA-evoked increase in BAT SNA following microinjection of 8-OH-DPAT (n=14) or DOI (n=5) into the T4 IML (mean + S.E.M., expressed as a percentage of the paired NMDA-only response). *p<0.05 compared to the NMDA-only response. Vertical scale bars for BAT SNA represent 40 μV in A and 50 μV in B.
Table 2.
Effects of 8-OH-DPAT and 5-hydroxytryptamine receptor antagonists on the peak magnitude and duration of brown adipose tissue sympathetic nerve activity (BAT SNA) evoked by microinjection of NMDA into the intermediolateral cell column
| NMDA only (n=14) | NMDA after 8-OH-DPAT (n=14) | NMDA after 8-OH-DPAT following | |||
|---|---|---|---|---|---|
| WAY-100635 (n=5) | SB-269970 (n=5) | Ketanserin (n=4) | |||
| Peak BAT SNA (% control) | +479 ± 70 | +935 ± 129* | +397 ± 139† | +785 ± 153* | +889 ± 170* |
| BAT SNA Response duration (s) | 142 ± 10 | 241 ± 14* | 161 ± 21† | 169 ± 23† | 188 ± 13* |
Effects on brown adipose tissue sympathetic nerve activity (BAT SNA) of microinjection into the intermediolateral cell column at the fourth thoracic segment (T4 IML) of NMDA; NMDA after 8-OH-DPAT; or NMDA after 8-OH-DPAT following the 5-HT1A antagonist, WAY-100635, or the 5-HT7 antagonist, SB-269970, or the 5-HT2 antagonist, Ketanserin. Values are mean ± SE.
indicates p<0.05, repeated measure comparison to the NMDA only response.
indicates p<0.05, repeated measure comparison to the NMDA after 8-OH-DPAT response.
Microinjection of the selective 5-HT1A receptor antagonist, WAY-100635, into the T4 IML prior to microinjection of 8-OH-DPAT attenuated the 8-OH-DPAT-mediated potentiation of the NMDA-evoked increase in BAT SNA (Fig. 3A and D, Fig. 4A and Table 2). Attenuation of the 8-OH-DPAT-mediated potentiation of the NMDA-evoked response by prior treatment with WAY-100635 was manifested as a reduction of both the enhancement of the peak amplitude and of the lengthening of the duration of the response (Fig. 3A, Fig. 4A, and Table 2). Microinjection of WAY-100635 alone (without subsequent administration of 8-OH-DPAT) had no consistent effect on the NMDA-evoked changes in BAT SNA (the AUC of the NMDA response following WAY-100635 was 121 ± 39% of the control NMDA response, n=6).
Figure 3.
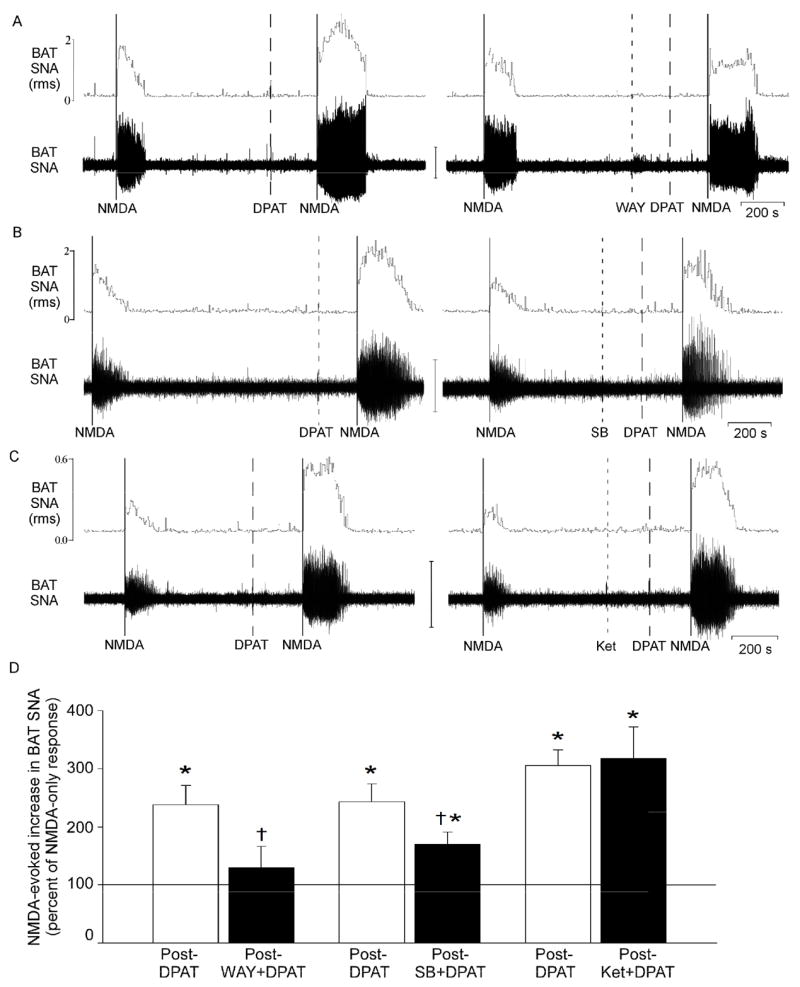
Effect of selective 5-HT receptor antagonists on the ability of 8-OH-DPAT to potentiate the increase in BAT SNA evoked by microinjection of NMDA into the T4 IML. (A) microinjection of the 5-HT1A receptor antagonist, WAY-100635, prior to 8-OH-DPAT attenuated the 8-OH-DPAT potentiation of the NMDA response. (B) prior microinjection of the 5-HT7 receptor antagonist, SB-269970, attenuated the 8-OH-DPAT potentiation of the NMDA-evoked increase in BAT SNA. (C) the 5-HT2A receptor antagonist, ketanserin, did not attenuate the 8-OH-DPAT potentiation of the NMDA-evoked increase in BAT SNA. Vertical scale bar for BAT SNA represents 50μV in panels A and C, and 100 μV in panel B. (D) Magnitudes of the BAT SNA excitatory responses to NMDA were determined as the area under the curve (AUC) of the root mean square (rms) values of BAT SNA. For each treatment, the bar height represents the mean magnitude of the NMDA-evoked increases in BAT SNA expressed as a percent of the corresponding control response elicited by microinjection of NMDA alone (i.e., prior to 8-OH-DPAT). Pre-treatment groups are: 8-OH-DPAT (DPAT, open bar, n=5), WAY-100635 followed by 8-OH-DPAT (WAY + DPAT, filled bar, n=5), DPAT (open bar, n=5), SB-269970 followed by 8-OH-DPAT (SB + DPAT, filled bar, n=5), and DPAT (open bar, n=4), Ketanserin followed by 8-OH-DPAT (Ket + DPAT, filled bar n=4).
* p<0.05 repeated measure comparison to the NMDA-only response.
† p<0.05 repeated measure comparison to the NMDA after 8-OH-DPAT response.
Figure 4.
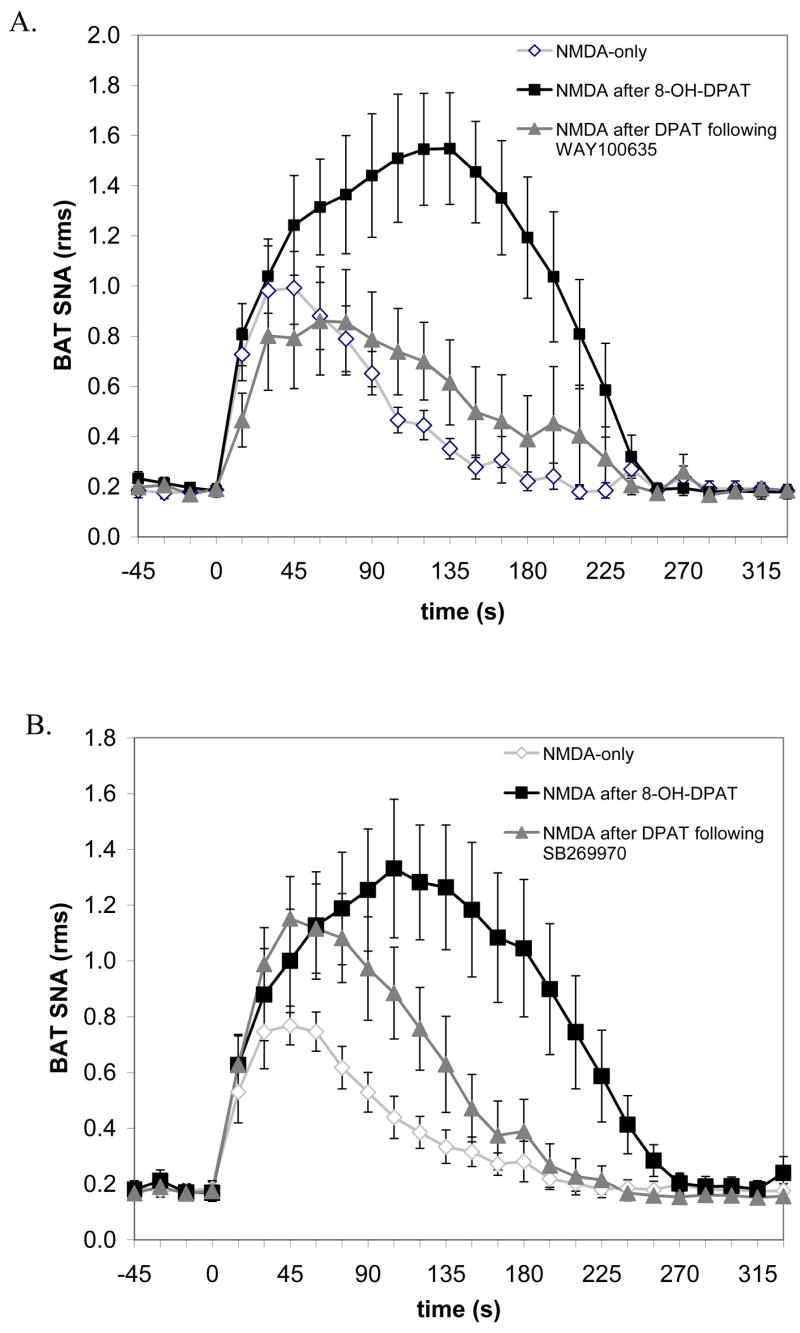
The mean time courses of the effects of selective 5-HT receptor antagonists on the 8-OH-DPAT-mediated potentiation of the increase in BAT SNA evoked by microinjection of NMDA into the T4 IML. (A) The 5-HT1A receptor antagonist, WAY-100635, attenuated both the amplitude and the duration of the 8-OH-DPAT potentiation. (B) The 5-HT7 receptor antagonist, SB269970, attenuated the duration but not the amplitude of the 8-OH-DPAT potentiation. Data represent group means and SEM for the BAT SNA (root mean square, rms) measured in 15-second bins over the time course of the NMDA-evoked responses to microinjection of NMDA-only into the T4 IML (open diamonds, n=5), microinjection of NMDA into the T4 IML after microinjection of 8-OH-DPAT into the T4 IML (closed squares, n=5), and microinjection of NMDA into the T4 IML following microinjection of 8-OH-DPAT into the T4 IML after microinjection of (A) WAY-100635 or (B) SB269970 into the T4 IML (closed triangles, n=5).
Microinjection of the selective 5-HT7 receptor antagonist, SB-269970 into the T4 IML prior to microinjection of 8-OH-DPAT attenuated the 8-OH-DPAT potentiation of the NMDA-evoked increase in BAT SNA through an effect on the duration rather than the amplitude of the response (Fig. 3B and D, Fig. 4B, and Table 2). Prior treatment with SB-269970 shortened the duration of the NMDA-evoked response following 8-OH-DPAT (190 s, Fig. 4B) compared to the response to NMDA after 8-OH-DPAT only (263 s, Fig. 4B; group data provided in Table 2). In contrast, the peak amplitude of the NMDA-evoked response following 8-OH-DPAT was not different from that following 8-OH-DPAT preceded by SB-269970 (Table 2). Microinjection of the 5-HT2 antagonist, ketanserin, did not affect the 8-OH-DPAT potentiation of the NMDA-evoked increase in BAT SNA (Fig. 3C and D, and Table 2).
Histological localization of microinjection sites
Representative examples illustrating the locations of the microinjection sites in the T4 IML and the dorsal horn are shown in Figure 5. All microinjection sites targeting the T4 IML were found in the IML or just lateral to the IML in the white matter within 100μm of the IML. Microinjection sites targeting the dorsal horn were found in lamina I or lamina II.
Figure 5.
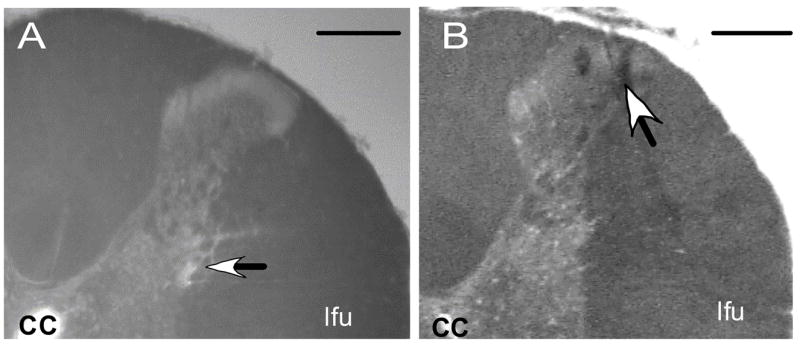
Representative examples of the dorsal right quadrant of coronal sections through the spinal cord illustrating the histological localization of the position (arrow) of the micropipette tip targeting (A) the intermediolateral cell column and (B) the dorsal horn within the fourth thoracic segment. Scale bars = 250 μm. cc, central canal; lfu, lateral funiculus
Discussion
The results of the present study demonstrate that microinjection of 5-HT, or the 5-HT1A/5-HT7 receptor agonist, 8-OH-DPAT, into the IML potentiates NMDA-evoked BAT SNA and that these potentiations are attenuated by 5-HT1A and 5-HT7 receptor antagonists. We conclude that activation of 5-HT1A/5-HT7 receptors can act synergistically with NMDA receptor activation within the IML to markedly potentiate the glutamate receptor-mediated increase in BAT SNA. In contrast, microinjection of the selective 5-HT2 agonist, DOI, into the IML did not potentiate the NMDA-evoked increase in BAT SNA, nor did the selective 5-HT2 antagonist, Ket, attenuate the 5-HT- or 8-OH-DPAT-evoked potentiations of the increase in BAT SNA evoked by NMDA. Thus, the current findings extend our previous observations by indicating a significant role for spinal 5-HT1A and 5-HT7 receptors, but not those of the 5-HT2 subtype, in mediating the 5-HT-evoked potentiation of NMDA-evoked increases in BAT SNA.
In the present study, spinal administration of the 5-HT1A/5-HT7 receptor agonist, 8-OH-DPAT, enhanced the increase in BAT SNA evoked by microinjection of NMDA into the T4 IML. We have previously demonstrated that the enhancement of BAT SNA following activation of 5-HT receptors within the spinal cord results in enhanced thermogenesis in that tissue (Madden and Morrison, 2006), therefore the present observation of an enhancement of BAT SNA by spinal administration of 8-OH-DPAT would seem to be at odds with the hypothermic effect, via activation of both 5-HT1A and 5-HT7 receptors, of systemically administered 8-OH-DPAT (Faure et al., 2006; Hedlund et al., 2004). This apparent discrepancy highlights the differing effects of 5-HT released at different sites in the central thermoregulatory network controlling body temperature. For example, while systemically administered 8-OH-DPAT may influence BAT thermogenesis and body temperature through actions in the spinal cord, it also has access to central sites such as the preoptic area, hypothalamus and medial medulla that play significant roles in thermoregulation (Morrison, 2004b; Romanovsky, 2007). Along these lines, microinjection of 8-OH-DPAT into the raphe pallidus area (RPa) inhibits BAT SNA and BAT thermogenesis (Morrison, 2004a) and indeed the anti-thermogenic effect of systemically administered 8-OH-DPAT is reversed by administration of the selective 5-HT1A receptor antagonist, WAY-100635 into the RPa (S.F.M., unpublished observation). Additionally, the hypothermic effect of systemically administered 8-OH-DPAT is likely to be mediated, at least in part, via cutaneous vasodilation resulting in increased heat loss to the environment (Ootsuka and Blessing, 2003). In fact, the reduction in cutaneous vasoconstriction evoked by systemic administration of 8-OH-DPAT is also reversed by microinjection of WAY-100635 into the RPa (Ootsuka and Blessing, 2006a); further supporting the interpretation that the hypothermic effect of systemically administered 8-OH-DPAT is mediated by an action at sites other than the spinal cord.
In the present study microinjection of the 5-HT1A/5-HT7 agonist, 8-OH-DPAT, alone did not increase BAT SNA; however, it did elicit a potent facilitation of the NMDA-evoked increase in BAT SNA. Similarly, Lewis and Coote, recording from sympathetic preganglionic neurons (SPNs) in the upper thoracic spinal segments of rats demonstrated that application of the 5-HT1A/5-HT7 agonist, 5-carboxamidotryptamine (5-CT) alone was not capable of exciting SPNs that had no spontaneous activity (Lewis and Coote, 1990). However, when a stable basal firing rate was maintained by exogenous application of excitatory amino acids the majority of SPNs increased their firing rate in response to application of 5-CT (Lewis and Coote, 1990).
Systemic administration of a 5-HT2A receptor agonist results in hyperthermia (Gudelsky et al., 1986), at least in part via an increase in sympathetic outflow to the cutaneous vasculature (Ootsuka et al., 2004) and possibly also via an increase in BAT thermogenesis (Ootsuka and Blessing, 2006b). In the present study the inability of a microinjection of DOI into the T4 IML to affect BAT SNA suggests that the BAT SPNs are unlikely to be the site at which systemically administered DOI increases BAT thermogenesis, an interpretation that depends on the efficacy of the dose of DOI chosen in the current study. In this regard, it is important to note that the concentration of DOI used in the current study (0.28 mM) is equivalent to the concentration that, when administered systemically (1ml/kg), increased BAT temperature (Ootsuka and Blessing, 2006b) and cutaneous sympathetic vasomotor outflow (Ootsuka et al., 2004). Systemically administered DOI may increase body temperature and BAT thermogenesis by acting at sites upstream of the SPNs, for example areas of the hypothalamus that have been implicated in 5-HT2 receptor-mediated hyperthermia (Chio et al., 2005). Alternatively, since DOI is hallucinogenic (Glennon et al., 1984), systemic administration of this agent in awake animals could result in a “stress”-induced thermogenesis that is secondary to its hallucinogenic properties.
Although the cellular mechanisms through which 5-HT1A/5HT7 agonists potentiate the NMDA-evoked activation of BAT were not addressed in the present study, administration of the 5-HT1A receptor antagonist, WAY-100635, or the 5-HT7 receptor antagonist, SB-269970, was found to attenuate the 8-OH-DPAT-evoked increase in the NMDA-evoked BAT activation whereas the 5-HT2 receptor antagonist, ketanserin, did not affect this potentiation. These data demonstrate that the potentiation evoked by 8-OH-DPAT is a specific receptor mediated effect and that the full potentiating effect of 8-OH-DPAT requires activation of both 5-HT1A and 5-HT7 receptors. 5-HT1A and 5-HT7 receptors are both G-protein coupled receptors; however their signal transduction systems differ significantly. 5-HT1A receptors are negatively coupled to adenylyl cyclase (Barnes and Sharp, 1999; Zifa and Fillion, 1992). In addition, 5-HT1A receptors can be coupled to K+ channels (with activation opening the channels) (Barnes and Sharp, 1999; Zifa and Fillion, 1992). These 5-HT1A receptor mediated responses would tend to inhibit cellular activity. In contrast, 5-HT7 receptors are positively coupled to adenylyl cyclase via Gs-proteins (Barnes and Sharp, 1999). Given the similarity in their ability to enhance the NMDA-evoked increase in BAT SNA, despite the opposing effects of 5-HT1A and 5-HT7 receptors on signal transduction mechanisms, it seems reasonable to speculate that these receptors are located on separate populations of neurons within the spinal cord. The simplest model to explain the current results would consist of a 5-HT1A receptor-mediated inhibition of a GABAergic input to BAT SPNs coupled with a 5-HT7 receptor-mediated increase in the excitability of SPNs. It will be of interest to determine the neurochemical phenotype of neurons within the IML that express the 5-HT1A and 5-HT7 receptor subtypes.
It is important to note that even though metabolism in BAT is not the principle contributor to thermogenesis in adult humans (Astrup et al., 1985), BAT has been shown to be present in adult humans (Heaton, 1972) and several studies have begun to suggest that genetic variations in BAT effector systems (Clement et al., 1996) or expression levels of uncoupling protein 1 (UCP1) in BAT (Oberkofler et al., 1997) can result in a propensity to gain weight in humans (for reviews see: Avram et al., 2005; Del Mar Gonzalez-Barroso et al., 2000). Although serotonergic agents are known to affect metabolic regulation, remarkably little is know about the neural pathways through which 5-HT can influence energy expenditure. The current results provide a potential site at which serotonergic agents could affect metabolic regulation.
In conclusion, the findings of the present study demonstrate that combined activation of NMDA and 5-HT1A/5-HT7 receptors within the IML can act synergistically to markedly increase sympathetic activation of BAT. Further studies will be required to investigate the detailed neurocircuitry responsible for the interactions between excitatory amino acid and serotonergic mechanisms within the IML and to determine the specific physiological conditions under which these mechanisms play a role in regulating body temperature and metabolism.
Acknowledgments
This work was supported by NIH grants NS40987 (SFM) and DK065401 (CJM). We are grateful to Joseph Rathner for his comments on this manuscript and to Brad Sugden for histological assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen GV, Cechetto DF. Serotoninergic and nonserotoninergic neurons in the medullary raphe system have axon collateral projections to autonomic and somatic cell groups in the medulla and spinal cord. Journal of Comparative Neurology. 1994;350:357–366. doi: 10.1002/cne.903500303. [DOI] [PubMed] [Google Scholar]
- Astrup A, Bulow J, Madsen J, Christensen NJ. Contribution of BAT and skeletal muscle to thermogenesis induced by ephedrine in man. American Journal of Physiology. 1985;248:E507–515. doi: 10.1152/ajpendo.1985.248.5.E507. [DOI] [PubMed] [Google Scholar]
- Avram AS, Avram MM, James WD. Subcutaneous fat in normal and diseased states: 2. Anatomy and physiology of white and brown adipose tissue. Journal of the American Academy of Dermatology. 2005;53:671–683. doi: 10.1016/j.jaad.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bowker RM, Westlund KN, Coulter JD. Origins of serotonergic projections to the spinal cord in rat: an immunocytochemical-retrograde transport study. Brain Research. 1981;226:187–199. doi: 10.1016/0006-8993(81)91092-1. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. Journal of Comparative Neurology. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- Chio CC, Tsai SM, Wang JJ, Lin MT. 5-HT2A-mu opioid receptor mechanisms in the hypothalamus mediate interleukin-1beta fever in rats. Neuroscience Letters. 2005;381:6–11. doi: 10.1016/j.neulet.2005.01.074. [DOI] [PubMed] [Google Scholar]
- Clark WG, Lipton JM. Changes in body temperature after administration of adrenergic and serotonergic agents and related drugs including antidepressants: II. Neuroscience & Biobehavioral Reviews. 1986;10:153–220. doi: 10.1016/0149-7634(86)90025-4. [DOI] [PubMed] [Google Scholar]
- Clement K, Ruiz J, Cassard-Doulcier AM, Bouillaud F, Ricquier D, Basdevant A, Guy-Grand B, Froguel P. Additive effect of A-->G (−3826) variant of the uncoupling protein gene and the Trp64Arg mutation of the beta 3-adrenergic receptor gene on weight gain in morbid obesity. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1996;20:1062–1066. [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. Journal of Comparative Neurology. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Del Mar Gonzalez-Barroso M, Ricquier D, Cassard-Doulcier AM. The human uncoupling protein-1 gene (UCP1): present status and perspectives in obesity research. Obesity Reviews. 2000;1:61–72. doi: 10.1046/j.1467-789x.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- Doly S, Fischer J, Brisorgueil MJ, Verge D, Conrath M. 5-HT5A receptor localization in the rat spinal cord suggests a role in nociception and control of pelvic floor musculature. Journal of Comparative Neurology. 2004;476:316–329. doi: 10.1002/cne.20214. [see comment] [DOI] [PubMed] [Google Scholar]
- Faure C, Mnie-Filali O, Scarna H, Debonnel G, Haddjeri N. Effects of the 5-HT7 receptor antagonist SB-269970 on rat hormonal and temperature responses to the 5-HT1A/7 receptor agonist 8-OH-DPAT. Neuroscience Letters. 2006;404:122–126. doi: 10.1016/j.neulet.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Gelderd JB, Chopin SF. The vertebral level of origin of spinal nerves in the rat. Anatomical Record. 1977;188:45–47. doi: 10.1002/ar.1091880106. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sciences. 1984;35:2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Koenig JI, Meltzer HY. Thermoregulatory responses to serotonin (5-HT) receptor stimulation in the rat. Evidence for opposing roles of 5-HT2 and 5-HT1A receptors. Neuropharmacology. 1986;25:1307–1313. doi: 10.1016/0028-3908(86)90101-2. [DOI] [PubMed] [Google Scholar]
- Heaton JM. The distribution of brown adipose tissue in the human. Journal of Anatomy. 1972;112:35–39. [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. European Journal of Pharmacology. 2004;487:125–132. doi: 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Helke CJ, Capuano S, Tran N, Zhuo H. Immunocytochemical studies of the 5-HT(1A) receptor in ventral medullaryneurons that project to the intermediolateral cell column and contain serotonin or tyrosine hydroxylase immunoreactivity. Journal of Comparative Neurology. 1997;379:261–270. [PubMed] [Google Scholar]
- Lewis DI, Coote JH. The influence of 5-hydroxytryptamine agonists and antagonists on identified sympathetic preganglionic neurones in the rat, in vivo. British Journal of Pharmacology. 1990;99:667–672. doi: 10.1111/j.1476-5381.1990.tb12987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton JM, Clark WG. Neurotransmitters in temperature control. Annual Review of Physiology. 1986;48:613–623. doi: 10.1146/annurev.ph.48.030186.003145. [DOI] [PubMed] [Google Scholar]
- Lopachin RM, Rudy TA. The thermoregulatory effects of noradrenaline, serotonin and carbachol injected into the rat spinal subarachnoid space. Journal of Physiology. 1982;333:511–529. doi: 10.1113/jphysiol.1982.sp014466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue in rats. Journal of Physiology. 2005;566:559–573. doi: 10.1113/jphysiol.2005.086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Serotonin potentiates sympathetic responses evoked by spinal NMDA. Journal of Physiology. 2006;577:525–537. doi: 10.1113/jphysiol.2006.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima T, Ito R, Hamada S, Senzaki K, Hamaguchi-Hamada K, Shutoh F, Okado N. The cellular localization of 5-HT2A receptors in the spinal cord and spinal ganglia of the adult rat. Brain Research. 1998;797:118–124. doi: 10.1016/s0006-8993(98)00360-6. [DOI] [PubMed] [Google Scholar]
- Marlier L, Teilhac JR, Cerruti C, Privat A. Autoradiographic mapping of 5-HT1, 5-HT1A, 5-HT1B and 5-HT2 receptors in the rat spinal cord. Brain Research. 1991;550:15–23. doi: 10.1016/0006-8993(91)90400-p. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Activation of 5-HT1A receptors in raphe pallidus inhibits leptin-evoked increases in brown adipose tissue thermogenesis. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2004a;286:R832–837. doi: 10.1152/ajpregu.00678.2003. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News in Physiological Sciences. 2004b;19:67–74. doi: 10.1152/nips.01502.2003. [DOI] [PubMed] [Google Scholar]
- Oberkofler H, Dallinger G, Liu YM, Hell E, Krempler F, Patsch W. Uncoupling protein gene: quantification of expression levels in adipose tissues of obese and non-obese humans. Journal of Lipid Research. 1997;38:2125–2133. [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW. 5-Hydroxytryptamine 1A receptors inhibit cold-induced sympathetically mediated cutaneous vasoconstriction in rabbits. Journal of Physiology. 2003;552:303–314. doi: 10.1113/jphysiol.2003.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW. Activation of 5-HT1A receptors in rostral medullary raphe inhibits cutaneous vasoconstriction elicited by cold exposure in rabbits. Brain Research. 2006a;1073–1074:252–261. doi: 10.1016/j.brainres.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW. Thermogenesis in brown adipose tissue: increase by 5-HT2A receptor activation and decrease by 5-HT1A receptor activation in conscious rats. Neuroscience Letters. 2006b;395:170–174. doi: 10.1016/j.neulet.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Nalivaiko E, Blessing WW. Spinal 5-HT2A receptors regulate cutaneous sympathetic vasomotor outflow in rabbits and rats; relevance for cutaneous vasoconstriction elicited by MDMA (3,4-methylenedioxymethamphetamine, “Ecstasy”) and its reversal by clozapine. Brain Research. 2004;1014:34–44. doi: 10.1016/j.brainres.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. American Journal of Physiology -Regulatory Integrative & Comparative Physiology. 2007;292:R37–46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Drace CD, Williams GW, Crider JY. Cloned human 5-HT1A receptor pharmacology determined using agonist binding and measurement of cAMP accumulation. Journal of Pharmacy & Pharmacology. 2004;56:1267–1274. doi: 10.1211/0022357044346. [DOI] [PubMed] [Google Scholar]
- Stowe RL, Barnes NM. Selective labelling of 5-HT7 receptor recognition sites in rat brain using [3H]5-carboxamidotryptamine. Neuropharmacology. 1998;37:1611–1619. doi: 10.1016/s0028-3908(98)00117-8. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Atkinson PJ, Ho M, Bromidge SM, Lovell PJ, Villani AJ, Hagan JJ, Middlemiss DN, Price GW. [(3)H]-SB-269970--A selective antagonist radioligand for 5-HT(7) receptors. British Journal of Pharmacology. 2000;130:409–417. doi: 10.1038/sj.bjp.0703318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor KB, Nickolaus S, Helke CJ. Autoradiographic localization of 5-hydroxytryptamine1A, 5-hydroxytryptamine1B and 5-hydroxytryptamine1C/2 binding sites in the rat spinal cord. Neuroscience. 1993;55:235–252. doi: 10.1016/0306-4522(93)90469-v. [DOI] [PubMed] [Google Scholar]
- Zifa E, Fillion G. 5-Hydroxytryptamine receptors. Pharmacological Reviews. 1992;44:401–458. [PubMed] [Google Scholar]