Abstract
N,N-dipropyltryptamine (DPT) is a synthetic tryptamine hallucinogen which has been used psychotherapeutically in humans, but has been studied preclinically only rarely. In the present studies, DPT was tested in a drug-elicited head twitch assay in mice, and in rats trained to discriminate lysergic acid diethylamide (LSD), N,N-dimethyl-4-phosphoryloxytryptamine (psilocybin), or 3,4-methylenedioxymethamphetamine (MDMA). A separate group of rats was also trained to recognize DPT itself as a discriminative stimulus, and in all cases, the behavioral effects of DPT were challenged with the selective serotonin (5-HT)2A antagonist M100907, the 5-HT1A selective antagonist WAY-100635, or their combination. In the head twitch assay, DPT elicited dose-dependent effects, producing a biphasic dose-effect curve. WAY-100635 produced a parallel rightward shift in the dose-effect curve for head twitches, indicative of surmountable antagonism, but the antagonist effects of M100907 were functionally insurmountable. DPT produced partial to full substitution when tested in rats trained to discriminate LSD, psilocybin or MDMA, and served as a discriminative stimulus. In all cases, the antagonist effects of M100907 were more profound than were those of WAY-100635. DPT is thus active in two rodent models relevant to 5-HT2 agonist activity. The effectiveness with which M100907 antagonizes the behavioral actions of this compound strongly suggests that the 5-HT2A receptor is an important site of action for DPT, but the modulatory actions of WAY-100635 also imply a 5-HT1A-mediated component to the actions of this compound.
Keywords: hallucinogens, drug-discrimination, head twitch response, serotonin receptors
Introduction
N,N-dipropyltryptamine (DPT, Figure 1A) is a synthetic hallucinogen with structural similarities to the serotonin (5-HT)-like psychedelics lysergic acid diethylamide (LSD, Figure 1B) and N,N-dimethyl-4-phosphoryloxytryptamine (psilocybin, Figure 1C). As a recreational drug of abuse, DPT is known as “The Light” and anecdotal reports of its hallucinogenicity in man are abundant on the internet (erowid.org, lycaeum.org). DPT was used as an adjunct to psychotherapy in the 1960s and 1970s (Soskin, Grof and Richards, 1973; Soskin 1975), but few peer-reviewed experimental studies have been conducted with this compound. However, two recent reports have demonstrated molecular effects of DPT relevant to the study of hallucinogens, including a strong inhibition of 5-HT reuptake into rat synaptosomes (Nagai et al., 2007) as well as moderate affinity partial agonism at the human 5-HT1A receptor (Thiagaraj et al., 2005). As regulatory interest in the synthetic hallucinogens increases, as exemplified by the recent placement of some structurally related tryptamines into Schedule I of the Controlled Substances Act (Leonhart 2004), a greater understanding of the pharmacology of these compounds is needed.
Figure 1.
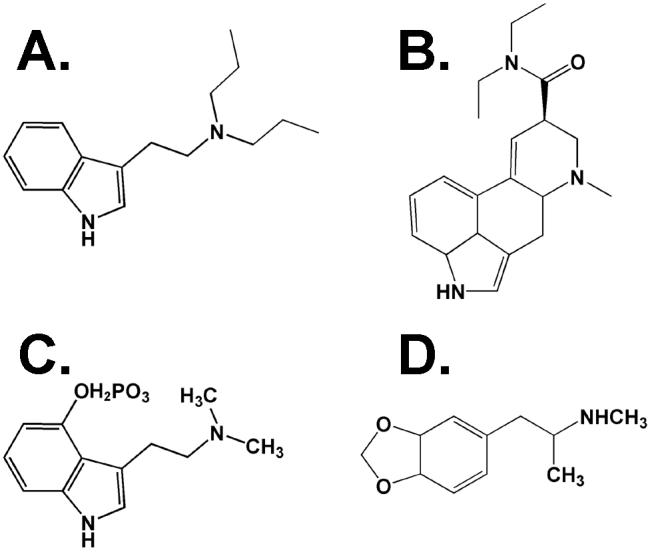
Chemical structures of the compounds studied. A. N,N-dipropyltryptamine (DPT), B. lysergic acid diethylamide (LSD), C. N,N-dimethyl-4-phosphoryloxytryptamine (psilocybin), D. 3,4-methylenedioxymethamphetamine (MDMA).
Many of the fourteen currently recognized 5-HT receptor subtypes are important mediators of the effects of hallucinogenic drugs, but the contributions of specific classes of 5-HT receptors are not well established (Winter et al., 1999). The discriminative stimulus properties of hallucinogens such as mescaline, DOI and LSD have been extensively investigated in several different animal species and it has been shown that, in agreement with studies in humans, these drugs generalize with one another (Winter, 1978; Glennon et al., 1983; Fiorella et al., 1995a). Furthermore, antagonist correlation analysis has determined that the stimulus effects of phenylisopropylamine and indolealkylamine hallucinogens are mediated by agonist activity at 5-HT2A receptors (Fiorella et al., 1995b) and possibly modulated by agonist activity at 5-HT2C (Fiorella et al., 1995c) and 5-HT1A (Reissig et al., 2005) receptors. However, previous work has revealed that affinity at the 5-HT2A receptor only partially predicts the potency of an antagonist in terms of blockade of the stimulus effects of LSD (Fiorella et al. 1995a), while high affinity 5-HT2A agonists such as quipazine and lisuride which seem to lack hallucinogenic effects in man nonetheless substitute in LSD-trained animals (Appel et al., 1999; Fiorella et al. 1995b; Egan et al. 1998). Thus, while 5-HT2A receptor stimulation is a necessary component of the behavioral effects of hallucinogens, other mechanisms must also contribute to the cluster of effects induced by these compounds.
The drug-elicited head twitch response (HTR) (Corne et al., 1963; Corne and Pickering, 1969) is considered to be a selective behavioral model for 5-HT2 agonist activity in the rodent, and several previous studies have established that direct and indirect 5-HT agonists induce this effect (Peroutka et al., 1981; Colpaert and Janssen, 1983; Green et al., 1983; Goodwin and Green, 1985; Darmani et al., 1990a;1990b;1992; Fantegrossi et al., 2004;2005;2006). Further, 5-HT2 receptor antagonists selectively block head twitch behavior (Lucki et al., 1984; Handley and Singh, 1986; Fantegrossi et al., 2004;2005;2006), and their potency in this regard is highly correlated with the antagonist's affinity for 5-HT2 receptors (Peroutka et al., 1981; Ortmann et al., 1982). Importantly, HTR is not elicited by non-hallucinogenic 5-HT2A agonists such as lisuride and quipazine in the mouse (Gonzalez-Maeso et al., 2003; Gonzalez-Maeso et al., 2007), although reports of quipazine-induced HTR in the rat are abundant (e.g., Seeger et al., 1995; Eison and Yocca, 1985; Yocca et al., 1990). Nevertheless, these findings perhaps suggest that the HTR is a specific murine analogue of hallucinogen-like effects.
Thus, we established dose-effect functions for DPT in the absence and presence of the selective 5-HT2A antagonist (+)-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine-methanol (M100907, formerly MDL100907) and the selective 5-HT1A antagonist N-(2-(1-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridyl) cyclohexane-carboxamide (WAY-100635) in the head twitch assay in mice in order to gauge the involvement of 5-HT1A and 5-HT2A receptors in the induction of this behavior. A parallel series of drug discrimination experiments was conducted in rats trained to discriminate LSD, psilocybin, or 3,4-methylenedioxymethamphetamine (MDMA, Figure 1D) in order to characterize the similarity of the discriminative stimulus effects of DPT with those of these three hallucinogen-like training drugs. Similarly, DPT itself was trained as a discriminative stimulus, and the effects of M100907, WAY-100635, or their combination on drug-appropriate responding were tested in all groups of drug discrimination rats receiving active doses of DPT.
Materials and Methods
Animals
Male Swiss Webster mice (Charles River Laboratories, Inc., Wilmington, MA) weighing approximately 20-30 g were housed 5 animals per 44.5 × 22.3 × 12.7 cm Plexiglas cage and used in drug-elicited head twitch experiments. Mice were housed in a temperature-controlled room within the Yerkes National Primate Research Center rodent vivarium that was maintained at an ambient temperature of 22±2°C at 45-50% humidity. Lights were set to a 12-h light/dark cycle. Animals were fed Lab Diet rodent chow (Laboratory Rodent Diet #5001, PMI Feeds, Inc., St. Louis, MO) and water ad libitum until immediately before testing. Animals were not used in experiments until at least 2 days after arrival in the laboratory. Each animal was used only once, and was sacrificed immediately after use.
Male Fischer-344 rats obtained from Harlan Sprague-Dawley Inc. (Indianapolis, IN, USA) at an age of approximately 6 weeks were used in drug discrimination experiments. Rats were housed in pairs with free access to water in a temperature-controlled room at the State University of New York at Buffalo under a constant 12-h light/dark cycle (all experiments were conducted during the light phase.) Caloric intake was controlled to yield a mean body weight of approximately 300 grams; supplemental feedings of standard rat chow were provided following experimental sessions.
All studies were carried out in accordance with the Declaration of Helsinki and with the Guide for Care and Use of Laboratory animals as adopted and promulgated by the National Institutes of Health. Experimental protocols were approved by the Animal Care and Use Committees at Emory University or the State University of New York at Buffalo.
Procedure
Drug-elicited head-twitch response in mice
On experimental days, mice were weighed, marked, and returned to the home cage. Doses were then calculated and prepared for injection. Individual animals were subsequently removed from the home cage, injected i.p. with saline, 1.0 or 3.0 mg/kg WAY-100635, or 0.01 mg/kg M100907, then placed into a 15.24 × 25.40 × 12.70 cm Plexiglas mouse cage. These antagonist doses were chosen based upon our previous demonstrations of their effectiveness against the behavioral effects of other tryptamines (e.g., Fantegrossi et al., 2006). Methods for measuring drug-elicited head twitch behavior have been previously described (Corne et al., 1963; Corne and Pickering, 1969; Boulton and Handley, 1973; Fozard and Palfreyman, 1979; Green et al., 1983; Fantegrossi et al., 2004). For the present experiments, ten minutes after the initial injection, mice were injected with various doses of DPT or saline, then returned to the small observation cage. Five minutes after this second injection, a camera mounted above the observation cage began recording behavior, and continued to do so for 10-min. Videotapes were later scored by two blind observers for HTR, here defined as a rapid rotational jerk of the head that is not contiguous with any grooming or scratching behaviors. All HTR experiments were conducted in a proximate behavioral laboratory at an ambient temperature of 22±2°C, and neither food nor water were available during the tests.
LSD-like, MDMA-like, and psilocybin-like discriminative stimulus effects in rats
Six small animal test chambers (Med-Associates Model ENV-008), each equipped with a house light and an exhaust fan, and housed in larger lightproof Malaguard sound attenuating cubicles (Med-Associates Model ENV-022M) were used for these experiments. Each chamber contained two levers mounted on opposite sides of one wall. Centered between the levers was a dipper that delivered 0.1 ml of sweetened condensed milk diluted 2:1 with tap water.
Twelve subjects were trained to discriminate LSD (0.1 mg/kg) from saline, 11 rats were trained to discriminate MDMA (1.5 mg/kg) from saline, and 12 animals were trained to discriminate psilocybin (0.5 mg/kg, which was successfully trained previously in studies reported in Winter et al., [in press]) from saline as described previously (e.g., Fiorella et al., 1995). All training injections were administered i.p. 15 min pretreatment prior to the start of the experimental session. A non-resetting fixed ratio 10 (FR10) schedule of reinforcement was employed using the MED-PC version IV behavioral programming application. Drug-induced stimulus control was assumed to be present when, in five consecutive sessions, 83% or more of all responses prior to the delivery of the first reinforcer were on the appropriate lever. The training dose of each drug produced greater than 95% drug-appropriate responding. After stimulus control was established with the training agents, tests with DPT in the presence or absence of the antagonists M100907, WAY100635, or their combination were conducted once per week in each animal so long as performance did not fall below the criterion level of 83% correct responding in any one of the previous three training sessions. Half of the test sessions were conducted the day after saline training sessions with the remainder following drug training sessions. During test sessions, no responses were reinforced and the session was terminated after the emission of ten responses on either lever. The distribution of responses between the two levers was expressed as a percentage of total responses emitted on the drug-appropriate lever. Response rate was calculated for each session by dividing the total number of responses emitted on both levers by the elapsed time prior to 10 responses on either lever.
DPT-induced stimulus control in rats
The general procedure was that described above for training with LSD, MDMA, and psilocybin in rats. To establish the stimulus effects of DPT in a group of 12 rats, animals were trained to respond on the drug-appropriate lever following administration of 1.5 mg/kg DPT (15 minute pretreatment). The role of 5-HT2A receptors was then assessed following injection of 0.1 mg/kg M100907 15 minutes before DPT, i.e., 30 minutes before testing. Similarly, the role of 5-HT1A receptors was investigated via administration of 0.3 mg/kg WAY100635 15 min prior to DPT injection.
In all drug discrimination experiments, complete generalization of a training drug to a test drug is said to be present when (a) a mean of 80% or more of all test responses occurs on the drug-appropriate lever; (b) there is no statistically significant difference between the response distributions of the training drug and the test drug; and (c) there is a statistically significant difference between the response distributions of the test drug and saline control sessions. An intermediate degree of generalization is defined as being present when response distributions after a test drug are less than 80% drug-appropriate, and are significantly different from both training conditions. Finally, when the response distribution after a test drug is not statistically significantly different from that in saline control sessions, an absence of generalization of the training drug to the test drug is assumed. Similar criteria are applied to the definitions of full, partial, and no antagonism. Thus, full antagonism is assumed to be present when (a) less than 20% of all test responses are on the training drug-appropriate lever; (b) there is no significant difference between the response distributions in the test of antagonism and the saline control, and (c) there is a statistically significant difference between the response distributions of the test drug alone and in combination with the antagonist.
Data analysis
Data from the HTR experiments are presented as mean±SEM and were compared to values obtained from equivolume saline controls using a Kruskal-Wallis one way analysis of variance (ANOVA) and Tukey's post-hoc tests. These statistical tests were performed using commercially available software, and significance was judged at P<0.05. Drug discrimination data are expressed as percent drug-appropriate responding, which is the number of responses emitted on the drug-appropriate lever as a percentage of the total number of responses emitted. Response rates are expressed as the number of responses per minute, calculated for each session by dividing the total number of responses emitted (prior to the emission of 10 responses on either lever) by elapsed time. Data for any subjects failing to emit 10 responses within the constraints of the 10-min test session were not considered in the calculation of the percent drug-appropriate responding but were included in the analysis of response rates. Generalization was said to occur if 80% or more of the responses were on the drug-appropriate lever. The statistical significance of the generalization of a training drug to DPT, and the antagonism of DPT by M100907, WAY-100635, or their combination was determined using one-way ANOVA to compare the two training conditions with DPT and with DPT in the presence of the antagonists, respectively. Subsequent multiple comparisons were made by the method of Student-Newman-Keuls. Differences were considered to be statistically significant if the probability of their having arisen by chance was < 0.05. All analyses were conducted using commercially available software. Control data were repeated for each comparison and statistical analyses were applied using the appropriate control sessions. However, for purposes of clarity, mean values for control data are shown in all figures.
Drugs
(+)-LSD, SR(±)-MDMA and psilocybin were supplied by the National Institute on Drug Abuse (Research Technology Branch, Research Triangle Park, NC) and dissolved in 0.9% physiological saline solution. M100907 and DPT were synthesized at the Laboratory of Medicinal Chemistry at the National Institutes of Diabetes, Digestive and Kidney Disorders at the National Institutes of Health (Bethesda, MD), and dissolved in sterile water and 0.5 N HCl. WAY-100635 was purchased from Tocris (Ellisville, MO) and dissolved in sterile saline. All injections were administered intraperitoneally at a volume of 1.0 ml/kg (rats) or 1.0 ml/100g (mice).
Results
Drug-elicited head-twitch response in mice
DPT induced a dose-dependent HTR in mice, producing a maximum of approximately 15 twitches during the 10 min observation period at a dose of 3.0 mg/kg (Figure 2, closed circles). Doses of 3.0 and 10.0 mg/kg DPT elicited significantly more head twitch behavior than did saline (P < 0.05 for both doses). Pretreatment with 1.0 mg/kg WAY-100635 (Figure 2, open inverted triangles) produced a 3-fold parallel rightward shift in the HTR dose-effect curve, suggesting competitive antagonism. Following pretreatment with WAY-100635, 10.0 mg/kg DPT induced significantly more head twitch behavior than did saline (P < 0.05). Importantly, administration of a 3-fold higher dose of WAY-100635 (Figure 2, filled inverted triangle) did not produce any further antagonism of the HTR. A more dramatic shift in the dose-effect curve was observed following administration of 0.1 mg/kg M100907 (Figure 2, open triangles). Following pretreatment with M100907, no dose of DPT elicited significantly more HTR than did saline. Doses higher than 10.0 mg/kg DPT were not tested due to the observation of convulsant effects at 30.0 mg/kg during pilot studies.
Figure 2.
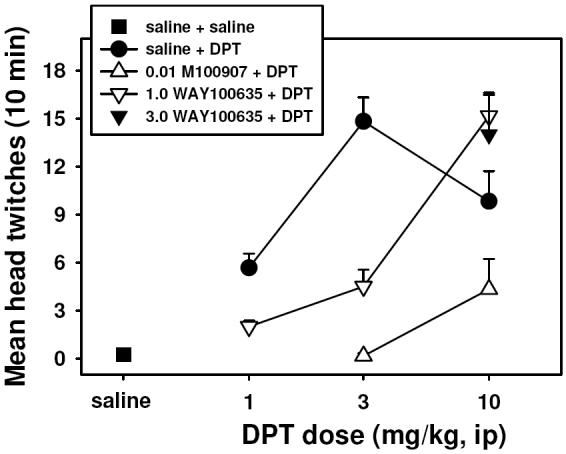
Effects of DPT on head twitch behavior in mice (filled circles), antagonism of these effects by 1.0 mg/kg (unfilled inverted triangles) and 3.0 mg/kg (filled inverted triangle) of the selective 5-HT1A antagonist WAY100635, and by 0.01 mg/kg of the selective 5-HT2A antagonist M100907 (unfilled triangles). All points represent the mean ± SEM (N = 6 mice per group), and any points without error bars indicate instances in which the SEM is encompassed by the data point. Abscissa: Dose of DPT expressed as mg/kg on a log scale. Ordinate: Mean head twitches recorded over a 10 minute observation period.
LSD-like discriminative stimulus effects in rats
An intermediate degree of generalization of LSD to DPT was observed with approximately 60% and 40% LSD-appropriate responding at doses of 1.5 and 3.0 mg/kg, respectively (P < 0.05 for both doses) (Figure 3, closed circles). DPT also suppressed responding, and doses higher than 3.0 mg/kg were not tested due to the greater than 50% reduction in response rates observed at that dose (Table 1). Pretreatment with 0.3 mg/kg WAY-100635 (Figure 3, open inverted triangles) produced a parallel rightward shift in the dose-effect curve, reducing the LSD-like effects of 1.5 mg/kg DPT, but increasing the LSD-like effects of 3.0 mg/kg DPT. Interestingly, the LSD-like discriminative stimulus effects of 1.5 and 3.0 mg/kg DPT were completely blocked (P < 0.05 for both doses) by the 5-HT2A selective antagonist M100907 (Figure 3, open triangles).
Figure 3.
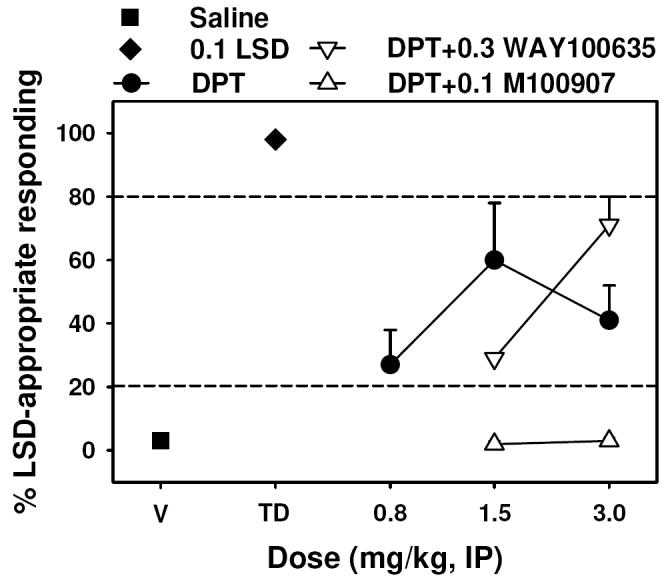
Effects of DPT alone (circles) and in combination with 0.3 mg/kg WAY-100,635 (inverted triangles) or 0.1 mg/kg M100907 (triangles) in rats trained with 0.1 mg/kg LSD (N = 12) as a discriminative stimulus. All points represent the mean ± SEM. Abscissa: Dose of DPT expressed as mg/kg and plotted on a log scale. The points at V and TD represent saline and LSD training sessions. Ordinate: Percent LSD-appropriate responding.
Table 1.
Rates of responding (per minute) following injection with various doses of DPT and antagonists in rats trained to discriminate 0.1 mg/kg LSD from saline.
| Injection | Rate | SEM | # completing |
|---|---|---|---|
| Saline | 17.67 | 5.41 | 12 |
| LSD training dose | 56.20 | 6.79 | 12 |
| 0.8 DPT | 5.91 | 1.34 | 12 |
| 1.5 DPT | 8.43 | 1.97 | 12 |
| 3.0 DPT | 5.09 | 0.94 | 12 |
| 1.5 DPT + M100907 | 7.87 | 0.88 | 12 |
| 3.0 DPT + M100907 | 7.35 | 1.11 | 10 |
| 1.5 DPT + WAY-100635 | 4.84 | 1.31 | 12 |
| 3.0 DPT + WAY-100635 | 5.26 | 0.98 | 12 |
MDMA-like discriminative stimulus effects in rats
DPT dose-dependently and fully substituted for MDMA (Figure 4, closed circles) at a dose of 3.0 mg/kg (P < 0.05), and suppressed response rates (Table 2). Pretreatment with 0.3 mg/kg WAY-100635 (Figure 3, open inverted triangles) did not alter the MDMA-like discriminative effects of DPT at doses of 1.5 or 3.0 mg/kg (P > 0.05 for both doses). In contrast, the MDMA-like discriminative stimulus effects of 1.5 and 3.0 mg/kg DPT were completely blocked (P < 0.05 for both doses) by the 5-HT2A selective antagonist M100907 (Figure 3, open triangles).
Figure 4.
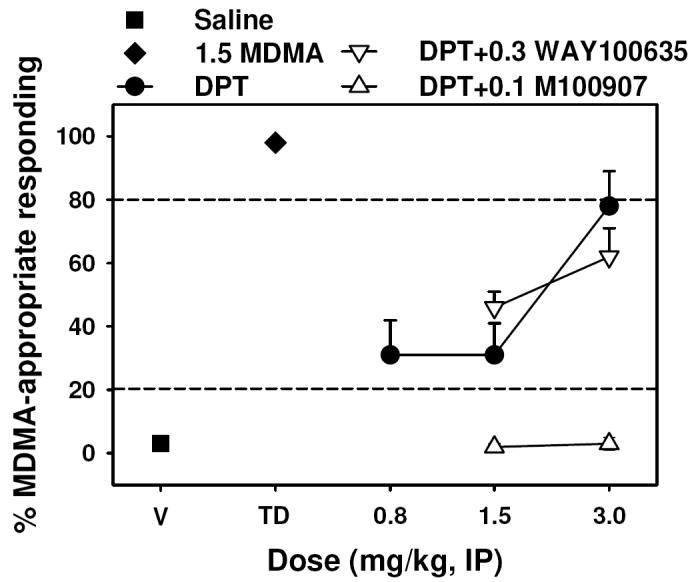
Effects of DPT alone (circles) and in combination with 0.3 mg/kg WAY-100,635 (inverted triangles), 0.1 mg/kg M100907 (triangles), or the combination of both antagonists (plus) in rats trained with 1.5 mg/kg MDMA (N = 11) as a discriminative stimulus. Graph properties as described in Figure 3.
Table 2.
Rates of responding (per minute) following injection with various doses of DPT and antagonists in rats trained to discriminate 1.5 mg/kg MDMA from saline.
| Injection | Rate | SEM | # completing |
|---|---|---|---|
| Saline | 26.76 | 6.33 | 11 |
| MDMA training dose | 35.98 | 4.87 | 11 |
| 0.8 DPT | 11.45 | 3.16 | 11 |
| 1.5 DPT | 18.87 | 4.41 | 11 |
| 3.0 DPT | 10.33 | 1.83 | 11 |
| 1.5 DPT + M100907 | 8.69 | 2.33 | 11 |
| 3.0 DPT + M100907 | 10.93 | 3.27 | 11 |
| 1.5 DPT + WAY-100635 | 6.00 | 0.99 | 11 |
| 3.0 DPT + WAY-100635 | 5.76 | 1.45 | 9 |
| 3.0 DPT + both antagonists | 7.25 | 1.88 | 11 |
Psilocybin-like discriminative stimulus effects in rats
An intermediate degree of generalization of psilocybin to DPT was observed with approximately 55% psilocybin-appropriate responding at a dose of 3.0 mg/kg (P < 0.05) (Figure 6, closed circles), as was a suppressant effect on response rates (Table 3). Interestingly, neither 0.3 mg/kg WAY-100635 (Figure 6, open inverted triangles) nor 0.1 mg/kg M100907 (Figure 6, open triangles) altered the psilocybin-like effects of DPT (P > 0.05 for all comparisons), but their combination (Figure 6, open crosses) completely abolished the interoceptive effects of DPT at 3.0 mg/kg (P < 0.05).
Figure 6.
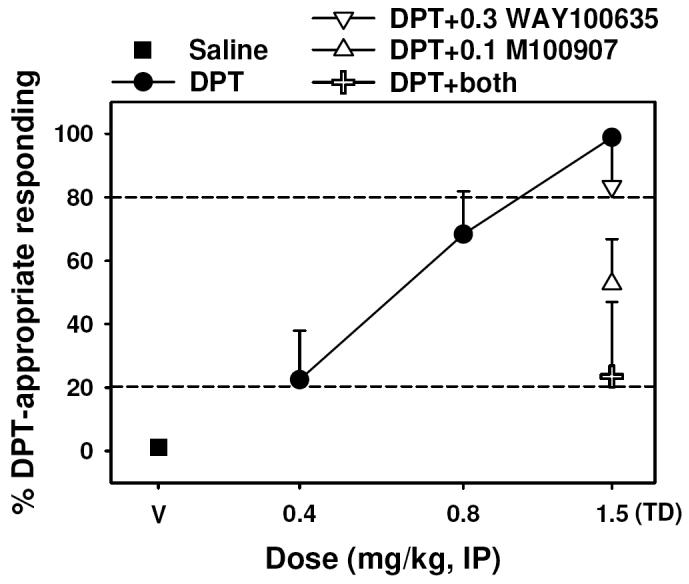
Effects of DPT alone (circles) and in combination with 0.3 mg/kg WAY-100,635 (inverted triangles), 0.1 mg/kg M100907 (triangles), or the combination of both antagonists (plus) in rats trained with 1.5 mg/kg DPT (N = 12) as a discriminative stimulus. Graph properties as described in Figure 3.
Table 3.
Rates of responding (per minute) following injection with various doses of DPT and antagonists in rats trained to discriminate 0.5 mg/kg psilocybin from saline.
| Injection | Rate | SEM | # completing |
|---|---|---|---|
| Saline | 27.01 | 6.46 | 12 |
| Psilocybin training dose | 36.23 | 4.78 | 12 |
| 0.8 DPT | 8.23 | 2.44 | 12 |
| 1.5 DPT | 11.66 | 1.89 | 12 |
| 3.0 DPT | 7.43 | 1.33 | 11 |
| 6.0 DPT | 0.00 | 0.00 | 0 |
| 1.5 DPT + M100907 | 8.11 | 0.98 | 12 |
| 3.0 DPT + M100907 | 5.96 | 0.79 | 10 |
| 3.0 DPT + WAY-100635 | 6.66 | 1.46 | 12 |
| 1.5 DPT + both antagonists | 10.33 | 1.45 | 12 |
| 3.0 DPT + both antagonists | 3.32 | 1.88 | 9 |
DPT-induced stimulus control in rats
Rats trained to discriminate 1.5 mg/kg DPT from saline performed in an almost completely drug-appropriate manner when tested with the training dose, and DPT-appropriate responding decreased in a dose-dependent manner when rats were injected with lower test doses (Figure 7, closed circles). No apparent effects on response rate were observed following administration of DPT (Table 4). The discriminative stimulus effects of the DPT training dose were unaltered by prior treatment with 0.3 mg/kg WAY-100635 (Figure 7, open inverted triangles) (P < 0.05), but there was a trend towards an attenuation of these effects following administration of 0.1 mg/kg M100907 (Figure 7, open triangles) (P = 0.073). The combination of both antagonists (Figure 7, open crosses) significantly blunted the interoceptive effects of the DPT training dose (P < 0.05).
Table 4.
Rates of responding (per minute) following injection with various doses of DPT and antagonists in rats trained to discriminate 1.5 mg/kg DPT from saline.
| Injection | Rate | SEM | # completing |
|---|---|---|---|
| Saline | 27.15 | 5.95 | 12 |
| 0.4 DPT | 23.40 | 4.78 | 12 |
| 0.8 DPT | 25.73 | 5.08 | 12 |
| 1.5 DPT (training dose) | 29.61 | 3.45 | 12 |
| 3.0 DPT + M100907 | 19.99 | 3.32 | 12 |
| 3.0 DPT + WAY-100635 | 6.37 | 2.45 | 12 |
| 3.0 DPT + both antagonists | 10.62 | 9.19 | 5 |
Discussion
The presently reported results suggest that DPT is behaviorally active in two rodent assays which model hallucinogen effects. The capacity of this compound to induce the head twitch response in the mouse, and the potent antagonism of this effect by prior injection of M100907, suggests that a primary site of action for DPT is the 5-HT2A receptor. Similarly, the LSD-like, psilocybin-like, and MDMA-like stimulus effects elicited by DPT in the rat, coupled with the greater antagonism of these effects by M100907 than by WAY-100635, are also consistent with a 5-HT2A-mediated mechanism of action for this compound. This receptor has previously been implicated in the mediation of hallucinogen effects for the ergoline (LSD-like), indolealkylamine (DMT-like), and phenylisopropylamine (DOI-like) hallucinogens (Sadzot et al., 1989; Aghajanian and Marek, 1999; Nichols, 2004). It should be noted that the dose of M100907 employed in the present discrimination studies have previously been shown to completely antagonize LSD-induced stimulus control (Winter et al., 2004) while the dose of WAY-100635 chosen for use in the present studies has previously been shown to completely antagonize stimulus control by the prototypic 5-HT1A agonist, 8-OH-DPAT (Reissig et al., 2005). It is important to note that recent evidence has suggested that the affinity of WAY-100635 for 5-HT1A receptors is only 10-fold lower than for D4 receptors (Chemel et al., 2006). Thus, administration of WAY-100635 doses higher than those given in the present studies would likely result in significant dopaminergic antagonism. Similarly, the selectivity of M100907 among 5-HT2 receptors is not absolute, and so the present studies can not completely rule out a 5-HT2C component to the actions of DPT.
Behaviors mediated by 5-HT2A receptors have been shown to be modulated by 5-HT1A receptor activity in a variety of experimental paradigms, but with regards to drug-elicited head twitch behavior, the effects of 5-HT1A ligands are unclear. In the rat, quipazine-induced head twitches are exacerbated by the 5-HT1A agonist gepirone (Eison and Yocca 1985; Yocca et al. 1990), but administration of the prototypical 5-HT1A receptor agonist 8-hydroxy-2-(di-N-propylamino)tetralin (8-OH-DPAT) attenuates the HTR elicited by DOI in the mouse (Darmani et al., 1990). In the present studies, DPT induced a dose-dependent HTR which was significantly antagonized by 1.0 mg/kg WAY-100635, suggesting that some component of HTR is indeed 5-HT1A-mediated. However, administration of 3.0 mg/kg WAY-100635 did not produce any further antagonism of the HTR, indicating that the contribution of 5-HT1A receptors to DPT-elicited twitch behavior is only partial.
Although a majority of our information regarding the effects of newer hallucinogens is largely anecdotal in nature, accounts of the effects of DPT in human subjects by Shulgin and Shulgin (1991), as well as those posted online (for example, erowid.org and lycaeum.org), strongly attest to the psychoactive properties of this drug. Based upon those reports and the previously described cross-generalization of hallucinogens in rats (Winter and Rabin, 1988; Glennon et al. 1983), we would expect DPT to substitute for LSD, psilocybin and MDMA. The data of Figures 3 - 5 only partially fulfill that prediction. A maximum of only 60% LSD-appropriate or psilocybin-appropriate responding followed the administration of 1.5 or 3.0 mg/kg DPT, respectively, and most doses of this compound produced significant suppression of the rate of responding in both groups. In contrast, DPT fully substituted for MDMA at a dose of 3.0 mg/kg, although response rates were again suppressed as compared to control conditions.
Figure 5.
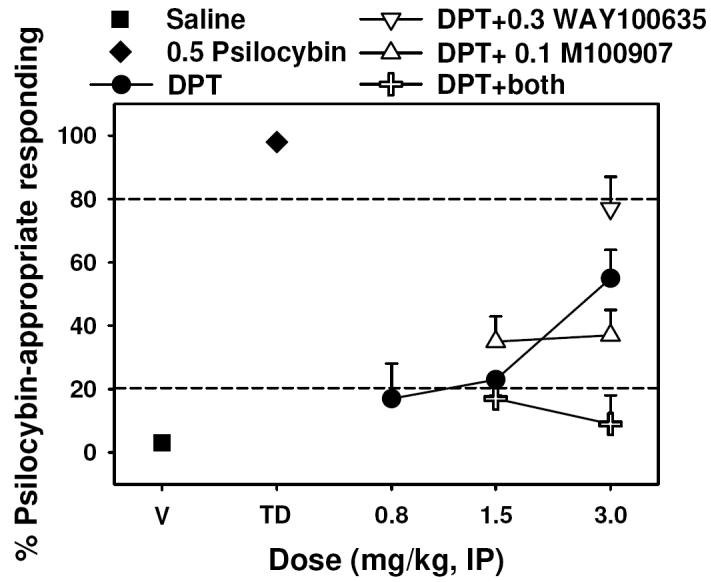
Effects of DPT alone (circles) and in combination with 0.3 mg/kg WAY-100,635 (inverted triangles), 0.1 mg/kg M100907 (triangles), or the combination of both antagonists (plus) in rats trained with 0.5 mg/kg psilocybin (N = 12) as a discriminative stimulus. Graph properties as described in Figure 3.
The previously reported affinity of DPT for 5-HT1A receptors (Thiagaraj et al., 2005) is perhaps expected given the structural similarity of this compound to other hallucinogenic tryptamines with high affinity for this receptor, such as 5-methoxydimethyltryptamine and psilocin (McKenna et al., 1990). This affinity may explain the surmountable antagonism of the behavioral effects of DPT by WAY-100635 in the head twitch assay. With regards to the presently reported drug discrimination studies, it may be the case that 5-HT1A-mediated components of DPT's stimulus effects are more salient at lower doses (i.e., the attenuation of LSD-appropriate responding by WAY-100635 at a dose of 1.5 mg/kg DPT), while the stimulus effects of higher doses are more likely to be dependent on 5-HT2A receptors (i.e., a restoration of LSD-appropriate responding at a dose of 3.0 mg/kg DPT by WAY-100635, but complete abolition by M100907). The appreciable affinities for 5-HT1A receptors displayed by the tryptamine-like hallucinogens have long distinguished them from the phenylisopropylamine hallucinogens (Nichols, 1999; Winter et al., 2000). Further research into these intruiging effects, particularly as they may relate to subtle differences in the subjective effects induced by chemically distinct hallucinogens in man, would be informative.
Acknowledgements
This research was supported by by USPHS grants DA03385 (JCW) and DA020645 (WEF), National Research Service Award DA016457 (CJR) as well as by the College on Problems of Drug Dependence. The authors express their gratitude to the Emory University and SUNY Buffalo units for laboratory animal medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aghajanian GK, Marek GJ. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21(2S):16S–23S. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- Appel JB, West WB, Rolandi WG, Alici T, Pechersky K. Increasing the selectivity of drug discrimination procedures. Pharmacol Biochem Behav. 1999;64(2):353–8. doi: 10.1016/s0091-3057(99)00089-1. [DOI] [PubMed] [Google Scholar]
- Chemel BR, Roth BL, Armbruster B, Watts VJ, Nichols DE. WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology. 2006;188(2):244–51. doi: 10.1007/s00213-006-0490-4. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Janssen PAJ. The head twitch response to intraperitoneal injection of 5-hydroxytryptophan in the rat: Antagonist effects of purported 5-hydroxytryptamine antagonists and of pirenperone, an LSD antagonist. Neuropharmacology. 1983;22(8):993–1000. doi: 10.1016/0028-3908(83)90215-0. [DOI] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW, Warner BT. A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Brit. J. Pharmacol. 1963;20:106–120. doi: 10.1111/j.1476-5381.1963.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW. A possible correlation between drug-induced hallucinations in man and a behavioral response in mice. Psychopharmacologia. 1967;11:65–78. doi: 10.1007/BF00401509. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Glennon RA. Withdrawal from chronic treatment with (±)-DOI causes supersensitivity to 5-HT2 receptor-induced head-twitch behavior in mice. Eur. J. Pharmacol. 1990a;186:115–118. doi: 10.1016/0014-2999(90)94066-7. [DOI] [PubMed] [Google Scholar]
- Darmani NR, Martin b.R., Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol. Biochem. Behav. 1990b;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Glennon RA. Behavioral evidence for differential adaptation of the serotonergic system after acute and chronic treatment with (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) or ketanserin. J. Pharmacol. Exp. Ther. 1992;262(2):692–698. [PubMed] [Google Scholar]
- Eison AS, Yocca FD. Reduction in cortical 5HT2 receptor sensitivity after continuous gepirone treatment. Eur J Pharmacol. 1985;111(3):389–92. doi: 10.1016/0014-2999(85)90648-x. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Kiessel CL, Leach PT, Martin CV, Karabenick RL, Chen X, Ohizumi Y, Ullrich T, Rice KC, Woods JH. Nantenine: an antagonist of the behavioral and physiological effects of MDMA in mice. Psychopharmacology. 2004;173(34):270–7. doi: 10.1007/s00213-003-1741-2. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology. 2005;181(3):496–503. doi: 10.1007/s00213-005-0009-4. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Kiessel CL, Eckler JR, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol Biochem Behav. 2006;83(1):122–9. doi: 10.1016/j.pbb.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. Role of 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. II: Reassessment of LSD false positives. Psychopharmacology. 1995a;121(3):357–63. doi: 10.1007/BF02246075. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. I: Antagonist correlation analysis. Psychopharmacology. 1995b;121(3):347–56. doi: 10.1007/BF02246074. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Helsley SE, Lorrain DS, Palumbo PA, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs III: The mechanistic basis for supersensitivity to the LSD stimulus following serotonin depletion. Psychopharmacology. 1995c;121(3):364–372. doi: 10.1007/BF02246076. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Jacyno JM, Slusher M, Rosecrans JA. DOM-stimulus generalization to LSD and other hallucinogenic indolealkylamines. Eur. J. Pharmacol. 1983;86(34):453–9. doi: 10.1016/0014-2999(83)90196-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, Sealfon SC. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003;23(26):8836–43. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53(3):439–52. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Green AR. A behavioural and biochemical study in mice and rats of putative selective agonists and antagonists for 5-HT1 and 5-HT2 receptors. Br J Pharmacol. 1985;84(3):743–53. doi: 10.1111/j.1476-5381.1985.tb16157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, O'Shaughnessy K, Hammond M, Schachter M, Grahame-Smith DG. Inhibition of 5-hydroxytryptamine-mediated behaviours by the putative 5-HT2 receptor antagonist pirenperone. Neuropharmacology. 1983;22:573–578. doi: 10.1016/0028-3908(83)90147-8. [DOI] [PubMed] [Google Scholar]
- Handley SL, Singh L. Neurotransmitters and shaking behavior: More than a “gut bath” for the brain. Trends Pharmacol. Sci. 1986;7:324–328. [Google Scholar]
- Leonhart MM. Schedules of Controlled Substances: Placement of alpha-methyltryptamine and 5-methoxy-N,N-diisopropyltryptamine Into Schedule I of the Controlled Substances Act. Final rule. Fed. Regist. 2004;69(188):58050–58053. [PubMed] [Google Scholar]
- Lucki I, Nobler MS, Frazer A. Differential actions of serotonin antagonists on two behavioral models of serotonin receptor activation in the rat. J. Pharmacol. Exp. Ther. 1984;228:133–139. [PubMed] [Google Scholar]
- McKenna DJ, Repke DB, Peroutka SJ. Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropsychopharmacology. 1990;29:193–198. doi: 10.1016/0028-3908(90)90001-8. [DOI] [PubMed] [Google Scholar]
- Nagai F, Nonaka R, Satoh Hisashi Kamimura K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559(23):132–7. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- Nichols DE . Role of serotonergic neurons and 5-HT receptors in the action of hallucinogens. In: : Baumgarten HG, Gothert M, editors. Serotonergic Neurons and 5-HT Receptors in the CNS. Springer; Berlin: 1999. pp. 563–585. [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol. Therapeutics. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Ortmann R, Biscoff S, Radeke E, Bueche O, Delini-Stula A. Correlation between different measures of antiserotonin activity of drugs. Naunyn Schmiedeberg's Arch. Pharmacol. 1982;321:265–270. doi: 10.1007/BF00498511. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Lebovitz RM, Snyder SH. Two distinct central serotonin receptors with different physiological functions. Science (Wash. DC) 1981;212:827–829. doi: 10.1126/science.7221567. [DOI] [PubMed] [Google Scholar]
- Reissig CJ, Eckler JR, Rabin RA, Winter JC. The 5-HT1A receptor and the stimulus effects of LSD in the rat. Psychopharmacology. 2005;182(2):197–204. doi: 10.1007/s00213-005-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadzot B, Baraban JM, Glennon RA, Lyon RA. Hallucinogenic drug interactions at human brain 5-HT2 receptors: Implications for treating LSD-induced hallucinogenesis. Psychopharmacology. 1989;98(4):495–499. doi: 10.1007/BF00441948. [DOI] [PubMed] [Google Scholar]
- Seeger TF, Seymour PA, Schmidt AW, Zorn SH, Schulz DW, Lebel LA, McLean S, Guanowsky V, Howard HR, Lowe JA, 3rd, Heym J. Ziprasidone (CP-88,059): a new antipsychotic with combined dopamine and serotonin receptor antagonist activity. J Pharmacol Exp Ther. 1995;275(1):101–13. [PubMed] [Google Scholar]
- Shulgin A, Shulgin A. TIHKAL: the continuation. Transform Press; Berkley: 1991. pp. 527–531. [Google Scholar]
- Soskin RA. Dipropyltryptamine in psychotherapy. Curr Psychiatr Ther. 1975;15:147–56. [PubMed] [Google Scholar]
- Soskin RA, Grof S, Richards WA. Low doses of Dipropyltryptamine in psychotherapy. Arch Gen Psychiatry. 1973;28(6):817–21. doi: 10.1001/archpsyc.1973.01750360047006. [DOI] [PubMed] [Google Scholar]
- Thiagaraj HV, Russo EB, Burnett A, Goldstein E, Thompson CM, Parker KK. Binding properties of dipropyltryptamine at the human 5-HT1a receptor. Pharmacology. 2005;74(4):193–9. doi: 10.1159/000085649. [DOI] [PubMed] [Google Scholar]
- Winter JC. Stimulus properties of phenethylamine hallucinogens and lysergic acid diethylamide: the role of 5-hydroxytryptamine. J. Pharmacol. Exp. Ther. 1978;204:416–423. [PubMed] [Google Scholar]
- Winter JC, Rabin RA. Interactions between serotonergic agonists and antagonists in rats trained with LSD as a discriminative stimulus. Pharmacol. Biochem. Behav. 1988;30:617–624. doi: 10.1016/0091-3057(88)90074-3. [DOI] [PubMed] [Google Scholar]
- Winter JC, Filipink RF, Timineri D, Helsley SE, Rabin RA. The paradox of 5-methoxy-N,N-dimethyltryptamine: A hallucinogen which induces stimulus control via 5-HT1A receptors. Pharmacol Biochem Behav. 2000;65:75–82. doi: 10.1016/s0091-3057(99)00178-1. [DOI] [PubMed] [Google Scholar]
- Winter JC, Eckler JR, Rabin RA. Serotonergic/glutamatergic interactions: The effects of mGlu2/3 receptor ligands in rats trained with phencyclidine and LSD as discriminative stimuli. Psychopharmacology. 2004;172:233–240. doi: 10.1007/s00213-003-1636-2. [DOI] [PubMed] [Google Scholar]
- Winter JC, Rice KC, Amorosi DJ, Rabin RA. Psilocybin-induced stimulus control in the rat. Pharmacol Biochem Behav. 2007 doi: 10.1016/j.pbb.2007.06.003. doi: 10.1016/j.pbb.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocca FD, Wright RN, Margraf RR, Eison AS. 8-OH-DPAT and buspirone analogs inhibit the ketanserin-sensitive quipazine-induced head shake response in rats. Pharmacol Biochem Behav. 1990;35(1):251–4. doi: 10.1016/0091-3057(90)90234-9. [DOI] [PubMed] [Google Scholar]


