Abstract
Objectives
Normal pregnancy is characterized by activation of the innate immunity and suppression of the adaptive limb of the immune response. However, pregnant women are more susceptible to the effects of infection and microbial products than non-pregnant women. CD30 is a member of the tumor necrosis factor receptor superfamily and is preferentially expressed by activated T cells producing Th2-type cytokines. Its soluble form (sCD30) is proposed to be an index of Th2 immune response. High serum concentrations of sCD30 have been found in the acute phase of viral infections, such as HIV-1 and hepatitis B. There is, however, conflicting evidence about serum sCD30 concentration in patients with bacterial infections. The objective of this study was to determine whether there are changes in the serum concentration of sCD30 in pregnant women with pyelonephritis.
Methods
This cross-sectional study included normal pregnant women (N=89) and pregnant women with pyelonephritis (N=41). Maternal serum concentration of sCD30 was measured by a specific and sensitive enzyme-linked immunoassay. Non-parametric tests were used for comparisons. A p value <0.05 was considered statistically significant.
Results
(1) Pregnant women with pyelonephritis had a significantly higher median serum concentration of sCD30 than those with a normal pregnancy (median: 44.3 U/ml, range: 16–352.5 vs. median: 29.7 U/ml, range: 12.2–313.2, respectively; p<0.001); and (2) No significant differences were found in the median maternal serum concentration of sCD30 between pregnant women with pyelonephritis who had a positive blood culture compared to those with a negative blood culture (median:47.7 U/mL, range: 17.1–118.8 vs. median: 42.6 U/mL, range: 16–352.5, respectively; p=0.86).
Conclusions
Acute pyelonephritis during pregnancy is associated with a higher maternal serum concentration of sCD30 than normal pregnancy. This finding is novel, and suggests that pregnant women with pyelonephritis may have a complex immune state in which there is activation of some components of what is considered a Th2 immune response.
Keywords: sCD30, cytokines, Th2 immune response, SIRS, sepsis, pro-inflammatory, anti-inflammatory, pregnancy
Introduction
Normal pregnancy is considered a unique immunological state characterized by activation of the innate immunity and suppression of the adaptive limb of the immune response in order to promote tolerance to the fetus, as well as protect the mother against infection[1,2]. Of interest, pregnant women [3–7] and pregnant animals are more prone to infection and the effects of microbial products. Evidence supporting this view includes: (1) pregnant animals develop generalized Shwartzman reaction after a single injection of endotoxin, while non-pregnant animals require a priming dose [8–10], and (2) acute pyelonephritis during pregnancy is more likely to be associated with acute respiratory distress syndrome (ARDS) [11–21] than pyelonephritis in the non-pregnant state.
CD30, a member of the tumor necrosis factor receptor superfamily [22–24], is preferentially expressed by activated T cells producing T helper (Th) type-2 cytokines [25,26]. Its soluble form (sCD30) is originated from the cleavage of the CD30 extracellular domain by a metalloproteinase [27,28], and it is proposed as an index of Th2 immune response [26]. It has been recently reported that: (1) women with a normal pregnancy have a significantly higher serum concentration of sCD30 compared to non-pregnant women, and (2) patients with preeclampsia as well as those who delivered small for gestational age (SGA) neonates have a significantly lower maternal sCD30 serum concentration than those with a normal pregnancy [29].
High serum concentrations of sCD30 have been found in the acute phase of viral infections, such as HIV-1 [30] and chronic hepatitis B [31,32]. However, conflicting evidence have been reported concerning serum sCD30 concentration in patients with bacterial infections [33–35]. This study was conducted to determine whether there are changes in the maternal serum concentration of sCD30 in an acute bacterial infection during pregnancy: pyelonephritis.
Methods
Study population
A cross-sectional study was conducted by searching our clinical database and bank of biological samples, including patients in the following groups: (1) normal pregnancy (N=89); and (2) pyelonephritis (N=41). Women with multiple pregnancies and fetal anomalies were excluded. Patients were considered to have a normal pregnancy outcome if they did not have any obstetrical, medical, or surgical complication of pregnancy, and delivered a term neonate of appropriate birth weight for gestational age [36] without complications. Pyelonephritis was diagnosed in the presence of fever (temperature ≥ 38ºC), clinical signs of an upper urinary tract infection (e.g., flank pain, costovertebral angle tenderness), pyuria, and a positive urine culture for microorganisms. Blood cultures were also obtained. Systemic inflammatory response syndrome (SIRS) was diagnosed in the presence of two or more of the following signs: 1) temperature ≥38°C or ≤36°C, (2) pulse ≥90 beats/min, (3) respiration of ≥20 per minute or a PaCO2 <32 mm Hg, and (4) white blood cell (WBC) count ≥12,000 or ≤4000 or >10% immature neutrophils [37]. Preterm delivery was defined as delivery before 37 weeks of gestation, while SGA neonate was defined as birthweight below the 10th percentile for the gestational age at birth according to a national birth weight distribution [36].
All women provided written informed consent prior to the collection of maternal blood samples. The utilization of samples for research purposes was approved by the Institutional Review Boards of both Wayne State University and the National Institute of Child Health and Human Development (NICHD/NIH/DHHS). Many of these samples have been employed to study the biology of inflammation, hemostasis, angiogenesis regulation, and growth factor concentrations in normal pregnant women and those with complications, such as Pyelonephritis [2,38,39].
Sample collection and soluble human CD30 (sCD30) immunoassays
Samples of peripheral blood from pregnant women were obtained by venipuncture. The samples were stored at −70°C until assay. A specific and sensitive enzyme-linked immunoassay was used for the quantitation of human sCD30 in maternal serum. Immunoassay kits for human sCD30 were obtained from Bender MedSystems (Vienna, Austria). The concentrations of sCD30 were measured as previously described [29]. The calculated inter- and intra-assay coefficients of variation (CVs) for sCD30 immunoassay in our laboratory were 6.72% and 5.20%, respectively. The lower limit of detection (sensitivity) was calculated to be 0.655 U/ml.
Statistical analysis
The Kolmogorov–Smirnov test was used to determine whether the data was normally distributed. Comparisons among groups were performed using Mann-Whitney U test for continuous variables, as well as Chi-square or Fisher’s exact test for categorical variables. A p value <0.05 was considered statistically significant. The statistical package used was SPSS v.14.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
The demographic and clinical characteristics of the study groups are shown in Table I. Patients with pyelonephritis delivered neonates with a significantly lower birthweight compared to those of normal pregnant women (median: 3140 g, range 1080–4090 vs. median: 3342 g, range: 2550–4050, respectively; p=0.02). Among patients with pyelonephritis, 12.8% (5/39) had preterm delivery, and the same proportion of patients delivered a SGA neonate. The most common microorganism isolated from urine cultures was Escherichia coli [75.6% (31/41)]. Other microorganisms included Gram negative bacilli (n=1), Klebsiella pneumoniae (n=2), Proteus mirabilis (n=1), Enterobacter aerogenes (n=1), Pseudomona aeruginosa (n=1), Streptococcus viridans (n=1), Citrobacter koseri (n=1), Streptococcus agalactiae (n=1), and mixed flora (n=2). One patient had more than one germ isolated from the urine culture.
Table I.
Demographic and clinical characteristics of the study groups
| Normal pregnancy (n=89) | Pyelonephritis (n=41) | p | |
|---|---|---|---|
| Maternal age (years) | 23 (17 – 34) | 22 (17 – 41) | NS |
| Nulliparity | 21.3 (19/89) | 19.5 (8/41) | NS |
| Smoking | 20.5 (17/83) | 21.4 (6/28) | NS |
| Race | |||
| African-American | 83.1 (74/89) | 75.6 (31/41) | NS |
| Caucasian | 12.4 (11/89) | 14.6 (6/41) | NS |
| Others | 4.5 (4/89) | 9.8 (4/41) | NS |
| Gestational age at blood draw (weeks) | 31.1 (19.4 – 38.3) | 31.3 (17 – 41.9) | NS |
| Gestational age at delivery (weeks) | 39.6 (37 – 42) | 39.1 (28.7 – 42.7) | NS |
| Birthweight (grs) | 3342 (2550 – 4050) | 3140 (1080 – 4090) | 0.02 |
Values are expressed as percentage (number) or median (range).
NS: not significant.
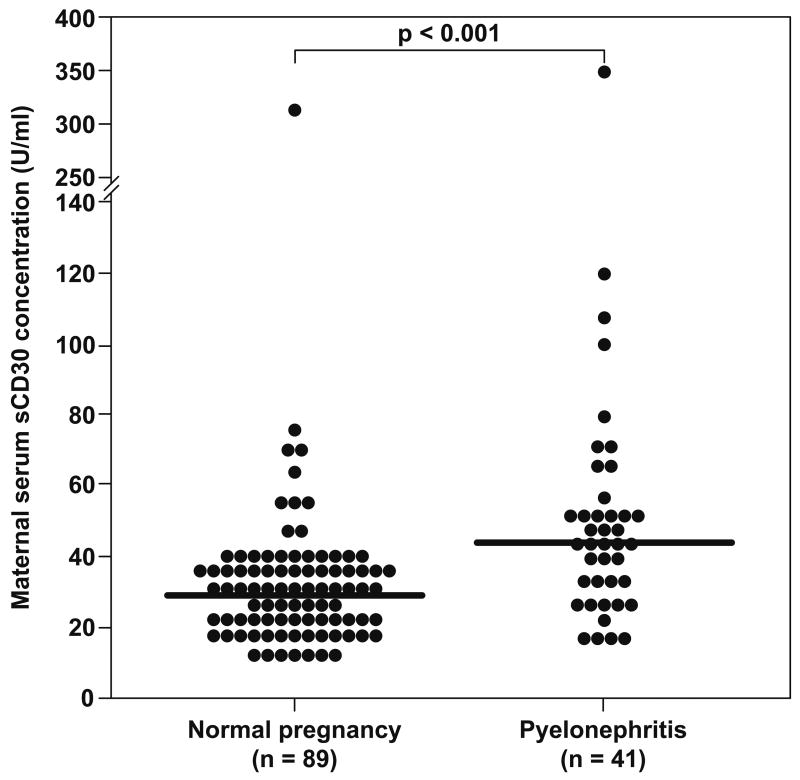
Pregnant women with pyelonephritis have a significantly higher median serum concentration of sCD30 than those with a normal pregnancy (median: 44.3 U/ml, range: 16–352.5 vs. median: 29.7 U/ml, range: 12.2–313.2, respectively; p<0.001) (Figure 1).
Figure 1.
Maternal serum sCD30 concentration in women with a normal pregnancy and those complicated with pyelonephritis. Pregnant women with pyelonephritis have a significantly higher median serum concentration of sCD30 than those with a normal pregnancy (median: 44.3 U/ml, range: 16–352.5 vs. median: 29.7 U/ml, range: 12.2–313.2, respectively; p<0.001).
Blood cultures were performed in 87.8% (36/41) of patients with pyelonephritis, and 44.4% (16/36) of them were positive for microorganisms. Again, Escherichia coli was the most common microorganism isolated from blood cultures [68.8% (11/16)]. Other microorganisms isolated from maternal blood were Gram positive cocci (n=1), Klebsiella pneumoniae (n=1), Enterobacter aerogenes (n=1), and Coagulase-negative staphylococcus (n=2). Maternal temperature, heart rate, and WBC count was obtained in all pregnant women with pyelonephritis at the time of admission. Of note, using only those clinical signs, 90.2% (37/41) of them fulfilled the criteria of SIRS. There were no patients with ARDS.
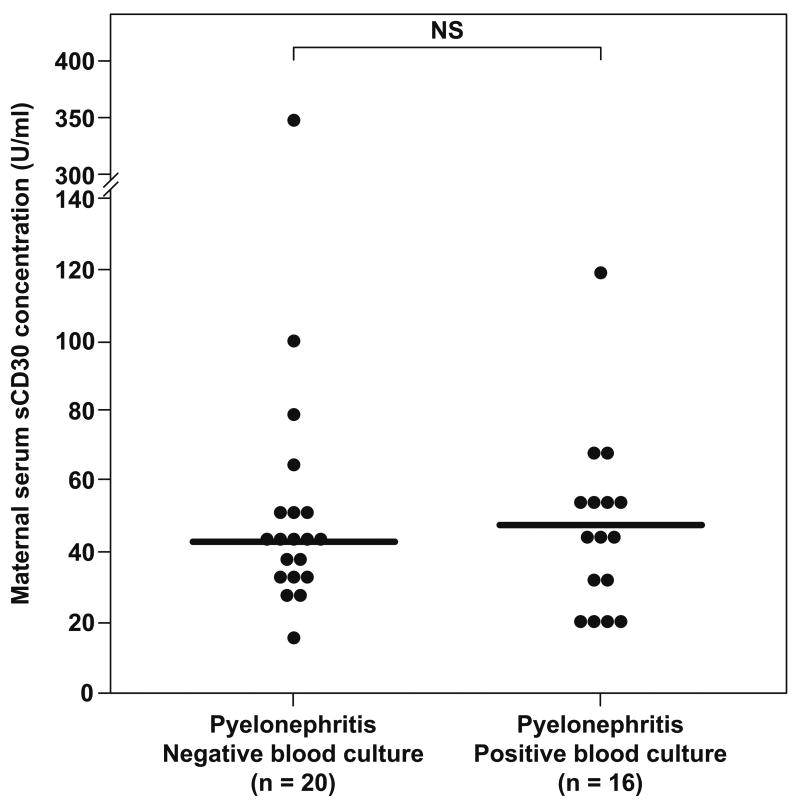
No significant differences were found in the median maternal serum concentration of sCD30 between pregnant women with pyelonephritis who had a positive blood culture and those with a negative blood culture (median: 47.7 U/ml, range: 17.1–118.8 vs. median: 42.6 U/ml, range: 16–352.5, respectively; p=0.86) (Figure 2).
Figure 2.
Maternal serum sCD30 concentration in pregnant women with pyelonephritis. No significant differences were found in the median serum concentration of sCD30 between patients with negative and positive blood cultures (median 42.6 U/ml, range: 16–352.5 vs. median 47.7 U/ml, range: 17.1–118.8, respectively; p=0.86). NS: not significant.
DISCUSSION
Principal findings of this study
(1) Pregnant women with acute pyelonephritis have a significantly higher median serum concentration of sCD30 than normal pregnant women, and (2) No significant differences were found in the median maternal serum concentration of sCD30 between patients with and without positive blood cultures.
Intravascular inflammation: a physiologic finding in normal pregnancy
Normal pregnancy is considered a pro-inflammatory state. Evidence supporting of this view includes: (1) the total white blood cell count in maternal blood increases with advancing gestational age [40]; (2) there is an increased concentration of acute phase proteins, such as fibrinogen and clotting factors [41,42]; (3) the complement system is activated in normal pregnancy [43,44]; (4) leukocytes from normal pregnant women have phenotypic and metabolic changes of monocytes and granulocytes, that are consistent with either priming or activation of the cells [2,45,46]; (5) impaired neutrophil apoptosis [47]; and (6) in the course of conducting longitudinal studies, the laboratory of Luppi et al at the University of Pittsburgh has demonstrated that monocytes during pregnancy have higher production of interleukin (IL)-12 and IL-1β compared to those in non-pregnant women [48]. Similar results were reported by Sacks et al [49] in a cross-sectional study. This cytokine (IL-12) is a major mediator of the type 1 (cell mediated) immune-response which favors a pro-inflammatory response [50].
Pyelonephritis during pregnancy
Pyelonephritis complicates 1–2% of pregnancies [21], is diagnosed approximately 70% of the time during the second and third trimester [51], and is one of the most common medical causes of pregnancy-associated hospitalizations [52]. With adequate antimicrobial treatment, 95% of patients become afebrile after 72 hours [53]. Despite the favorable clinical evolution of most patients, pregnant women with pyelonephritis are at risk of developing sepsis [54–56] and ARDS [11–21], complications that are rare in young non-pregnant women with urinary tract infections. Indeed, pyelonephritis-related sepsis is the most frequent medical complication of admission to obstetric intensive care unit [57], as well as the most common cause of septic shock during pregnancy [55,56].
Systemic inflammatory response in bacterial infections
A systemic inflammatory response can be elicited in patients with trauma, major surgery, pancreatitis, burns, and localized or generalized infection [37]. SIRS is characterized by the production of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, IL-1β, interferon (IFN)-γ, IL-6, and IL-12. This early inflammatory response is followed by a compensatory production of anti-inflammatory cytokines, such as IL-4, IL-10, IL-13, and IL-1 receptor antagonist (IL-1ra) [58]. This condition is known as compensatory anti-inflammatory response syndrome (CARS), which commonly appears after SIRS is established [59]. It has been proposed that plasma concentrations of pro-inflammatory cytokines are proportional to sepsis severity [60,61], and that an exaggerated and persistent inflammatory response is associated with increased organ injury and mortality [62,63]. It is important to stress that this traditional view in patients with sepsis has been challenged [64]. It is now clear that some patients with sepsis have features that are characteristic of immunossuppression [60,65,66], and as sepsis persists, there is a shift towards an anti-inflammatory immunosuppressive state, which could be as detrimental as an exaggerated systemic inflammatory response [64,67–70].
The importance of a counter inflammatory response in sepsis
Recent evidence has suggested that if sepsis persists, the compensatory anti-inflammatory state down-regulates host defense against infection, which is consistent with an immunosuppressive state [64]. Indeed, a shift from a Th1 to Th2 response has been proposed as a potential mechanism for immunossuppression in patients with sepsis [64]. Evidence in support of this view includes: (1) compared to non-infected critically ill controls, patients with severe sepsis have a significantly decreased number of Th1 cells, while Th2 cells are significantly increased [71]; (2) IL-10 concentration is increased in septic patients, and predicts mortality [69,72]; (3) the expression of Th1 response is reduced by IL-4 and IL-10 from Th2 cells [73]; (4) reversal of Th2 response improves survival among patients with sepsis [64,66,74]; (5) in murine models, suppressed bacterial clearance is associated with a significantly lower serum concentration of IL-12 and IFN-γ and a higher serum concentration of IL-10 [75]. Finally, it has been proposed that the timing and magnitude of the anti-inflammatory response, rather than the pro-inflammatory response, correlates with the severity of infection in sepsis [70].
What is sCD30?
CD30 is a 120-kD glycoprotein [76,77] member of the TNF/nerve growth factor receptor superfamily [22,23,78]. The extracellular domain of CD30 is cleaved by a metalloproteinase [27,28] in a 88-kD soluble CD30 antigen (sCD30) which is released from activated T cells in vitro and in vivo [79]. The conventional view is that CD30 expression is restricted to lymphocytes [80,81] and endometrial cells with decidual changes [82], and that monocytes and macrophages do not express CD30 [78].
The interaction between CD30 and its ligand (CD153) can be associated with cell activation, proliferation, differentiation, and death [78,83,84]. On activated T cells, CD30 can act as a co-stimulatory receptor, induce cell surface molecules (CD54, CD80, CD86) and cytokine expression, and promote Th2 lymphocytes [85]. In vitro experiments have demonstrated that sCD30 is preferentially released by human T cells producing Th2-type cytokines. CD4+ T cell clones obtained from peripheral blood mononuclear cells of healthy donors showed that most of those Th2 clones have both CD30 mRNA and surface CD30 expression, and also released sCD30. In contrast, while Th0 clones had an intermediate pattern, Th1 clones did not or poorly expressed CD30 mRNA and surface CD30, and had low or undetectable concentrations of sCD30 [25].
Serum concentrations of sCD30 have been found to correlate with CD30 expression in T lymphocytes [86], as well as being closely associated with disease activity, stage, and prognosis in Hodgkin’s disease, HIV-1 infection, and anaplastic large-cell lymphoma [87–91]. In addition, high serum concentrations of sCD30 have been detected in patients during the acute phase of viral infections, such as HIV-1[30], Epstein-Barr virus infection [92], and chronic hepatitis B [31,32]. However, conflicting results have been reported about serum concentrations of sCD30 in patients with bacterial infections, such as tuberculosis [35] and Lyme disease [34].
sCD30 and pregnancy
Few studies have evaluated the role of sCD30 during pregnancy. Hoshimoto et al [93] reported that the plasma concentration of sCD30 did not change in early pregnancy compared to non-pregnant women, while lower concentrations of sCD30 were observed in pregnant women during the third trimester. Ostensen et al [94] observed that pregnant patients with rheumatoid arthritis had higher plasma concentrations of sCD30 than those with normal pregnancies, and Ekstrom et al [95] reported that atopic pregnant patients have a significantly higher serum concentration of sCD30 than non-atopic pregnant women. These results, however, were not reflected in changes between the groups in umbilical cord blood and the placental expression of CD30 and its ligand.
Recently, we have demonstrated that normal pregnancy is associated with a higher maternal serum concentration of sCD30 than the non-pregnant state, and that patients with preeclampsia and those who delivered an SGA neonate have a significantly lower serum concentration of sCD30 compared to those with normal pregnancies. These observations support the view that normal pregnancy is associated with a shift towards a Th2 immune response, and that preeclampsia and SGA are associated with a polarized Th1 response [29].
sCD30 in pyelonephritis
The finding that pregnant women complicated with pyelonephritis have a higher maternal serum concentration of sCD30 than those with a normal pregnancy is novel, and concurs with the observations that SIRS is followed by a compensatory anti-inflammatory state [59].
In 1991, The American College of Chest Physicians and the Society of Critical Care Medicine proposed the consensus guidelines to unify definitions commonly used in patients with septic states [96]. The term ‘systemic inflammatory response syndrome’ (SIRS) was introduced to identify patients with clinical findings suggestive of activation of the immune system, regardless of the cause [37]. The diagnosis of SIRS is based on the presence of two or more of the following signs: (1) temperature ≥38°C or ≤36°C; (2) pulse ≥90 beats/min; (3) respiration of ≥20 per minute, or a PaCO2 <32 mm Hg; or (4) WBC count ≥12,000 or ≤4000, or >10% immature neutrophils. It should be noted that these criteria do not consider the physiologic changes of normal pregnancy [97], and there is no modified definition of SIRS for pregnant women. Therefore, the diagnosis of SIRS in pregnant patients could be inaccurate if based upon the criteria used for non-pregnant patients. It is interesting to note, however, that in this study 90% of the pregnant women with pyelonephritis fulfilled the criteria of SIRS at admission. Thus, our data suggest that most patients with pyelonephritis meet the criteria of SIRS for non-pregnant subjects. These findings are similar to those of Hill et al [21] who, in a study that included 440 cases of antepartum pyelonephritis, reported that 17% of pregnant women with pyelonephritis were complicated with sepsis at the time of diagnosis, 10% required admission to an extended care unit, and that 7% had respiratory insufficiency.
The fact that pregnant women are more susceptible to the effects of microbial products than non-pregnant women remains a fascinating biological observation. It is tempting to postulate that this may be due either to a priming/activation of monocytes and neutrophils [2,45,46,48] and/or a Th2 biased immune response during normal pregnancy [98]. Though the limitations of the old and simplistic model of Th1/Th2 immune responses are well recognized [99,100], this model may still be helpful in communicating broad concepts about the nature of the immune response.
Specifically, we proposed that an increased sCD30 in maternal serum may reflect a Th2 response in pregnant patients with acute pyelonephritis, and that this represents evidence of an anti-inflammatory state which may predispose to complications. Although the measurement of Th2-type cytokines such as IL-4 and IL-10 could be a different approach to demonstrate a Th2 response in pregnant women with pyelonephritis, sCD30 has been widely proposed and used as an index of a Th2 immune response. The mechanisms whereby sCD30 is elevated in the course of an acute infection during pregnancy require further investigation. It is of considerable interest that in another condition where there is intravascular inflammation (preeclampsia) the maternal serum concentration of sCD30 is significantly lower than in normal pregnancy, suggesting that the systemic inflammatory state of preeclampsia and pyelonephritis differ. Whether this is due to the acuity of the inflammatory process in pyelonephritis and/or the chronic nature of the process in preeclampsia will require further study [101–103].
Conclusions
Acute pyelonephritis during pregnancy is associated with a higher maternal serum concentration of sCD30 than normal pregnancy. This finding is novel, and suggests that pregnant women with pyelonephritis may have a complex immune state in which there is activation of some components of what is considered a Th2 immune response.
Acknowledgments
The authors wish to acknowledge the contributions of the Nursing staff of the Perinatology Research Branch and Detroit Medical Center: Ms Nancy Hauff, Ms Sandy Field, Ms Lorraine Nikita, Ms Vicky Ineson, Ms Mahbubeh Mahmoudieh, Ms Julie McKinley, Ms Sue Rehel, Ms Shannon Donegan, Ms Carolyn Sudz, Ms Sylvia Warren, Ms Gail Barley, Ms Denise Bayoneto, Ms Judy Kerman, Ms Barbara Steffy and Lynn Laity.
This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Sacks G, Sargent I, Redman C. An innate view of human pregnancy. Immunol Today. 1999:114–8. doi: 10.1016/s0167-5699(98)01393-0. [DOI] [PubMed] [Google Scholar]
- 2.Naccasha N, Gervasi MT, Chaiworapongsa T, Berman S, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol. 2001:1118–23. doi: 10.1067/mob.2001.117682. [DOI] [PubMed] [Google Scholar]
- 3.Kort BA, Cefalo RC, Baker VV. Fatal influenza A pneumonia in pregnancy. Am J Perinatol. 1986:179–82. doi: 10.1055/s-2007-999862. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues J, Niederman MS. Pneumonia complicating pregnancy. Clin Chest Med. 1992:679–91. [PubMed] [Google Scholar]
- 5.Kochar DK, Thanvi I, Joshi A, Shubhakaran Agarwal N, Jain N. Mortality trends in falciparum malaria--effect of gender difference and pregnancy. J Assoc Physicians India. 1999:774–8. [PubMed] [Google Scholar]
- 6.Laibl VR, Sheffield JS. Influenza and pneumonia in pregnancy. Clin Perinatol. 2005:727–38. doi: 10.1016/j.clp.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox S, Posner SF, McPheeters M, Jamieson DJ, Kourtis AP, Meikle S. Hospitalizations with respiratory illness among pregnant women during influenza season. Obstet Gynecol. 2006:1315–22. doi: 10.1097/01.AOG.0000218702.92005.bb. [DOI] [PubMed] [Google Scholar]
- 8.Muller-Berghaus G, Obst R. Induction of the generalized Shwartzman reaction in pregnant and nonpregnant rats by colchicine. Am J Pathol. 1972:131–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Muller-Berghaus G, Schmidt-Ehry B. The role of pregnancy in the induction of the generalized Shwartzman reaction. Am J Obstet Gynecol. 1972:847–9. doi: 10.1016/0002-9378(72)90085-3. [DOI] [PubMed] [Google Scholar]
- 10.Mori W. The Shwartzman reaction: a review including clinical manifestations and proposal for a univisceral or single organ third type. Histopathology. 1981:113–26. doi: 10.1111/j.1365-2559.1981.tb01772.x. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham FG, Leveno KJ, Hankins GD, Whalley PJ. Respiratory insufficiency associated with pyelonephritis during pregnancy. Obstet Gynecol. 1984:121–5. [PubMed] [Google Scholar]
- 12.Elkington KW, Greb LC. Adult respiratory distress syndrome as a complication of acute pyelonephritis during pregnancy: case report and discussion. Obstet Gynecol. 1986:18S–20S. doi: 10.1097/00006250-198603001-00006. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham FG, Lucas MJ, Hankins GD. Pulmonary injury complicating antepartum pyelonephritis. Am J Obstet Gynecol. 1987:797–807. doi: 10.1016/0002-9378(87)90335-8. [DOI] [PubMed] [Google Scholar]
- 14.Pruett K, Faro S. Pyelonephritis associated with respiratory distress. Obstet Gynecol. 1987:444–6. [PubMed] [Google Scholar]
- 15.Gurman G, Schlaeffer F, Kopernic G. Adult respiratory distress syndrome as a complication of acute pyelonephritis during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1990:75–80. doi: 10.1016/0028-2243(90)90052-3. [DOI] [PubMed] [Google Scholar]
- 16.Amstey MS. Frequency of adult respiratory distress syndrome in pregnant women who have pyelonephritis. Clin Infect Dis. 1992:1260–1. doi: 10.1093/clinids/14.6.1260. [DOI] [PubMed] [Google Scholar]
- 17.Mabie WC, Barton JR, Sibai BM. Adult respiratory distress syndrome in pregnancy. Am J Obstet Gynecol. 1992:950–7. doi: 10.1016/s0002-9378(12)80018-4. [DOI] [PubMed] [Google Scholar]
- 18.Catanzarite VA, Willms D. Adult respiratory distress syndrome in pregnancy: report of three cases and review of the literature. Obstet Gynecol Surv. 1997:381–92. doi: 10.1097/00006254-199706000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Catanzarite V, Willms D, Wong D, Landers C, Cousins L, Schrimmer D. Acute respiratory distress syndrome in pregnancy and the puerperium: causes, courses, and outcomes. Obstet Gynecol. 2001:760–4. doi: 10.1016/s0029-7844(00)01231-x. [DOI] [PubMed] [Google Scholar]
- 20.Cole DE, Taylor TL, McCullough DM, Shoff CT, Derdak S. Acute respiratory distress syndrome in pregnancy. Crit Care Med. 2005:S269–S278. doi: 10.1097/01.ccm.0000182478.14181.da. [DOI] [PubMed] [Google Scholar]
- 21.Hill JB, Sheffield JS, McIntire DD, Wendel GD., Jr Acute pyelonephritis in pregnancy. Obstet Gynecol. 2005:18–23. doi: 10.1097/01.AOG.0000149154.96285.a0. [DOI] [PubMed] [Google Scholar]
- 22.Smith CA, Davis T, Anderson D, Solam L, Beckmann MP, Jerzy R, Dower SK, Cosman D, Goodwin RG. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990:1019–23. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- 23.Durkop H, Latza U, Hummel M, Eitelbach F, Seed B, Stein H. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin’s disease. Cell. 1992:421–7. doi: 10.1016/0092-8674(92)90180-k. [DOI] [PubMed] [Google Scholar]
- 24.Smith CA, Gruss HJ, Davis T, Anderson D, Farrah T, Baker E, Sutherland GR, Brannan CI, Copeland NG, Jenkins NA, et al. CD30 antigen, a marker for Hodgkin’s lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell. 1993:1349–60. doi: 10.1016/0092-8674(93)90361-s. [DOI] [PubMed] [Google Scholar]
- 25.Del Prete G, De CM, Almerigogna F, Daniel CK, D’Elios MM, Zancuoghi G, Vinante F, Pizzolo G, Romagnani S. Preferential expression of CD30 by human CD4+ T cells producing Th2-type cytokines. FASEB J. 1995:81–6. [PubMed] [Google Scholar]
- 26.Romagnani S, Del PG, Maggi E, Chilosi M, Caligaris-Cappio F, Pizzolo G. CD30 and type 2 T helper (Th2) responses. J Leukoc Biol. 1995:726–30. doi: 10.1002/jlb.57.5.726. [DOI] [PubMed] [Google Scholar]
- 27.Hansen HP, Kisseleva T, Kobarg J, Horn-Lohrens O, Havsteen B, Lemke H. A zinc metalloproteinase is responsible for the release of CD30 on human tumor cell lines. Int J Cancer. 1995:750–6. doi: 10.1002/ijc.2910630524. [DOI] [PubMed] [Google Scholar]
- 28.Hansen HP, Dietrich S, Kisseleva T, Mokros T, Mentlein R, Lange HH, Murphy G, Lemke H. CD30 shedding from Karpas 299 lymphoma cells is mediated by TNF-alpha-converting enzyme. J Immunol. 2000:6703–9. doi: 10.4049/jimmunol.165.12.6703. [DOI] [PubMed] [Google Scholar]
- 29.Kusanovic JP, Romero R, Hassan SS, Gotsch F, Edwin S, Chaiworapongsa T, Erez O, Mittal P, Mazaki-Tovi S, Soto E, et al. Maternal serum soluble CD30 is increased in normal pregnancy, but decreased in preeclampsia and small for gestational age pregnancies. J Matern Fetal Neonatal Med. 2007:1–12. doi: 10.1080/14767050701482993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pizzolo G, Vinante F, Nadali G, Krampera M, Morosato L, Chilosi M, Raiteri R, Sinicco A. High serum level of soluble CD30 in acute primary HIV-1 infection. Clin Exp Immunol. 1997:251–3. doi: 10.1046/j.1365-2249.1997.d01-1005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fattovich G, Vinante F, Giustina G, Morosato L, Alberti A, Ruol A, Pizzolo G. Serum levels of soluble CD30 in chronic hepatitis B virus infection. Clin Exp Immunol. 1996:105–10. doi: 10.1046/j.1365-2249.1996.915607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monsalve F, Romero A, Estevez J, Costa L, Callejas D. Serum levels of soluble CD30 molecule in hepatitis B virus infection. Rev Med Chil. 2001:1248–52. [PubMed] [Google Scholar]
- 33.Wang G, Hansen H, Tatsis E, Csernok E, Lemke H, Gross WL. High plasma levels of the soluble form of CD30 activation molecule reflect disease activity in patients with Wegener’s granulomatosis. Am J Med. 1997:517–23. doi: 10.1016/s0002-9343(97)00049-1. [DOI] [PubMed] [Google Scholar]
- 34.Grusell M, Widhe M, Ekerfelt C. Increased expression of the Th1-inducing cytokines interleukin-12 and interleukin-18 in cerebrospinal fluid but not in sera from patients with Lyme neuroborreliosis. J Neuroimmunol. 2002:173–8. doi: 10.1016/s0165-5728(02)00255-2. [DOI] [PubMed] [Google Scholar]
- 35.Lienhardt C, Azzurri A, Amedei A, Fielding K, Sillah J, Sow OY, Bah B, Benagiano M, Diallo A, Manetti R, et al. Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. Eur J Immunol. 2002:1605–13. doi: 10.1002/1521-4141(200206)32:6<1605::AID-IMMU1605>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 37.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 38.Soto E, Richani K, Romero R, Espinoza J, Chaiworapongsa T, Nien JK, Edwin S, Kim YM, Hong JS, Goncalves L, et al. Increased concentration of the complement split product C5a in acute pyelonephritis during pregnancy. J Matern Fetal Neonatal Med. 2005:247–52. doi: 10.1080/14767050500072805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gotsch F, Romero R, Espinoza J, Kusanovic JP, Mazaki-Tovi S, Erez O, Than NG, Edwin S, Mazor M, Yoon BH, et al. Maternal serum concentrations of the chemokine CXCL10/IP-10 are elevated in acute pyelonephritis during pregnancy. J Matern Fetal Neonatal Med. 2007:735–44. doi: 10.1080/14767050701511650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.EFRATI P, PRESENTEY B, MARGALITH M, ROZENSZAJN L. LEUKOCYTES OF NORMAL PREGNANT WOMEN. Obstet Gynecol. 1964:429–32. [PubMed] [Google Scholar]
- 41.Stirling Y, Woolf L, North WR, Seghatchian MJ, Meade TW. Haemostasis in normal pregnancy. Thromb Haemost. 1984:176–82. [PubMed] [Google Scholar]
- 42.Comeglio P, Fedi S, Liotta AA, Cellai AP, Chiarantini E, Prisco D, Mecacci F, Parretti E, Mello G, Abbate R. Blood clotting activation during normal pregnancy. Thromb Res. 1996:199–202. doi: 10.1016/0049-3848(96)00176-4. [DOI] [PubMed] [Google Scholar]
- 43.Hopkinson ND, Powell RJ. Classical complement activation induced by pregnancy: implications for management of connective tissue diseases. J Clin Pathol. 1992:66–7. doi: 10.1136/jcp.45.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richani K, Soto E, Romero R, Espinoza J, Chaiworapongsa T, Nien JK, Edwin S, Kim YM, Hong JS, Mazor M. Normal pregnancy is characterized by systemic activation of the complement system. J Matern Fetal Neonatal Med. 2005:239–45. doi: 10.1080/14767050500072722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998:80–6. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 46.Luppi P, Haluszczak C, Trucco M, DeLoia JA. Normal pregnancy is associated with peripheral leukocyte activation. Am J Reprod Immunol. 2002:72–81. doi: 10.1034/j.1600-0897.2002.1o041.x. [DOI] [PubMed] [Google Scholar]
- 47.von Dadelszen P, Watson RW, Noorwali F, Marshall JC, Parodo J, Farine D, Lye SJ, Ritchie JW, Rotstein OD. Maternal neutrophil apoptosis in normal pregnancy, preeclampsia, and normotensive intrauterine growth restriction. Am J Obstet Gynecol. 1999:408–14. doi: 10.1016/s0002-9378(99)70570-3. [DOI] [PubMed] [Google Scholar]
- 48.Luppi P, Haluszczak C, Betters D, Richard CA, Trucco M, DeLoia JA. Monocytes are progressively activated in the circulation of pregnant women. J Leukoc Biol. 2002:874–84. [PubMed] [Google Scholar]
- 49.Sacks GP, Redman CW, Sargent IL. Monocytes are primed to produce the Th1 type cytokine IL-12 in normal human pregnancy: an intracellular flow cytometric analysis of peripheral blood mononuclear cells. Clin Exp Immunol. 2003:490–7. doi: 10.1046/j.1365-2249.2003.02082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trinchieri G, Gerosa F. Immunoregulation by interleukin-12. J Leukoc Biol. 1996:505–11. doi: 10.1002/jlb.59.4.505. [DOI] [PubMed] [Google Scholar]
- 51.Gilstrap LC, III, Cunningham FG, Whalley PJ. Acute pyelonephritis in pregnancy: an anterospective study. Obstet Gynecol. 1981:409–13. [PubMed] [Google Scholar]
- 52.Bacak SJ, Callaghan WM, Dietz PM, Crouse C. Pregnancy-associated hospitalizations in the United States, 1999–2000. Am J Obstet Gynecol. 2005:592–7. doi: 10.1016/j.ajog.2004.10.638. [DOI] [PubMed] [Google Scholar]
- 53.Mittal P, Wing DA. Urinary tract infections in pregnancy. Clin Perinatol. 2005:749–64. doi: 10.1016/j.clp.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Bubeck RW. Acute pyelonephritis during pregnancy with anuria, septicemia and thrombocytopenia. Del Med J. 1968:143–7. [PubMed] [Google Scholar]
- 55.Mabie WC, Barton JR, Sibai B. Septic shock in pregnancy. Obstet Gynecol. 1997:553–61. doi: 10.1016/s0029-7844(97)00352-9. [DOI] [PubMed] [Google Scholar]
- 56.Sheffield JS. Sepsis and septic shock in pregnancy. Crit Care Clin. 2004:651–60. doi: 10.1016/j.ccc.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Zeeman GG, Wendel GD, Jr, Cunningham FG. A blueprint for obstetric critical care. Am J Obstet Gynecol. 2003:532–6. doi: 10.1067/mob.2003.95. [DOI] [PubMed] [Google Scholar]
- 58.Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997:235–43. doi: 10.1378/chest.112.1.235. [DOI] [PubMed] [Google Scholar]
- 59.Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. 1996:1125–8. doi: 10.1097/00003246-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 60.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 61.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006:1967–74. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 62.Bone RC. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS) Ann Intern Med. 1996:680–7. doi: 10.7326/0003-4819-125-8-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 63.Takahashi H, Tsuda Y, Kobayashi M, Herndon DN, Suzuki F. CCL2 as a trigger of manifestations of compensatory antisinflammatory response syndrome in mice with severe systemic inflammatory response syndrome. J Leukoc Biol. 2006:789–96. doi: 10.1189/jlb.0705372. [DOI] [PubMed] [Google Scholar]
- 64.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003:138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 65.Meakins JL, Pietsch JB, Bubenick O, Kelly R, Rode H, Gordon J, MacLean LD. Delayed hypersensitivity: indicator of acquired failure of host defenses in sepsis and trauma. Ann Surg. 1977:241–50. doi: 10.1097/00000658-197709000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lederer JA, Rodrick ML, Mannick JA. The effects of injury on the adaptive immune response. Shock. 1999:153–9. doi: 10.1097/00024382-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 67.Rogy MA, Coyle SM, Oldenburg HS, Rock CS, Barie PS, Van Zee KJ, Smith CG, Moldawer LL, Lowry SF. Persistently elevated soluble tumor necrosis factor receptor and interleukin-1 receptor antagonist levels in critically ill patients. J Am Coll Surg. 1994:132–8. [PubMed] [Google Scholar]
- 68.Neidhardt R, Keel M, Steckholzer U, Safret A, Ungethuem U, Trentz O, Ertel W. Relationship of interleukin-10 plasma levels to severity of injury and clinical outcome in injured patients. J Trauma. 1997:863–70. doi: 10.1097/00005373-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 69.Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000:176–80. doi: 10.1086/315214. [DOI] [PubMed] [Google Scholar]
- 70.Ashare A, Powers LS, Butler NS, Doerschug KC, Monick MM, Hunninghake GW. Anti-inflammatory response is associated with mortality and severity of infection in sepsis. Am J Physiol Lung Cell Mol Physiol. 2005:L633–L640. doi: 10.1152/ajplung.00231.2004. [DOI] [PubMed] [Google Scholar]
- 71.Ferguson NR, Galley HF, Webster NR. T helper cell subset ratios in patients with severe sepsis. Intensive Care Med. 1999:106–9. doi: 10.1007/s001340050795. [DOI] [PubMed] [Google Scholar]
- 72.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000:1162–72. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 73.Romagnani S. Understanding the role of Th1/Th2 cells in infection. Trends Microbiol. 1996:470–3. doi: 10.1016/s0966-842x(97)82906-x. [DOI] [PubMed] [Google Scholar]
- 74.O’Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995:482–90. doi: 10.1097/00000658-199522240-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murphey ED, Lin CY, McGuire RW, Toliver-Kinsky T, Herndon DN, Sherwood ER. Diminished bacterial clearance is associated with decreased IL-12 and interferon-gamma production but a sustained proinflammatory response in a murine model of postseptic immunosuppression. Shock. 2004:415–25. doi: 10.1097/00024382-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 76.Froese P, Lemke H, Gerdes J, Havsteen B, Schwarting R, Hansen H, Stein H. Biochemical characterization and biosynthesis of the Ki-1 antigen in Hodgkin-derived and virus-transformed human B and T lymphoid cell lines. J Immunol. 1987:2081–7. [PubMed] [Google Scholar]
- 77.Nawrocki JF, Kirsten ES, Fisher RI. Biochemical and structural properties of a Hodgkin’s disease-related membrane protein. J Immunol. 1988:672–80. [PubMed] [Google Scholar]
- 78.Falini B, Pileri S, Pizzolo G, Durkop H, Flenghi L, Stirpe F, Martelli MF, Stein H. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995:1–14. [PubMed] [Google Scholar]
- 79.Josimovic-Alasevic O, Durkop H, Schwarting R, Backe E, Stein H, Diamantstein T. Ki-1 (CD30) antigen is released by Ki-1-positive tumor cells in vitro and in vivo. I. Partial characterization of soluble Ki-1 antigen and detection of the antigen in cell culture supernatants and in serum by an enzyme-linked immunosorbent assay. Eur J Immunol. 1989:157–62. doi: 10.1002/eji.1830190125. [DOI] [PubMed] [Google Scholar]
- 80.Stein H, Mason DY, Gerdes J, O’Connor N, Wainscoat J, Pallesen G, Gatter K, Falini B, Delsol G, Lemke H, et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985:848–58. [PubMed] [Google Scholar]
- 81.Schwarting R, Gerdes J, Durkop H, Falini B, Pileri S, Stein H. BER-H2: a new anti-Ki-1 (CD30) monoclonal antibody directed at a formol-resistant epitope. Blood. 1989:1678–89. [PubMed] [Google Scholar]
- 82.Ito K, Watanabe T, Horie R, Shiota M, Kawamura S, Mori S. High expression of the CD30 molecule in human decidual cells. Am J Pathol. 1994:276–80. [PMC free article] [PubMed] [Google Scholar]
- 83.Gruss HJ, Boiani N, Williams DE, Armitage RJ, Smith CA, Goodwin RG. Pleiotropic effects of the CD30 ligand on CD30-expressing cells and lymphoma cell lines. Blood. 1994:2045–56. [PubMed] [Google Scholar]
- 84.Lee SY, Park CG, Choi Y. T cell receptor-dependent cell death of T cell hybridomas mediated by the CD30 cytoplasmic domain in association with tumor necrosis factor receptor-associated factors. J Exp Med. 1996:669–74. doi: 10.1084/jem.183.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Horie R, Watanabe T. CD30: expression and function in health and disease. Semin Immunol. 1998:457–70. doi: 10.1006/smim.1998.0156. [DOI] [PubMed] [Google Scholar]
- 86.Chilosi M, Facchetti F, Notarangelo LD, Romagnani S, Del PG, Almerigogna F, De CM, Pizzolo G. CD30 cell expression and abnormal soluble CD30 serum accumulation in Omenn’s syndrome: evidence for a T helper 2-mediated condition. Eur J Immunol. 1996:329–34. doi: 10.1002/eji.1830260209. [DOI] [PubMed] [Google Scholar]
- 87.Pizzolo G, Vinante F, Chilosi M, Dallenbach F, Josimovic-Alasevic O, Diamantstein T, Stein H. Serum levels of soluble CD30 molecule (Ki-1 antigen) in Hodgkin’s disease: relationship with disease activity and clinical stage. Br J Haematol. 1990:282–4. doi: 10.1111/j.1365-2141.1990.tb02664.x. [DOI] [PubMed] [Google Scholar]
- 88.Nadali G, Vinante F, Ambrosetti A, Todeschini G, Veneri D, Zanotti R, Meneghini V, Ricetti MM, Benedetti F, Vassanelli A, et al. Serum levels of soluble CD30 are elevated in the majority of untreated patients with Hodgkin’s disease and correlate with clinical features and prognosis. J Clin Oncol. 1994:793–7. doi: 10.1200/JCO.1994.12.4.793. [DOI] [PubMed] [Google Scholar]
- 89.Pizzolo G, Vinante F, Morosato L, Nadali G, Chilosi M, Gandini G, Sinicco A, Raiteri R, Semenzato G, Stein H, et al. High serum level of the soluble form of CD30 molecule in the early phase of HIV-1 infection as an independent predictor of progression to AIDS. AIDS. 1994:741–5. doi: 10.1097/00002030-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 90.Nadali G, Tavecchia L, Zanolin E, Bonfante V, Viviani S, Camerini E, Musto P, Di RN, Carotenuto M, Chilosi M, et al. Serum level of the soluble form of the CD30 molecule identifies patients with Hodgkin’s disease at high risk of unfavorable outcome. Blood. 1998:3011–6. [PubMed] [Google Scholar]
- 91.Zinzani PL, Pileri S, Bendandi M, Buzzi M, Sabattini E, Ascani S, Gherlinzoni F, Magagnoli M, Albertini P, Tura S. Clinical implications of serum levels of soluble CD30 in 70 adult anaplastic large-cell lymphoma patients. J Clin Oncol. 1998:1532–7. doi: 10.1200/JCO.1998.16.4.1532. [DOI] [PubMed] [Google Scholar]
- 92.Vinante F, Morosato L, Siviero F, Nadali G, Rigo A, Veneri D, de SD, Vincenzi C, Chilosi M, Semenzato G, et al. Soluble forms of p55-IL-2R alpha, CD8, and CD30 molecules as markers of lymphoid cell activation in infectious mononucleosis. Haematologica. 1994:413–9. [PubMed] [Google Scholar]
- 93.Hoshimoto K, Ohta N, Ohkura T, Inaba N. Changes in plasma soluble CD26 and CD30 during pregnancy: markers of Th1/Th2 balance? Gynecol Obstet Invest. 2000:260–3. doi: 10.1159/000010328. [DOI] [PubMed] [Google Scholar]
- 94.Ostensen M, Forger F, Nelson JL, Schuhmacher A, Hebisch G, Villiger PM. Pregnancy in patients with rheumatic disease: anti-inflammatory cytokines increase in pregnancy and decrease post partum. Ann Rheum Dis. 2005:839–44. doi: 10.1136/ard.2004.029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ekstrom ES, Bengtsson A, Svensson A, Nilsson C, Ostlund E, Sandstedt B, Bremme K, Lilja G, Scheynius A. Presence of CD30(+) and CD30L(+) cells in human placenta and soluble CD30 levels in cord blood are independent of maternal atopy. Placenta. 2001:372–9. doi: 10.1053/plac.2000.0619. [DOI] [PubMed] [Google Scholar]
- 96.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992:864–74. [PubMed] [Google Scholar]
- 97.Martin SR, Foley MR. Intensive care in obstetrics: an evidence-based review. Am J Obstet Gynecol. 2006:673–89. doi: 10.1016/j.ajog.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 98.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 99.Chaouat G, Ledee-Bataille N, Dubanchet S, Zourbas S, Sandra O, Martal J. Reproductive immunology 2003: reassessing the Th1/Th2 paradigm? Immunol Lett. 2004:207–14. doi: 10.1016/j.imlet.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 100.Chaouat G, Ledee-Bataille N, Dubanchet S, Zourbas S, Sandra O, Martal J. TH1/TH2 paradigm in pregnancy: paradigm lost? Cytokines in pregnancy/early abortion: reexamining the TH1/TH2 paradigm. Int Arch Allergy Immunol. 2004:93–119. doi: 10.1159/000074300. [DOI] [PubMed] [Google Scholar]
- 101.Beller FK. Low-dose endotoxin infusion: a new model? Am J Obstet Gynecol. 1995:1634–5. doi: 10.1016/0002-9378(95)90508-1. [DOI] [PubMed] [Google Scholar]
- 102.Faas MM, Schuiling GA, Linton EA, Sargent IL, Redman CW. Activation of peripheral leukocytes in rat pregnancy and experimental preeclampsia. Am J Obstet Gynecol. 2000:351–7. doi: 10.1016/s0002-9378(00)70223-7. [DOI] [PubMed] [Google Scholar]
- 103.Faas MM, Broekema M, Moes H, van der SG, Heineman MJ, de VP. Altered monocyte function in experimental preeclampsia in the rat. Am J Obstet Gynecol. 2004:1192–8. doi: 10.1016/j.ajog.2004.03.041. [DOI] [PubMed] [Google Scholar]