Abstract
Heparin-induced thrombocytopenia is an immunologically mediated syndrome that is associated with potentially life-threatening arterial and venous thrombosis. Re-exposing patients who have heparin-induced thrombocytopenia to heparin during cardiopulmonary bypass may be hazardous. We describe the re-exposure to unfractionated heparin of a patient with a left ventricular assist device and evidence of heparin-induced thrombocytopenia who needed cardiac transplantation, which was accomplished without complications.
Key words: Cardiopulmonary bypass, heart transplantation, heparin/adverse effects, left ventricular assist device, platelet factor 4/immunology, thrombocytopenia/chemically induced
Heparin-induced thrombocytopenia (HIT), a side effect of prolonged heparin therapy, has life-threatening thrombotic consequences. Repeated and prolonged heparin exposure is common in hospitalized end-stage heart disease patients because of the frequent use in these patients of intra-aortic balloon pumps, heparin-coated pulmonary artery catheters, arterial line flushes, prophylaxis against deep vein thrombosis, multiple interventional cardiology procedures, coronary artery bypass grafting, hemodialysis, and insertion of left ventricular assist devices (LVADs). Heparin is used in preference to other anticoagulants because it has a short half-life and can be reversed with protamine.
Case Report
A 51-year-old, 69.9-kg white woman with nonischemic, dilated cardiomyopathy was admitted to the hospital after being resuscitated from cardiac arrest. She had a 4-year history of dyspnea on exertion and easy fatigability. Despite medical therapy, including weekly infusions of milrinone, she had developed orthopnea and paroxysmal nocturnal dyspnea. Comorbidities included non-insulin-dependent diabetes mellitus, hypertriglyceridemia, Gilbert's syndrome, and hypothyroidism. She had undergone cholecystectomy and total abdominal hysterectomy several years earlier.
Physical examination showed distended jugular veins, pedal edema, and S4 gallop rhythm. Two-dimensional echocardiography revealed global left ventricular (LV) hypokinesia, a low calculated LV ejection fraction (<0.10), a LV end-diastolic dimension of 5.5 cm, depressed right ventricular function, and mildly thickened mitral and aortic valves. Selective coronary angiography revealed normal arteries. Invasive hemodynamic testing revealed pulmonary hypertension: the baseline pulmonary artery pressure was 68/33 (mean, 48 mmHg), and the pulmonary capillary wedge pressure was 26 mmHg. Intravenous nitroglycerin lowered the patient's resting transpulmonary gradient from 19.6 to 9.3 mmHg and her pulmonary vascular resistance from 5.1 to 2.4 Wood units, indicating reversibility of the pulmonary hypertension. However, nitroglycerin did not change her pulmonary capillary wedge pressure of 25 mmHg or her cardiac index of 2.1 L/(min·m2). The patient's liver echotexture was heterogeneous, consistent with fatty infiltration seen on abdominal ultrasonography. Renal function was normal, but liver panel results suggested hepatic dysfunction (bilirubin, 3.7; gamma-glutamyl transpeptidase, 149 IU/L; alkaline phosphate, 106 IU/L; and lactate dehydrogenase, 224 IU/L).
The patient was accepted for cardiac transplantation, but because her clinical symptoms and hemodynamics were worsening and no donor heart was available, she received a Jarvik 2000® (Jarvik Heart Inc.; New York, NY) axial-flow LVAD as a bridge to transplantation. Standard systemic heparinization was used during cardiopulmonary bypass (CPB).
The patient's intraoperative and immediate postoperative course was uneventful. Intravenous heparin therapy began after the chest tubes were removed on postoperative day 2, in accordance with the standard anticoagulation regimen for Jarvik 2000 recipients. The platelet count was 184 × 109/L on postoperative day 1 and 92 × 109/L by day 7 (a 50% decrease). Because HIT was suspected, heparin was stopped and lepirudin infusion was begun (loading dose of 0.4 mg/kg given intravenously over 15–20 sec, then 0.15 mg/[kg-hr]). The platelet level continued to fall, reaching 47 × 109/L on postoperative day 10. An enzyme-linked immunosorbent assay (ELISA) detected heparin platelet factor 4 (HPF4) antibodies. A 2-dimensional echocardiogram strongly suggested thrombus formation around the Jarvik inlet cannula. The patient was upgraded to United Network for Organ Sharing Class IA on the transplant waiting list. A donor heart became available 14 days after LVAD insertion.
Before the heart-transplant surgery, lepirudin infusion was stopped, and the patient received plasmapheresis (3 L of the patient's plasma replaced with donor plasma). Her preoperative platelet count was 171 × 109/L. Because of concern that using alternative anticoagulants that cannot be readily reversed might cause intraoperative or postoperative hemorrhaging, the decision was made to administer a single, 300-mg dose (4 mg/kg) of unfractionated porcine heparin before CBP began. No more heparin was given during the 129-minute CPB period. The activated clotting time, evaluated every 30 minutes during CPB, remained greater than 400 seconds throughout. After decannulation, protamine was given to reverse the effect of heparin. There was no evidence of thrombosis in the bypass circuit or elsewhere.
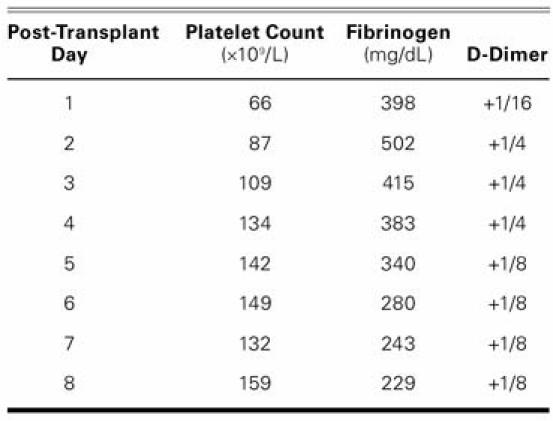
The surgery was completed without complications, including clinical evidence of thrombosis, and the patient was transferred to intensive care in stable condition. Postoperatively, heparin was not used for flushing of the arterial or venous lines, and the patient received no anticoagulants. D-Dimer (a marker of hypercoagulability), fibrinogen, and platelet levels were evaluated daily for 8 days (Table I). The patient was discharged from intensive care on postoperative day 2 and was discharged from the hospital on postoperative day 18 without further incident. Histologic examination of the explanted heart showed a 3.5 × 3.5-cm thrombus near the pump's LV outlet. The patient was alive and well at her 2-year follow-up.
TABLE I. Patient's Platelet Count, Fibrinogen Levels, and D-dimer Levels on the First 8 Days after Heart Transplantation
Discussion
In 1958, Weismann and Tobin1 first noted the association between heparin and thrombogenesis in certain patients. This problem was further associated with thrombocytopenia in 1973 by Rhodes and colleagues,2 who also suggested that HIT might have an immunologic cause. In the early 1970s,3 reported frequencies of HIT were as high as 25% because there was no clear distinction between the more common type I, which is not associated with thrombosis and does not appear to be immunologic in origin, and type II, which is immune-mediated and associated with hypercoagulability. Subsequently, other investigators have found incidences of HIT type II to be 1% to 4% overall4,5 and to be lower in surgical patients (0–3.5%)6,7 than in medical patients (2.7%–5.0%).8,9
The potentially lethal complications of HIT (for example, pulmonary embolism, cerebral stroke, and limb gangrene) are sometimes misattributed to unsatisfactory anticoagulation.10 To complicate the diagnosis, many patients with the HPF4 antibody do not express the clinical HIT syndrome. Although 20% to 50% of cardiac surgery patients form antibodies to HPF4, thrombocytopenia develops in only 3.8 % of these patients.11–13 In addition, because of previous heparin exposure, about 20% of patients who undergo CPB have heparin-associated antibodies detectable by ELISA.14 Recipients of ventricular assist devices (VADs) seem particularly likely to have HPF4 antibodies: in a study of 55 VAD recipients, 40 had HPF4 antibodies, and, in 35 of these, the antibodies were present before VAD implantation, suggesting that VAD recipients are at greater risk for HIT type II than are other cardiac surgical patients.15 Sources of previous heparin exposure include interventional cardiology procedures, cardiac or vascular surgery, arterial line flushes, and heparin-coated pulmonary artery catheters.
Anticoagulation Strategy
Most Jarvik 2000 LVAD patients at our institution receive sufficient intravenous heparin to maintain an activated partial thromboplastin time of 50 to 70 sec and an anti-Xa activity of 0.3 to 0.7 U/mL. Heparin is alternated with oral warfarin until the international normalized ratio (INR) reaches the therapeutic range (2.5–3.5). Platelet inhibitors (for example, aspirin or dipyridam-ole) are also administered.16,17 In LVAD recipients who are diagnosed with HIT type II, we substitute intravenous bivalirudin or argatroban for heparin, until the INR is brought into therapeutic range with warfarin.
Intraoperative Management Strategy in Patients with HIT
In patients diagnosed with HIT who need repeat cardiac surgery that requires CPB, heparin re-exposure is considered hazardous by many, and an alternative management strategy may be used. Antibodies to HPF4 tend to be self-limiting and are usually not detectable after 3 months; waiting this period of time has been associated with good outcomes on re-exposure to unfractionated heparin during CPB, followed by the postoperative use of alternative anticoagulants, if needed.
When waiting is not possible, as in the case reported here, some have advocated using alternative anticoagulants during CPB.18,19 However, the alternative anticoagulants available (for example, danaparoid sodium, recombinant hirudin, and bivalirudin) do not have corresponding reversal agents as heparin does, and they have been associated with severe hemorrhage in patients who have renal impairment.20
Another approach, before initiating CPB, is using unfractionated heparin after first administering an anti-platelet agent (for example, tirofiban20 or epoprostenol). However, in some reported cases, prostacyclin use has been complicated by severe hypotension. One reportedly successful strategy for preventing this problem is the inhibition of platelets with the short-acting platelet glycoprotein IIb/IIIa antagonist tirofiban (10 μg/kg, followed by an infusion of tirofiban at 0.15 μg/[kg·min], stopped 1 hour before CPB is ended20), before administering a 400-IU/kg bolus of unfractionated heparin. Supplementary heparin is administered as needed to keep activated clotting time above 500 seconds. We, too, have used this protocol successfully in a series of 6 patients (Cooper and Bracey, unreported data), but some degree of postoperative hemorrhage has complicated this approach, possibly because tirofiban's effects weaken with time.
In this particular case, because of concern about postoperative bleeding in a patient having a 2nd cardiac surgical procedure very shortly after the 1st, the risk s posed by using an irreversible antiplatelet agent were judged to be greater than the risk of thrombosis. This has been our standard approach in the past.
This case is unique in that there was laboratory documentation of an antibody to the HPF4 complex. The concurrent intradevice thrombus suggests that the patient had HIT with thrombosis syndrome. At present, the diagnosis of this malady remains chiefly a clinical one. Treatment requires carefully considering benefits versus risks, particularly in surgical patients who are at greater risk of bleeding complications when treated wit h direct thrombin inhibitors. In this case, the benefits of having a suitable donor with a negative crossmatch were thought to outweigh the risks associated with CPB. This successful approach shows that even in some patients who have a clinical picture consistent with HIT, heparin re-exposure does not lead to thrombosis, especially when the heparin exposure occurs in a high dose over a short time and is not recurrent.
Acknowledgment
Stephen N. Palmer, PhD, ELS, provided editorial assistance with this manuscript.
Footnotes
Address for reprints: Yasmin Wadia, MD, Center for Cardiac Support, MC 2-114A, Texas Heart Institute, P.O. Box 20345, Houston, TX 77225-0345 E-mail: yasminwadia@yahoo.com
References
- 1.Weismann RE, Tobin RW. Arterial embolism occurring during systemic heparin therapy. AMA Arch Surg 1958;76(2):219–27. [DOI] [PubMed]
- 2.Rhodes GR, Dixon RH, Silver D. Heparin induced thrombocytopenia with thrombotic and hemorrhagic manifestations. Surg Gynecol Obstet 1973;136(3):409–16. [PubMed]
- 3.Bell WR, Tomasulo PA, Alving BM, Duffy TP. Thrombocytopenia occurring during the administration of heparin. A prospective study in 52 patients. Ann Intern Med 1976;85(2):155–60. [DOI] [PubMed]
- 4.Cipolle RJ, Rodvold KA, Seifert R, Clarens R, Ramirez-Lassepas M. Heparin-associated thrombocytopenia: a prospective evaluation of 211 patients. Ther Drug Monit 1983;5(2):205–11. [PubMed]
- 5.Powers PJ, Kelton JG, Carter CJ. Studies on the frequency of heparin-associated thrombocytopenia. Thromb Res 1984;33(4):439–43. [DOI] [PubMed]
- 6.Chong BH. Heparin-induced thrombocytopenia. In: Michelson AD, editor. Platelets. 1st ed. New York: Academic Press; 2002. p. 571–91.
- 7.Schmitt BP, Adelman B. Heparin-associated thrombocytopenia: a critical review and pooled analysis. Am J Med Sci 1993;305(4):208–15. [DOI] [PubMed]
- 8.Leyvraz PF, Bachmann F, Hoek J, Buller HR, Postel M, Samama M, Vandenbroek MD. Prevention of deep vein thrombosis after hip replacement: randomised comparison between unfractionated heparin and low molecular weight heparin [published erratum appears in BMJ 1991;303(6812):1243]. BMJ 1991;303(6802):543–8. [DOI] [PMC free article] [PubMed]
- 9.Warkentin TE, Levine MN, Hirsh J, Horsewood P, Roberts RS, Gent M, Kelton JG. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med 1995;332(20):1330–5. [DOI] [PubMed]
- 10.Kleinschmidt S, Seyfert U T. Heparin-associated thrombocytopenia (HAT)–still a diagnostic and therapeutical problem in clinical practice. Angiology 1995;46(1):37–44. [DOI] [PubMed]
- 11.Pouplard C, May MA, Iochmann S, Amiral J, Vissac AM, Marchand M, Gruel Y. Antibodies to platelet factor 4-heparin after cardiopulmonary bypass in patients anticoagulated with unfractionated heparin or a low-molecular-weight heparin: clinical implications for heparin-induced thrombocytopenia. Circulation 1999;99(19):2530–6. [DOI] [PubMed]
- 12.Trossaert M, Gaillard A, Commin PL, Amiral J, Vissac AM, Fressinaud E. High incidence of anti-heparin/platelet factor 4 antibodies after cardiopulmonary bypass surgery. Br J Haematol 1998;101(4):653–5. [DOI] [PubMed]
- 13.Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia and cardiac surgery [corrected and republished in Ann Thorac Surg 2003;76(6):2121–31]. Ann Thorac Surg 2003;76(2):638–48. [DOI] [PubMed]
- 14.Bauer TL, Arepally G, Konkle BA, Mestichelli B, Shapiro SS, Cines DB, et al. Prevalence of heparin-associated antibodies without thrombosis in patients undergoing cardiopulmonary bypass surgery. Circulation 1997;95(5):1242–6. [DOI] [PubMed]
- 15.Koster A, Sanger S, Hansen R, Sodian R, Mertzlufft F, Harke C, et al. Prevalence and persistence of heparin/platelet factor 4 antibodies in patients with heparin coated and noncoated ventricular assist devices. ASAIO J 2000;46(3):319–22. [DOI] [PubMed]
- 16.Frazier OH, Myers TJ, Gregoric ID, Khan T, Delgado R, Croitoru M, et al. Initial clinical experience with the Jarvik 2000 implantable axial-flow left ventricular assist system. Circulation 2002;105(24):2855–60. [DOI] [PubMed]
- 17.Siegenthaler MP, Martin J, van de Loo A, Doenst T, Bothe W, Beyersdorf F. Implantation of the permanent Jarvik-2000 left ventricular assist device: a single-center experience. J Am Coll Cardiol 2002;39(11):1764–72. [DOI] [PubMed]
- 18.Kurup V, Transue S, Wu Y, Rinder HM, Barash P, Dewar M. Cardiac surgery in a patient with heparin-induced thrombocytopenia–cautions with use of the direct thrombin inhibitor, argatroban. Conn Med 2006;70(4):245–50. [PubMed]
- 19.Wasowicz M, Vegas A, Borger MA, Harwood S. Bivalirudin anticoagulation for cardiopulmonary bypass in a patient with heparin-induced thrombocytopenia. Can J Anaesth 2005;52(10):1093–8. [DOI] [PubMed]
- 20.Koster A, Kukucka M, Bach F, Meyer O, Fischer T, Mertz-lufft F, et al. Anticoagulation during cardiopulmonary bypass in patients with heparin-induced thrombocytopenia type II and renal impairment using heparin and the platelet glycoprotein IIb-IIIa antagonist tirofiban. Anesthesiology 2001;94(2):245–51. [DOI] [PubMed]


