Abstract
In patients who have cyanosis and dyspnea that are unrelated to a cardiopulmonary cause, 1 rare possible diagnosis is methemoglobinemia. This condition is generally asymptomatic, even when the methemoglobin level is as high as 40% of the total hemoglobin value. In the patient described herein, extensive pulmonologic and cardiologic investigations failed to yield the correct diagnosis, which was finally made on the basis of physical findings and arterial blood-gas analysis. Later, a DNA analysis, reported separately by others, showed that the patient's methemoglobinemia was caused by a novel mutation of the cytochrome b5 reductase gene.
Key words: Cytochrome-B(5) reductase; cytochrome reductases/blood; diagnosis, differential; hemoglobin/analysis; methemoglobin; methemoglobinemia/blood/diagnosis/genetics; NADH, NADPH oxidoreductases/blood; oxygen/blood
Methemoglobinemia is a rare possible diagnosis when patients present with cyanosis and dyspnea that are unrelated to cardiopulmonary causes. Methemoglobinemia is usually asymptomatic, even when methemoglobin (metHb) levels are as high as 40% of the total hemoglobin (Hb) value. Herein, we present the case of a patient in whom physical findings and blood-gas analysis led to a diagnosis of methemoglobinemia after extensive pulmonologic and cardiologic tests proved unrevealing.
Case Report
In October 2004, a 21-year-old Asian Indian man with a long history of mild cyanosis and exertional dyspnea was referred to our clinic. He had no history of orthopnea, chest pain, palpitations, cough, syncope, weight loss, early satiety, edema, hemoptysis, or exposure to pets or chemicals. His family history was negative for congenital heart disease and premature coronary artery disease. Childhood evaluations by multiple physicians and recent investigations by a pulmonologist had all been inconclusive.
On admission to our clinic, the patient had mild generalized cyanosis, flattened and cyanotic nailbeds (Fig. 1), normal vital signs, and a pulse oximetry value of 91%. The patient's venous blood was dark (Fig. 2). Treadmill stress-testing yielded normal results, but transesophageal echocardiography suggested a left upper-pulmonary arteriovenous malformation of questionable clinical significance. Intravenously injected agitated saline appeared to enter the left atrium in small amounts from the left upper pulmonary vein. No intracardiac shunt was identified. Cardiac catheterization for possible coil embolization of a pulmonary arteriovenous malformation was recommended.
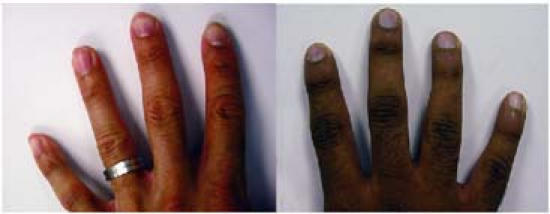
Fig. 1 The patient's hand (right) shows cyanosis of the nailbeds, compared with that of a normal adult (left) with normal nailbeds and similarly dark skin pigmentation.

Fig. 2 The patient's venous blood (right) shows dark discoloration due to methemoglobin, compared with venous blood from an adult who does not have methemoglobinemia (left).
Catheterization yielded femoral artery blood that was the color of dark chocolate. Arterial blood-gas analysis revealed the following values: pH, 7.44; Pco2, 38 mmHg; oxygen pressure, 91 mmHg; oxygen saturation (So2), 97%; and metHb concentration, 33% (normal level, <1.1%). Right-sided heart catheterization showed normal pressures. The mixed venous oxyhemoglobin (O2Hb) content ranged from 54% to 60% without increased pulmonary arterial saturation. Selective angiography of both pulmonary arteries revealed no arteriovenous malformation or anomalous vessel. Co-oximetry showed an O2Hb fraction of 75% and a metHb concentration of 29.5%. Laboratory tests revealed an NADH-cytochrome b5 reductase deficiency.
Discussion
Methemoglobinemia is an important cause of cyanosis. In methemoglobinemia, the concentration of metHb in the blood exceeds 1.5 g/dL (8%–12% of the normal Hb level), impairing oxygen transport and causing “anemic hypoxia.” The regulatory enzyme NADH-cytochrome b5 reductase keeps Hb in an oxidized state. Hereditary methemoglobinemia, characterized by a deficiency of NADH-dependent cytochrome b5 reductase, has a wide geographic distribution.1–3 Type-I deficiency, which is limited to red cells, presents solely as cyanosis, dating from birth. Affected individuals are “more blue than sick.”
Nussenzveig and colleagues3 reported that type-1 methemoglobinemia such as that in our patient is due to a previously unreported mutation in the cytochrome b5 reductase gene. A single T→C transition in exon 8 at position 25985 at the base of alpha-helix N alpha3 (a region of sequence highly conserved from yeast to man) was identified at an NADH binding domain; the transition changed codon 217 from leucine to proline (L217P). Upon quantitative evaluation at 37°C, the thermodynamic cost of this mutation was a 10-fold decrease in the free energy of stability, due to changes in the hydrogen bonding and solvent accessibility.
Two of the most common clinical measures of blood oxygen levels are the pulse oximetry-derived So2 and the arterial blood gas-derived Po2 and So2. However, neither of these tests is adequate for detecting or measuring metHb. Pulse oximetry measures the relative absorbance of 2 wavelengths of light (660 nm and 940 nm) that correspond to the absorption of O2Hb and deoxy-hemoglobin (HHb), respectively. Although metHb absorbance at 660 nm is similar to that of HHb, metHb absorbance at 940 nm is markedly greater than that of either HHb or O2Hb. T his increases the numerator and the denominator of the 660 nm-to-940 nm absorbance ratio and causes the derived So2 measurement to be in error. The arterial blood gas-derived Po2 reflects plasma-dissolved oxygen content, which does not correspond to the oxygen-carrying capacity of Hb. The reported Po2 may remain within the normal reference range in patients who have methemoglobinemia. The So2, when measured by means of arterial blood-gas analysis, is calculated from the blood pH, the Po2, and the standard Hb oxygen dissociation curve. Unfortunately, this approach to calculating the So2 assumes a normal oxygen dissociation curve, and metHb can falsely elevate the calculated So2. One possible clue to the diagnosis of methemoglobinemia is the presence of a “saturation gap.” This occurs when there is a difference between the So2 that has been measured by means of pulse oximetry (the lower value) and the So2 that has been calculated by means of arterial blood-gas analysis. Typically, this saturation gap is greater than 5% in cases of metHb.4
Co-oximetry is the appropriate test for detecting and measuring the metHb level. The co-oximeter measures light absorbance at 4 different wavelengths that correspond to t he absorption characteristics of HHb, O2Hb, carboxyhemoglobin, and metHb. Accordingly, co-oximetry can distinguish among these 4 configurations of Hb while providing a more accurate measurement of SO2. When cyanosis and a saturation gap are detected, co-oximetry should be ordered to confirm the presence of methemoglobinemia,5 thus avoiding more-invasive testing and a delayed diagnosis.6
Our patient remains mildly cyanotic but leads a normal life. In similar cases of unexplained cyanosis, met-hemoglobinemia should be considered, because early diagnosis by co-oximetry testing may obviate other tests that are costly, invasive, or misleading.
Footnotes
Address for reprints: Raymond F. Stainback, MD, 6624 Fannin, Suite 2480, Houston, TX 77030-2304 E-mail: rstainback@sleh.com
References
- 1.Kitao T, Sugita Y, Yoneyama Y, Hattori K. Methemoglobin reductase (cytochrome b5 reductase) deficiency in congenital methemoglobinemia. Blood 1974;44(6):879–84. [PubMed]
- 2.Schwartz JM, Paress PS, Ross JM, DiPillo F, Rizek R. Unstable variant of NADH methemoglobin reductase in Puerto Ricans with hereditary methemoglobinemia. J Clin Invest 1972;51(6):1594–601. [DOI] [PMC free article] [PubMed]
- 3.Nussenzveig RH, Lingam HB, Gaikwad A, Zhu Q, Jing N, Prchal JT. A novel mutation of the cytochrome-b5 reductase gene in an Indian patient: the molecular basis of type I methemoglobinemia. Haematologica 2006;91(11):1542–5. [PubMed]
- 4.Barker SJ, Tremper KK, Hyatt J. Effects of methemoglobinemia on pulse oximetry and mixed venous oximetry. Anesthesiology 1989;70(1):112–7. [DOI] [PubMed]
- 5.Haymond S, Cariappa R, Eby CS, Scott MG. Laboratory assessment of oxygenation in methemoglobinemia. Clin Chem 2005;51(2):434–44. [DOI] [PubMed]
- 6.Da-Silva SS, Sajan IS, Underwood JP 3rd. Congenital methemoglobinemia: a rare cause of cyanosis in the newborn–a case report. Pediatrics 2003;112(2):e158–61. [DOI] [PubMed]


