Abstract
The novel isoform of protein kinase C (PKC), PKCɛ, is an important regulator of ciliated cell function in airway epithelial cells, including cilia motility and detachment of ciliated cells after environmental insult. However, the mechanism of PKCɛ signaling in the airways and the potential role of the PKCɛ-interacting protein, receptor for activated C kinase 1 (RACK1), has not been widely explored. We used immunohistochemistry and Western blot analysis to show that RACK1 is localized exclusively to basal, non-ciliated (and non-goblet) bovine and human bronchial epithelial cells. Our immunohistochemistry experiments used the basal body marker pericentrin, a marker for cilia, β-tubulin, and an airway goblet cell marker, MUC5AC, to confirm that RACK1 was excluded from differentiated airway cell subtypes and is only expressed in the basal cells. These results suggest that PKCɛ signaling in the basal airway cell may involve RACK1; however, PKCɛ regulation in ciliated cells uses RACK1-independent pathways. (J Histochem Cytochem 56:7–14, 2008)
Keywords: receptor for activated C kinase 1, protein kinase C, airway, cilia
The receptor for activated C kinase 1 (RACK1) is a highly conserved adaptor protein that plays an important role in protein kinase C (PKC) signaling in a variety of cell types. RACK1 is a 36-kDa protein with a propeller-like structure of seven tryptophan-aspartate 40 (WD40) domains, enabling RACK1 to participate in complex protein–protein interactions among signaling molecules such as β integrins, phosphodiesterase 4D5, and Src tyrosine kinase (Schechtman and Mochly-Rosen 2001; McCahill et al. 2002; Sklan et al. 2006), as well as PKC. PKC is a family of serine/threonine kinases that are classified according to their cofactor requirements for activation: classical PKC isoforms require calcium, diacylglycerol (DAG), and phosphatidylserine (PS); novel PKC isoforms require DAG and PS; and atypical forms require only PS. Activation of certain isoforms such as the classical PKCβ isoform and the novel PKCɛ isoform induces translocation of the enzyme from the cytosolic cellular region to the membrane. In many cell types, RACK1 stabilizes PKC at the membrane, allowing PKC to phosphorylate target substrates and potentiate downstream signaling (McCahill et al. 2002).
PKC is present in bronchial epithelial cells and alveolar cells (Wyatt et al. 1997; Monick et al. 2001), and recent data from our laboratory strongly implicate the novel PKC isoform, PKCɛ, in regulation of crucial airway epithelial cell functions. For example, we have shown that inhibition of PKCɛ causes preferential ciliated cell detachment from the basal monolayer in cultured bovine bronchial epithelial cells (BBECs); however, the PKCɛ-dependent signaling mechanism controlling these events is not well understood (Slager et al. 2006). Additionally, we have recently shown that PKCɛ signaling mediates cytokine release from bronchial epithelial cells in response to organic dust exposure (Wyatt et al. in press). Although the PKCɛ/RACK1 interaction has been shown to be important for diverse cellular functions such as adhesion and migration (Besson et al. 2002), the role of the RACK1 adaptor protein in the airways has not been well characterized. Liedtke and colleagues have shown that RACK1 binding to PKCɛ along with the Na+/H+ exchange regulatory factor is necessary for cystic fibrosis transmembrane regulator protein function in the immortalized lung cell line Calu-3 (Liedtke et al. 2002,2004; Liedtke and Wang 2006). This group has also shown that RACK1 is apically localized in the membrane of the Calu-3 cell line (Auerbach and Liedtke 2007). However, to date, the localization of RACK1 in differentiated, primary airway cells and tissue has not been reported.
Before identification of PKCɛ downstream substrate targets, we hypothesized that RACK1 would be localized to the ciliary axoneme or basal body region of ciliated cells and basal cells, to regulate PKCɛ-dependent cilia motility and ciliated cell attachment. We used immunohistochemistry (IHC) and Western blot analysis to localize RACK1 in primary BBECs, bovine and human bronchial epithelial tissue, and the immortalized human bronchial epithelial cell line BEAS-2B. Surprisingly, we found that RACK1 was localized exclusively to the membrane of the basal epithelial cells and was not present in ciliated airway cells or goblet cells, suggesting that the mechanisms of PKC signaling are distinctly different in ciliated vs non-ciliated airway cells.
Materials and Methods
Bronchial Epithelial Cell Culture and Isolation
Primary BBECs were prepared from bovine lung obtained from a local abattoir, as described previously (Wyatt et al. 2003). Briefly, bronchi were dissected from the lung and digested overnight at 4C in 0.1% bacterial protease type IV in minimum essential media (M199 with Earl's salts; Gibco, Carlsbad, CA). The following day, the bronchi were repeatedly rinsed in M199 containing 10% FBS (Gibco) to collect a mixture of >95% viable ciliated and basal epithelial cells lining the lumen. Clumped, ciliated primary cells attached to a confluent basal monolayer were collected by a 40-μm mesh filter and grown on tissue culture dishes. This preparation resembles the ciliated, goblet, and basal cells that populate the normal airway. BBECs were washed in M199 media and counted with a hemacytometer, and 105 cells were cytospun (1200 rpm for 4 min) onto concanavalin A (5 mg/ml; Sigma-Aldrich, St. Louis, MO)–coated slides in preparation for IHC.
BEAS-2B, an SV40-immortalized human bronchial epithelial cell line (American Type Culture Collection; Manassas, VA), were cultured in serum-free LHC9-RPMI 1640 media at a 1:1 mix on 1% type I collagen-coated 100-mm plastic Petri plates as previously described (Romberger et al. 2002) or glass chamber slides (Nalge Nunc International; Naperville, IL). LHC9 media contains LHC basal media (Invitrogen; Carlsbad, CA), 0.5 μM phosphoethanolamine-ethanolamine (Sigma-Aldrich), 0.11 mM calcium (Fisher; Springfield, NJ), 50 U/ml penicillin and streptomycin (Gibco), 2 μg/ml fungizone (Gibco), trace elements, 5 μg/ml bovine insulin (Sigma), 5 ng/ml epidermal growth factor (Sigma), 10 μg/ml bovine transferrin (Sigma), 10 nM 3,3′,5-triiodothyronine (Invitrogen), bovine pituitary extract (50 μg protein/ml; Pel Freeze, Rogers, AR), 0.2 μM hydrocortisone (Invitrogen), 0.5 μg/ml epinephrine (Sigma), and 0.1 μg/ml retinoic acid (Sigma) (Beckmann et al. 1992). RPMI 1640 media was purchased from Gibco. Confluent monolayers were lysed for Western blot analysis. For IHC analysis, BEAS-2B cells were grown on collagen-coated glass chamber slides and fixed as described below.
Bovine and Human Trachea Isolation and Fixation
For some IHC experiments, bovine bronchus was fixed in 10% formalin, embedded in paraffin, and sectioned. Slides were deparaffinized in the following series: xylene twice for 5 min, 100% ethanol twice for 3 min, 95% ethanol twice for 3 min, 80% ethanol for 3 min, and 50% ethanol for 3 min. Slides were rehydrated in PBS twice for 5 min and treated with 0.1% trypsin in PBS, pH 7.4, at 37C for 30 min. Before IHC, slides were blocked with PBS containing 0.5% Triton X-100 and 2% BSA (PBSAT) for 1 hr at 37C.
Normal human bronchus slides (24-year-old male) were purchased from US Biomax (Rockville, MD), deparaffinized, rehydrated, and blocked as above, before IHC.
IHC
IHC localization experiments using airway epithelial cells were performed similar to published methods (Jurczyk et al. 2004; Stout et al. 2007). Briefly, BBECs or BEAS-2B cells were either permeabilized for 30 sec with 80 mM PIPES, pH 6.8, 5 mM EDTA, 1 mM MgCl2 and 0.5% Triton X-100 (permeabilization buffer) and fixed for 15 min in absolute methanol at −20C (Figures 1A–1F, Figure 5) or fixed for 15 min in absolute methanol and permeabilized with Triton X-100 containing buffer (Figures 1G and 1H). All slides were washed twice in PBS for 5 min and twice for 15 min in PBSAT. Slides were stained with a primary mouse monoclonal antibody to RACK1 (BD Biosciences; San Jose, CA), a rabbit polyclonal anti-pericentrin (Covance Research Products; Berkeley, CA), or a rabbit polyclonal β-tubulin antibody (AbCam; Cambridge, MA) diluted in PBSAT for 1 hr at room temperature in a humidity chamber. The slides were washed several times in PBSAT and incubated with goat anti-rabbit or goat anti-mouse IgG secondary antibodies labeled with Alexa488 or Alexa594 fluor (Molecular Probes; Carlsbad, CA) diluted in PBSAT. Slides were washed five times in PBS and five times in PBSAT and mounted in media (for 10 ml of mounting media: 5 ml of PBS, 5 ml glycerol, 0.1 g N-propyl gallate; Sigma-Aldrich) or in Vectashield containing 4',6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA). Cells were imaged by confocal microscopy at the UNMC Confocal Laser Scanning Microscopy Core Facility using Zeiss Confocal LSM410 or LSM510 equipped with an argon-krypton laser (Carl Zeiss; Thornwood, NY). Negative control slides for all experiments contained cells or tissue incubated with primary or secondary antibodies alone.
Figure 1.
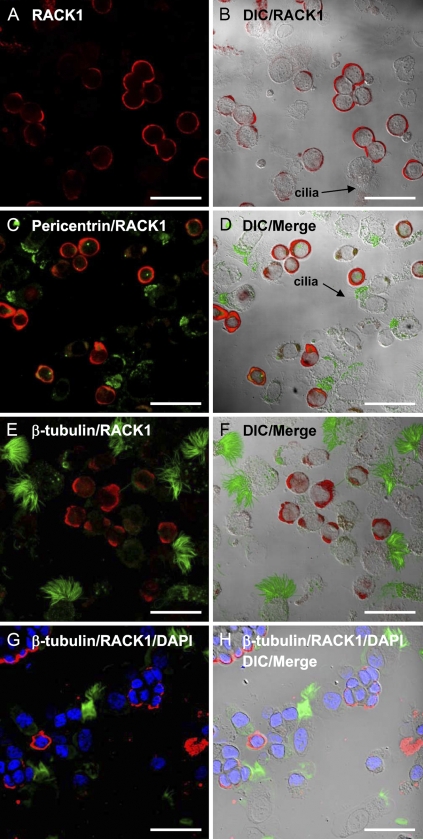
Immunohistochemical localization of receptor for activated C kinase 1 (RACK1) in bovine bronchial epithelial cells (BBECs). In the fluorescence (A) and differential interference contrast (DIC)/fluorescence-merged images (B), RACK1 (red) is localized exclusively to the membrane of BBECs and is not expressed in ciliated cells. This finding was confirmed by the combined fluorescence and merged images of BBECs incubated with primary antibodies against RACK1 (red) and a marker for the apical basal body region, pericentrin (green; C,D) and a marker for cilia, β-tubulin (green; E,F). In the fluorescence (G) and DIC/fluorescent-merged image in H, the BBECs were first fixed in methanol and then permeabilized in buffer containing Triton X-100 before staining with RACK1 (red) and β-tubulin (green) and mounting in Vectashield containing 4',6-diamidino-2-phenylindole (DAPI) as a nuclear marker (blue), showing that RACK1 localization is not affected by fixation artifact and the nuclei remain intact after cytospin. Bar = 50 μm.
Figure 5.
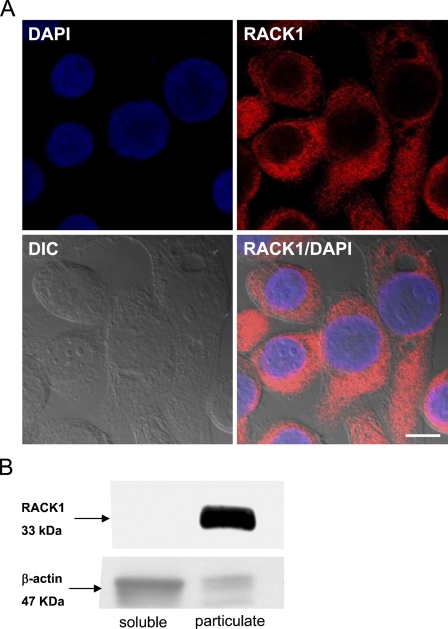
RACK1 localization in BEAS-2B. IHC (A) and Western blot analysis (B) were used to localize RACK1 in the cytosol and membrane (A) and lysates (B) of the immortalized human bronchial epithelial cell line BEAS-2B. DAPI (blue) was used as a nuclear marker in A. In B, RACK1 is present in the membrane-containing particulate fraction of BEAS-2B and the cytoplasm. β-actin was used as a loading control for the Western blot. Bar = 10 μm.
Bovine tracheal slides or human bronchus slides were incubated with primary antibody against RACK1, β-tubulin, or a rabbit polyclonal antibody to human MUC5AC (Santa Cruz Biotechnology; Santa Cruz, CA) diluted in PBSAT at 4C overnight, and washed repeatedly in PBSAT and incubated with secondary antibodies labeled with Alexa488 or Alexa594 fluor for 1 hr at room temperature, washed, mounted, and imaged as above.
Western Blot
Confluent 100-mm dishes of BEAS-2B cells were incubated on ice for 20–30 min in cell lysis buffer containing 35 mM Tris-HCl (pH 7.4), 0.4 mM EGTA, 10 mM MgCl2, and 1 μg/ml each of leupeptin A, phenylmethylsulphonylfluoride, and aprotinin. The cells were scraped with a cell lifter, sonicated for 5 sec, and centrifuged at 10,000 × g for 30 min at 4C. The supernatant was removed (soluble fraction), and the pellet was resuspended in cell lysis buffer containing 0.01% Triton X-100 and sonicated again (particulate fraction). Bovine airway cilia protein, isolated as previously described (Wyatt et al. 2005), were included as a negative control for cilia. Protein concentrations for soluble and particulate fractions were calculated using a Bradford protein assay, and 12 μg of protein for each sample was loaded onto a gel, electrophoresed on a precast SDS-PAGE gel (Bio-Rad; Hercules, CA) at 100 V for ∼1 hr, and transferred to a polyvinylidene difluoride (PVDF; Millipore, Billerica, MA) membrane. Membranes were probed with RACK1 antibody diluted 1:1000 or a rabbit polyclonal antibody to β-actin (Abcam; Cambridge, MA) diluted 1:5000 in Tris-buffered saline, pH 7.4 (TBS), containing 3% BSA overnight at 4C, washed four times in TBS for 1 hr, incubated with secondary anti-mouse IgG antibody conjugated to horseradish peroxidase diluted 1:3000 or anti-rabbit IgG antibody conjugated to horseradish peroxidase diluted 1:40,000 at room temperature for 1 hr, and washed five times in TBS. Proteins were resolved using SuperSignal West Pico Substrate (Pierce; Rockford, IL) and exposing the membrane to autoradiography film.
Results
In our localization studies of RACK1 in airway epithelial cells, we anticipated that a targeting protein for PKC might be localized to the apical region of both ciliated cells and basal airway cells, because PKCɛ is an important regulator of cilia motility and ciliated cell attachment (Slager et al. 2006). Contrary to this hypothesis, our IHC results showed that RACK1 is localized exclusively on non-ciliated basal mammalian airway cells. A mixed preparation of ciliated and BBECs were cytospun onto slides, fixed, and stained with a primary anti-RACK1 antibody, labeled fluorescent secondary antibodies, and analyzed with laser scanning confocal microscopy. In the fluorescent image in Figure 1A and the differential interference contrast (DIC)/fluorescent-merged image in Figure 1B, RACK1 (red) is present in the membrane of non-ciliated BBECs. We used a marker of the apical, basal body region of ciliated cells (pericentrin, green) in Figures 1C and 1D to show that RACK1 (red) is excluded from those airway cells that express basal bodies. Similar results were found in Figures 1E and 1F, when a marker for cilia, β-tubulin, was stained in green, and RACK1 was labeled in red. RACK1 is only expressed in cells that do not express pericentrin or β-tubulin. To confirm that there was no staining artifact from the cytospin preparation or permeabilization, we repeated the experiment above using an alternate protocol. In the fluorescence (Figure 1G) and DIC/fluorescent-merged image in Figure 1H, we used a similar cytospin preparation as Figures 1A–1F; however, the BBECs were first fixed in methanol and then permeabilized in buffer containing Triton X-100 before staining with RACK1 (red) and β-tubulin (green) and mounted in Vectashield containing DAPI as a nuclear marker (blue). The RACK1 localization in Figures 1G and 1H was similar to Figures 1E and 1F, and the DAPI-stained nuclei remained intact after the cytospin preparation.
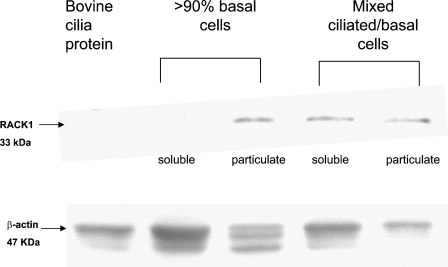
Western blot techniques were used to verify our IHC results. A mixed preparation of primary ciliated and non-ciliated BBECs (similar to the preparation used in Figure 1), a preparation of primary BBECs enriched for basal cells (>90%), and an enriched preparation of bovine cilia protein (Wyatt et al. 2005) were lysed and sonicated, and the soluble and particulate fractions were separated. The same amount of protein (12 μg) for each sample was electrophoresed, transferred to a membrane, stained with a monoclonal antibody against RACK1 or a rabbit polyclonal antibody against β-actin as a loading control, and visualized. As shown in Figure 2, an ∼33-kDa band (a typical molecular mass for RACK1 binding on Western blots) was present in the particulate fraction from the basal cells that were enriched for RACK1, which is in accordance with the observation that RACK1 is localized primarily to the membrane of basal, non-ciliated airway cells. A 47-kDa β-actin control band was present in all lanes. The mixed ciliated cell preparation contains some contaminating RACK1 protein, because basal cells are present, and, as expected, the bovine cilia was negative for RACK1 (Figure 2).
Figure 2.
Western analysis of BBECs and cilia protein. Soluble and particulate lysate fractions of a mixed ciliated/basal BBECs preparation, a preparation of primary basal BBECs (>90%), and bovine cilia protein were quantitated, electrophoresed, and transferred to a membrane for Western analysis and probed with antibodies against RACK1 and β-actin. The anti-RACK1 monoclonal antibody detected an ∼33-kDa band in the particulate basal cell fraction and the mixed ciliated/basal cell preparation. The basal cell particulate (membrane) fraction has the greatest amount of RACK1 and bovine cilia protein and is negative for RACK1. β-actin was present in all lanes.
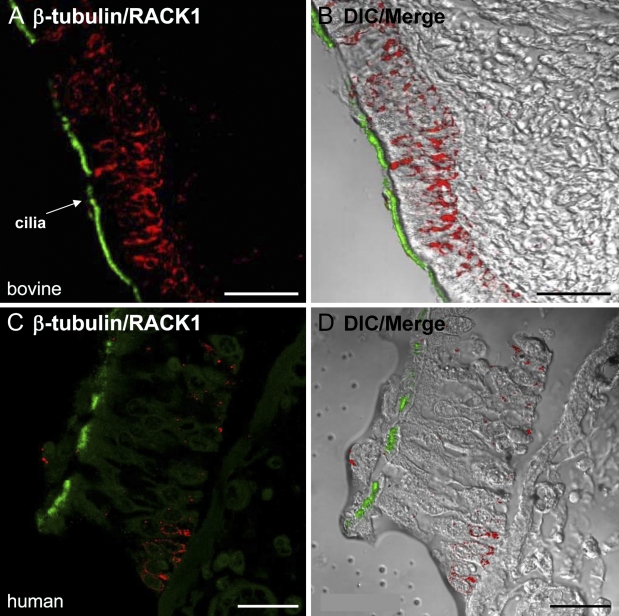
Because our previous IHC observations used cytospin preparations of bovine primary epithelial cells, we confirmed our results using intact, human bronchial tissue (slides purchased from US Biomax) and bovine bronchial epithelial tissue, fixed in formalin, sectioned, and stained with antibodies against RACK1 and β-tubulin. As shown in Figures 3A and 3B, RACK1 (red) was localized to basal airway cells in bovine tracheal tissue and was excluded from ciliated cells that express β-tubulin (green). A similar staining pattern to Figures 3A and 3B was present in the normal human bronchus stained with RACK1 (red) and β-tubulin (green; Figures 3C and 3D), confirming our cytospin results in Figure 1. In both the human and bovine tissue, the ciliated columnar epithelium was identified by the cilia stained for β-tubulin. The basal cells exclusively expressed RACK1 (red) and were located below the ciliated columnar cells.
Figure 3.
RACK1 and β-tubulin expression in bovine and human airway epithelial tissue. Immunohistochemistry (IHC) was performed on formalin-fixed, paraffin-embedded human bronchus tissue or fixed bovine tracheal tissue and stained with antibodies against RACK1 or β-tubulin. In the bovine tissue, RACK1 (red) localization is clearly distinguished in the two color fluorescence (A) and DIC/merge images (B), which show that RACK1 is expressed in the basal region and is excluded from the ciliated columnar epithelial cells that express β-tubulin (green). Similar results were seen in the human bronchial tissue stained with RACK1 (red) and β-tubulin (green) in the fluorescence (C) and DIC/merge images (D). Bar = 25 μm.
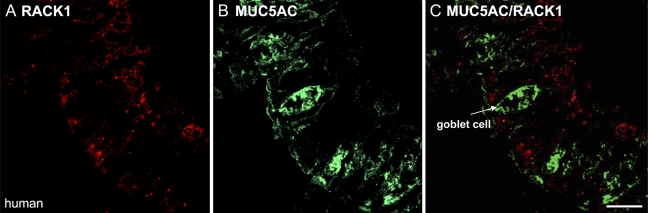
Additionally, to show that RACK1 does not localize to airway goblet cells, human bronchus was dual-stained with a rabbit polyclonal antibody against MUC5AC (green), which is localized to airway goblet cells (Rogers 2003) and RACK1 (red). In Figures 4A and 4B, no colocalization appeared with these markers. These results confirmed that RACK1 is present only in basal airway cells and not in the more differentiated airway cells.
Figure 4.
RACK1 is not localized to human airway goblet cells. IHC was performed on human bronchial tissue and stained with antibodies against RACK1 (red) or the human goblet cell marker MUC5AC (green). In the individual fluorescence images (A,B) and the two-color fluorescence image (C), RACK1 does not appear to be expressed in airway goblet cells, which express MUC5AC. Bar = 10 μm.
Because previous RACK1 localization studies in the human airway were performed using the Calu-3 lung cell line (Liedtke et al. 2002; Auerbach and Liedtke 2007), we also used an immortalized human bronchial epithelial cell line, BEAS-2B, to confirm our analysis. IHC and Western blot experiments were performed on BEAS-2B cells (Figure 5) (Ke et al. 1988; Reddel et al. 1995). In Figure 5A, BEAS-2B cells were grown on chamber slides, fixed and stained with RACK1 (red), and mounted with Vectashield containing DAPI. These results showed that RACK1 is expressed in the cytosol and membrane (Figure 5A). Western analysis showed that RACK1 is predominantly present in the particulate fraction (Figure 5B) of this cell line.
Discussion
To date, there have been few reports detailing RACK1 localization or function in primary airway cells. This important adaptor protein is expressed in the immortalized lung Calu-3 cell line, where RACK1 plays a necessary role in regulation of the cystic fibrosis transmembrane regulator through PKCɛ-dependent signaling and binding to the Na+/H+ exchange regulatory factor (Liedtke et al. 2002,2004; Auerbach and Liedtke 2007). Using IHC localization and Western blot analysis, we showed that the PKC targeting protein RACK1 is localized exclusively to non-ciliated and non-goblet bovine and human airway epithelial cells. In fixed primary cells and intact bovine and human tissue, RACK1 is excluded from columnar ciliated cells or goblet cells and is localized solely in the basal cells. These results have exciting implications for differential regulation of PKC signaling in the subtypes of airway epithelial cells.
We have previously shown that PKCɛ is an important regulator of several important airway functions, including cytokine release after environmental insult from organic dust (Wyatt et al. in press) and ciliated cell detachment from the basal cell layer (Slager et al. 2006) after epithelial injury. In the latter phenomenon, inhibition of PKCɛ by a novel PKC inhibitor, Ro 31-8220, mimics the effects seen in viral infections or cigarette smoke exposure (Sisson et al. 1994), producing widespread detachment of ciliated cells and cilia loss (Slager et al. 2006). We were interested in determining how this mechanism of ciliated cell detachment is regulated and hypothesized that RACK1 would be localized to the cilia or apical region of ciliated cells as a targeting protein for PKC. In some reports, activated PKCɛ has been reported to use RACK1 scaffolding at the membrane to initiate downstream signaling (Besson et al. 2002; McCahill et al. 2002). However, our data showed that RACK1 is not present in all airway cell types, specifically the more differentiated columnar airway cells subtypes that are ciliated or produce mucus. RACK1 is expressed in the less-differentiated basal cell subtype within the mammalian airway. Therefore, PKCɛ-mediated signaling may be regulated differently within these cell types. Although we originally hypothesized that ciliated cell detachment after environmental insult would be induced by the PKCɛ/RACK1 signaling within the damaged ciliated cell, we now revise this hypothesis because RACK1 is not present in the ciliated airway cell. We therefore speculate that PKCɛ-regulated preferential detachment of ciliated cell detachment may be modulated through PKCɛ/RACK1 interaction in the basal cell. For instance, after an epithelial injury, activated PKCɛ may translocate to the basal cell membrane, where it is anchored by RACK1 to specifically phosphorylate cell–cell contact substrates, causing a signaling cascade that results in the release of damaged ciliated cells. We recognize that there are several other possibilities in which PKCɛ signaling in airway ciliated cells could rely on other anchoring proteins such as A kinase anchoring proteins, which have also been reported to bind to PKC (Perkins et al. 2001; Higashida et al. 2005). Further study is needed to determine the mechanism of PKCɛ-dependent signaling after epithelial cell injury.
The possibility that the RACK1 adaptor protein is essential for basal airway epithelial cell signaling has not been widely explored. RACK1 has been shown to regulate adhesion and migration in human glioma cells through PKCɛ linkage to integrins (Besson et al. 2002); migration in keratinocytes also was enhanced by overexpression of RACK1 (Osmanagic-Myers et al. 2006). It would be intriguing if RACK1 also mediated wound healing and migration in airway epithelial cells and other crucial airway epithelial cell functions such as cytokine release. Developmentally, RACK1 expression may also be downregulated as the basal cell undergoes differentiation to become a columnar ciliated cell or goblet cell. Depletion of RACK1 by siRNA in basal epithelial cells will determine whether RACK1 is needed for PKCɛ-regulated signaling mechanisms that regulate airway cell migration and wound repair, as well as interleukin (IL)-8 and IL-6 cytokine release in response to organic dust exposure.
In conclusion, we showed that the adaptor protein RACK1 is excluded from ciliated airway epithelial cells, and its localization in basal cells indicates that PKC regulation in the airways is dependent on cell type. This novel observation, although largely descriptive, provides the basis for several interesting hypotheses regarding PKC signaling in the airways.
Acknowledgments
The authors thank Lisa Chudomelka for manuscript preparation and Janice Taylor and James Talaska of the UNMC Laser Scanning Microscope Core Facility for operation of the confocal microscopes.
References
- Auerbach M, Liedtke CM (2007) Role of the scaffold protein RACK1 in apical expression of CFTR. Am J Physiol Cell Physiol 293:294–304 [DOI] [PubMed] [Google Scholar]
- Beckmann JD, Takizawa H, Romberger D, Illig M, Claassen L, Rickard K, Rennard SI (1992) Serum-free culture of fractionated bovine bronchial epithelial cells. In Vitro Cell Dev Biol 28A:39–46 [DOI] [PubMed] [Google Scholar]
- Besson A, Wilson TL, Yong VW (2002) The anchoring protein RACK1 links protein kinase Cɛ to integrin β chains. Requirements for adhesion and motility. J Biol Chem 277:22073–22084 [DOI] [PubMed] [Google Scholar]
- Higashida H, Hoshi N, Zhang JS, Yokoyama S, Hashii M, Jin D, Noda M, et al. (2005) Protein kinase C bound with A-kinase anchoring protein is involved in muscarinic receptor-activated modulation of M-type KCNQ potassium channels. Neurosci Res 51:231–234 [DOI] [PubMed] [Google Scholar]
- Jurczyk A, Gromley A, Redick S, San Agustin J, Witman G, Pazour GJ, Peters DJ, et al. (2004) Pericentrin forms a complex with intraflagellar transport proteins and polycystin-2 and is required for primary cilia assembly. J Cell Biol 166:637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Reddel RR, Gerwin BI, Miyashita M, McMenamin M, Lechner JF, Harris CC (1988) Human bronchial epithelial cells with integrated SV40 virus T antigen genes retain the ability to undergo squamous differentiation. Differentiation 38:60–66 [DOI] [PubMed] [Google Scholar]
- Liedtke CM, Raghuram V, Yun CC, Wang X (2004) Role of a PDZ1 domain of NHERF1 in the binding of airway epithelial RACK1 to NHERF1. Am J Physiol Cell Physiol 286:C1037–1044 [DOI] [PubMed] [Google Scholar]
- Liedtke CM, Wang X (2006) The N-terminus of the WD5 repeat of human RACK1 binds to airway epithelial NHERF1. Biochemistry 45:10270–10277 [DOI] [PubMed] [Google Scholar]
- Liedtke CM, Yun CH, Kyle N, Wang D (2002) Protein kinase C ɛ-dependent regulation of cystic fibrosis transmembrane regulator involves binding to a receptor for activated C kinase (RACK1) and RACK1 binding to Na+/H+ exchange regulatory factor. J Biol Chem 277:22925–22933 [DOI] [PubMed] [Google Scholar]
- McCahill A, Warwicker J, Bolger GB, Houslay MD, Yarwood SJ (2002) The RACK1 scaffold protein: a dynamic cog in cell response mechanisms. Mol Pharmacol 62:1261–1273 [DOI] [PubMed] [Google Scholar]
- Monick M, Staber J, Thomas K, Hunninghake G (2001) Respiratory syncytial virus infection results in activation of multiple protein kinase C isoforms leading to activation of mitogen-activated protein kinase. J Immunol 166:2681–2687 [DOI] [PubMed] [Google Scholar]
- Osmanagic-Myers S, Gregor M, Walko G, Burgstaller G, Reipert S, Wiche G (2006) Plectin-controlled keratin cytoarchitecture affects MAP kinases involved in cellular stress response and migration. J Cell Biol 174:557–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins GA, Wang L, Huang LJ, Humphries K, Yao VJ, Martone M, Deerinck TJ, et al. (2001) PKA, PKC, and AKAP localization in and around the neuromuscular junction. BMC Neurosci 2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddel RR, De Silva R, Duncan EL, Rogan EM, Whitaker NJ, Zahra DG, Ke Y, et al. (1995) SV40-induced immortalization and ras-transformation of human bronchial epithelial cells. Int J Cancer 61:199–205 [DOI] [PubMed] [Google Scholar]
- Rogers DF (2003) The airway goblet cell. Int J Biochem Cell Biol 35:1–6 [DOI] [PubMed] [Google Scholar]
- Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA (2002) Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol 93:289–296 [DOI] [PubMed] [Google Scholar]
- Schechtman D, Mochly-Rosen D (2001) Adaptor proteins in protein kinase C-mediated signal transduction. Oncogene 20:6339–6347 [DOI] [PubMed] [Google Scholar]
- Sisson JH, Papi A, Beckmann JD, Leise KL, Wisecarver J, Brodersen BW, Kelling CL, et al. (1994) Smoke and viral infection cause cilia loss detectable by bronchoalveolar lavage cytology and dynein ELISA. Am J Respir Crit Care Med 149:205–213 [DOI] [PubMed] [Google Scholar]
- Sklan EH, Podoly E, Soreq H (2006) RACK1 has the nerve to act: structure meets function in the nervous system. Prog Neurobiol 78:117–134 [DOI] [PubMed] [Google Scholar]
- Slager RE, Sisson JH, Pavlik JA, Johnson JK, Nicolarsen JR, Jerrells TR, Wyatt TA (2006) Inhibition of protein kinase C ɛ causes ciliated bovine bronchial cell detachment. Exp Lung Res 32:349–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout SL, Wyatt TA, Adams JJ, Sisson JH (2007) Nitric oxide-dependent cilia regulatory enzyme localization in bovine bronchial epithelial cells. J Histochem Cytochem 55:433–442 [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Forget MA, Adams JM, Sisson JH (2005) Both cAMP and cGMP are required for maximal ciliary beat stimulation in a cell-free model of bovine ciliary axonemes. Am J Physiol Lung Cell Mol Physiol 288:L546–551 [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Forget MA, Sisson JH (2003) Ethanol stimulates ciliary beating by dual cyclic nucleotide kinase activation in bovine bronchial epithelial cells. Am J Pathol 163:1157–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TA, Ito H, Veys TJ, Spurzem JR (1997) Stimulation of protein kinase C activity by tumor necrosis factor-α in bovine bronchial epithelial cells. Am J Physiol 273:L1007–1012 [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Slager RE, Devasure J, Auvermann BW, Mulhern ML, Von Essen SG, Mathisen T, et al. (In Press) Feedlot dust stimulation of interleukin-6 and 8 requires protein kinase C-ɛ human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. Published online August 24, 2007 (DOI: 10.1152/ajplung.00103.2007) [DOI] [PubMed]