Abstract
A recent study from our laboratory has shown cellular and ultrastructural distribution of the gonadotropin-releasing hormone (GnRH) and the relative expression of its mRNA in the rat oviduct during the postimplantation period of pregnancy (days 7, 9, 16, and 20). To determine the possible autocrine/paracrine involvement of the oviductal GnRH during pregnancy in rats, the present investigation aims at the study of the relative expression of GnRH receptor (GnRHR) mRNA by real-time PCR followed by immunolocalization of the peptide in the oviduct during pregnancy. Semiquantitative analysis of the oviductal GnRHR expression by Western blot was done by densitometry of the signal intensity. Our results indicate the expression of GnRHR mRNA in the rat oviduct throughout the postimplantation period of pregnancy with no significant difference in expression between the selected time points. Immunoreactive GnRHR peptide was localized predominantly in the cytoplasm of the luminal epithelial cells, with less expression in the cytoplasm of the stromal cells and the smooth muscles throughout the oviduct. Signal intensity of GnRHR was significantly lower during day 16 when compared to days 7 and 20. These results, for the first time, support the transcription of GnRHR mRNA and its translation to protein in the rat oviduct throughout the postimplantation period of pregnancy. The lower protein content of GnRHR by day 16 may be indicative of ligand-induced downregulation of the receptor expression. The present investigation thus strengthens our previously postulated hypothesis regarding the receptor-mediated autocrine/paracrine role of oviductal GnRH during pregnancy in rats. (J Histochem Cytochem 56:25–31, 2008)
Keywords: gonadotropin-releasing hormone receptor, oviduct, pregnancy, rat
The mammalian oviduct is the venue for reproductive events that leads to establishment and maintenance of pregnancy (Miller and Leondires 2003). The mammalian oviduct is anatomically divided into the infundibulum, ampulla, and isthmus (Abe 1996; Lyons et al. 2006), each of which is composed of layers of smooth muscle surrounding a heterogeneous population of secretory and ciliated epithelial cells bounding the oviductal lumen (Lyons et al. 2006). These heterogeneous cell populations are reported to secrete several embryotropic factors like peptides (Sengupta et al. 2007) and glycoproteins (Komiya et al. 1996), which differentially influence several processes that lead to induction of pregnancy (Chegini 1996).
The hypothalamic peptide gonadotropin-releasing hormone (GnRH), which plays a central role in the regulation of mammalian reproductive functions by triggering the release of pituitary gonadotropins, has been reported to be expressed in human oviduct during the luteal phase of the menstrual cycle (Casan et al. 2000). Oviductal GnRH is predicted to be involved in autocrine/paracrine regulation of functions like fertilization, early embryonic development, and implantation in human (Casan et al. 2000). A recent study from our laboratory (Sengupta et al. 2007) has revealed evidence for the synthesis and expression of immunoreactive GnRH in the luminal epithelium (LE) and its release into the lumen of rat oviduct during the postimplantation period of pregnancy. This finding led us to hypothesize the existence of an autocrine/paracrine/endocrine mechanism through which GnRH of oviductal origin expressed during the postimplantation period of pregnancy may help in the maintenance of pregnancy in rats (Sengupta et al. 2007).
GnRH is reported to induce differential downstream effects at its target site by signaling through its specific receptor (GnRHR) (Han et al. 1998; Kraus et al. 2001; Millar 2005), an example of ligand-selective signaling. The expression pattern of this G-protein-coupled GnRHR in the rat oviduct during cyclicity/pregnancy in mammals remains unexplored. As an initial approach towards investigation of the existence of a receptor-mediated autocrine/paracrine role of oviductal GnRH during pregnancy in rats, we designed the present study that aims at elucidation of the expression pattern of immunoreactive GnRHR and its mRNA during the considered time points (days 7, 9, 16, and 20) of pregnancy. The selected time points during pregnancy correspond to changes in the serum profiles of progesterone, critical for induction and maintenance of pregnancy in rats related to changes in the circulating levels of luteinizing hormone and prolactin (Morishige et al. 1973; Sengupta et al. 2007).
Materials and Methods
Animals
All experimental procedures and protocols used in the present study were approved by the Institutional Animal Care and Use Committee (IACUC) of Morehouse School of Medicine. Accepted standards of NIH Guide for Humane Care and Use of Experimental Animals were followed. Timed-pregnant Sprague Dawley rats (8 weeks old) were obtained from Charles River Laboratories (Wilmington, MA) and maintained under defined conditions within a temperature- (23–25C) and light- (daily 14 h light:10 h darkness) controlled room in the Institutional Animal Care Facility. Purina rat chow and tap water were accessible to the animals ad libitum. Considering the day of insemination identified by the appearance of the sperm plug as the first day of pregnancy, animals were sacrificed subsequently on each of the 7th, 9th, 16th, and 20th days by exposing them to a chamber of carbon dioxide for 5–10 min.
Tissue Collection
Implantation of the embryo in the uterine wall of each animal was checked during each of the considered time points to ensure induction of pregnancy prior to tissue collection. The paired oviduct and pituitary of each animal (n=4) was dissected out on each considered time point of pregnancy. One oviduct of each pair was fixed in 4% formaldehyde neutral-buffered solution for 24 h and embedded in paraffin for histochemical analysis. The other was snap frozen in liquid nitrogen and stored at −80C for use in real-time PCR studies. Paired oviducts and pituitary tissues from a different set of timed pregnant animals (n=3) killed during the designated time points of pregnancy were snap frozen and stored at −80C for Western blot (WB) analysis.
Antibodies
The primary antibody used for immunohistochemistry (IHC) and WB analysis was monoclonal anti-GnRHR antibody raised in mouse obtained from Lab Vision Corporation (cat. #MS-1139-P, clone designation GNRH03; NeoMarkers, Fremont, CA) in dilutions of 1:200 for IHC and 1:100 for WB. The antibody was raised against a synthetic 1–29 aa-long peptide (MANSASPEQNQNHCSAINNSIPLMQGNLPY) from the N-terminal end of human GnRH receptor (Karande et al. 1995). A biotin-conjugated universal secondary antibody provided in Universal Vectastain ABC kit (cat. #PK-6200, 1:100 dilution; Vector Laboratories, Burlingame, CA) was used for IHC studies. Horseradish peroxidase-conjugated goat anti-mouse IgG (cat. #170-5047, Immun-Star GAM-HRP conjugate, 1:2000 dilution; Bio-Rad Laboratories, Hercules, CA) was used for WB analysis. Mouse monoclonal anti-β-A-tubulin antibody (cat. #T-5168, clone B-5-1-2, 1:100 dilution; Sigma Chemical Co., St Louis, MO) was used for expression of internal standard protein for WB analysis.
RNA Extraction and Reverse Transcription Reaction
Extractions of total RNA from frozen pre-weighed oviduct and pituitary tissues were done according to the method described by Sengupta et al. (2007). Purity of the RNA was assessed with an Eppendorf AG 2231 Biophotometer (Hamburg, Germany) using the 260:280 nM ratios, which were in the range of 1.8 and 2.1. 28S and 18S rRNA bands of representative samples loaded on an Agilent 2100 Bioanalyzer using RNA 6000 nano assay kit (Agilent Technologies; Santa Clara, CA) helped in assessing the integrity of the extracted RNA. Two μg of RNA samples from each tissue type (oviduct and pituitary) were reverse transcribed to cDNA following manufacturer's instructions provided in the Taqman Reverse Transcription Reagent kit (Applied Biosystems; Foster City, CA) with reaction conditions described previously by us (Sengupta et al. 2007). cDNA samples thus obtained were stored at −20C for real-time PCR studies.
Real-time PCR
Sequence of Rattus norvegicus GnRHR mRNA (accession number NM_031038) was obtained from the gene bank database of the National Center for Biotechnology Information of NIH (http://www.ncbi.nlm.nih.gov/cgi-bin/genbank). Using OligoPerfect Designer software (Invitrogen; Carlsbad, CA), primer sequences were selected to hybridize successfully and thus amplify target cDNA sequences for real-time PCR studies. Primer sequences and annealing temperatures of each primer are elaborated in Table 1. To avoid amplification of contaminating genomic DNA, primers specific for GnRHR and 18S rRNA (housekeeping gene) were designed across the exon/intron boundaries. Testing of the primer efficiency, quantitative PCR assay using 650 ng of cDNA for each reaction (determined from standard curve), and quantification of the results [expressed as cycle threshold (CT) value] have been done according to a detailed process described by Sengupta et al. (2007). A comparative CT method (Qiagen; Valencia, CA) was used to compare the relative expression of GnRHR mRNA in the rat oviduct during different days of pregnancy, using day 7 as the calibrator. Rat pituitary was used as a positive control for validation of the experiment. The detailed calculation method has been described elsewhere (Sengupta et al. 2007).
Table 1.
Sequences and annealing temperatures of sense and antisense primers used for amplification of GnRHR and 18S mRNA by real-time PCR studies and length of the amplification product
| Gene name | Sequence (5′ to 3′) | Product length | Primer temperature (C) |
|---|---|---|---|
| GnRHR | Sense: CTAACAATGCGTCTCTTGA | 101 | 54 |
| Antisense: TCCAGATAAGGTTAGAGTCG | |||
| 18S mRNA | Sense: AATTCCGATAACGAACGAGA | 141 | 54 |
| Antisense: ATCTAAGGGCATCACAGACC |
GnRHR, gonadotropin-releasing hormone receptor.
Protein Extraction and WB Analysis
Pre-weighed paired oviducts and pituitary from animals collected during each time point were used for extraction of total protein for WB analysis. Pituitary tissues collected during each time point were pooled together for protein extraction. Tissues were homogenized in 1 ml lysis buffer containing 0.15 M NaCl, 2 mM EDTA, 0.15% Triton X-100, and protease inhibitors (protease inhibitor cocktail, cat. #P8340; Sigma-Aldrich). The lysate was centrifuged at 18,000 × g for 20 min at 4C. The supernatant was collected, and protein content was estimated by the method of Lowry et al. (1951) using BSA standards. Linearization of the extracted total protein from each sample was done by boiling the supernatant with an equal volume of Laemmli sample buffer (cat. #161-0737; Bio-Rad Laboratories) for 10 min in a boiling water bath. Linearized protein samples were cooled on ice and recentrifuged at 11,000 × g for 10 min at 4C. The collected supernatant containing extracted protein was used for WB analysis.
Oviductal protein samples (35 μg) collected during each time point of pregnancy along with the pooled pituitary (positive control) sample were electrophoresed on 15% Tris–HCl pre-cast minigels (cat. #161-1157; Bio-Rad Laboratories) for 45 min under reducing conditions. Proteins were electrotransferred for 1 hr at 100 V to nitrocellulose membrane (0.2-μm pore size; Bio-Rad Laboratories), and WB analysis was done according to manufacturer's instructions using Immuno-Star HRP chemiluminescent starter kit (cat. #170-5055; Bio-Rad Laboratories). The primary antibody was used at a dilution of 1:100 in Tris-buffered saline (TBS) containing 0.1% Tween 20 and 2.5% non-fat dry milk. Secondary IgG (provided in the kit) diluted to 1:2000 in TBS-0.1% Tween 20 buffer was used for analysis. Immunoblotted signals were detected by enhanced chemiluminescence (ECL; a component of the kit) and visualized on films (Amersham; Buckinghamshire, UK). Individual band intensity was quantified by densitometry using Image J software (version 1.33U, a program inspired by NIH image; http://rsb.info.nih.gov/ij/docs/index.html). Signal intensities of the rat oviductal GnRHR protein were normalized by intensity of the α-tubulin (internal standard) in each sample and expressed in arbitrary densitometric units (ADU) (Shao et al. 2007). To standardize the assay for measurement of the GnRHR protein, we examined different starting protein concentrations for each sample. Results correspond to the average of three individual experiments.
Immunohistochemical Analysis and Microscopy
Deparaffinization and rehydration of the 10-μm paraffin sections of the oviduct were followed by antigen retrieval with 10 mM sodium citrate buffer (pH 6.0, 10 min in a 700-W microwave). Endogenous peroxidase activity and non-specific binding were minimized by incubation in 0.3% H2O2 solution and 1% universal horse serum (provided in Universal Vectastain ABC kit, cat. #PK 6200; Vector Laboratories) in PBS buffer, respectively, for 1 hr at room temperature. Incubation of sections with the primary antibody for GnRHR was done at 37C for 1 hr followed by an overnight incubation at 4C. This incubation step with the primary antibody was omitted in the negative control sections. Sections were treated with the primary antibody pre-absorbed with a synthetic GnRHR antigen (Santa Cruz Biotechnology; Santa Cruz, CA) for pre-absorption control study. Sections were subsequently stained using avidin–biotin–peroxide complex (Universal Vectastain ABC kit, cat. #PK6200; Vector Laboratories) according to manufacturer's instructions, followed by a 5-min treatment with DAB (0.03% DAB in PBS containing 0.01% hydrogen peroxide) in darkness for visualization of peroxidase activity at the antigen sites. Counterstaining of the sections with hematoxylin (QS, H3404; Vector Laboratories), dehydration in graded alcohols and xylene, and mounting with Vectashield mounting medium (Vector Laboratories) followed thereafter. Stained sections were imaged on a Zeiss microscope (Carl Zeiss MicroImaging; Thornwood, NY), and photomicrographs were prepared using Axioshop 2 plus software. Specificity of the primary antibody used for immunolocalization of GnRHR was checked by staining sagittal sections of rat pituitary that exhibited distinct immunoreactivity for GnRHR in the pituitary gonadotroph cells.
Statistical Analysis
All data represent the mean ± SEM. Statistical evaluation of the mean differences in data among the chosen time points of pregnancy was performed by one-way ANOVA with significance levels set at p≤0.05. Post-hoc analysis or pairwise multiple comparisons between groups was done by Student–Newman–Keuls Multiple Comparisons Test.
Results
Expression of GnRHR mRNA in the Rat Oviduct
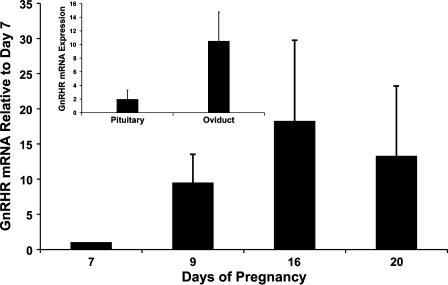
Expression of GnRHR mRNA in the rat oviduct during the postimplantation period of pregnancy was found to be equal to (if not greater) that in the pituitary (Figure 1, inset). Relative to day 7 (considered as 1), GnRHR mRNA was expressed during each time point (days 9, 16, and 20) with no statistically significant difference in the degree of expression during each considered point. 18S mRNA was expressed equally (p=0.07) across the samples throughout the entire experiment, reflecting the validity of the real-time PCR results and the integrity of the RNA samples used. Collectively, real-time PCR results indicate that GnRHR mRNA is transcribed in the rat oviduct throughout the postimplantation period of pregnancy at levels comparable to that in the pituitary.
Figure 1.
GnRHR mRNA expression (mean ± SEM; n=3/time point) in the rat oviduct during chosen time points of pregnancy. Values were standardized to respective 18S control values. Inset represents comparison between mRNA expression of GnRHR in the pituitary (n=8) and oviduct (n=12).
Expression of GnRHR Protein in Rat Oviduct by WB Analysis
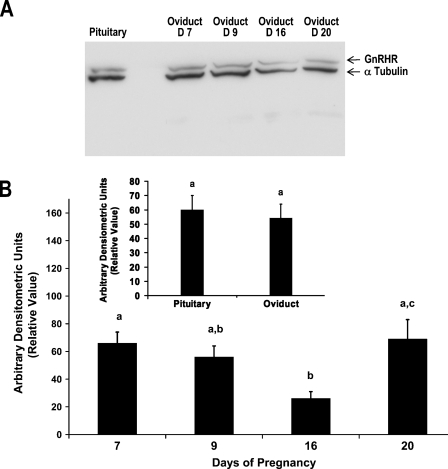
WB analysis for GnRHR using total protein extracts from the rat oviduct demonstrated expression of the receptor protein through strong signals corresponding to 62 kDa during each selected time point of pregnancy (Figure 2A). Whereas the expression of the internal standard, α-tubulin (signal corresponding to 50 kDa) remained approximately the same in all samples (confirming equal loading of protein samples and thus validating the WB analysis), expression of GnRHR was least abundant during day 16 when compared to days 7, 9, and 20 (Figure 2A). Relative levels of GnRHR protein in the oviduct were expressed in ADU (a ratio of the signal intensities of GnRHR to α-tubulin) during each time point of pregnancy (Figure 2B). A significant decrease (p≤0.05) in ADU for GnRHR was noted in the oviduct by day 16 when compared to days 7 and 20 (Figure 2B). Expression of GnRHR in the oviduct and pituitary (Figure 2A) and the relative levels of the signal intensity measured in ADU (Figure 2B, inset) were comparable during pregnancy in rats.
Figure 2.
(A) Representative Western blot of oviductal protein for expression of GnRHR (62 kDa) during days 7, 9, 16, and 20 of pregnancy in rats. Expression of GnRHR in the rat pituitary used as positive control validates the experiment. α-Tubulin (50 kDA) used as an internal standard protein and for normalization is expressed in all samples. (B) Relative levels of GnRHR in the rat oviduct expressed as a ratio of the densitometric values of GnRHR to α-tubulin during chosen time points of pregnancy. Data represent mean ± SEM of three animals per group. Inset represents comparison between the mean relative value of GnRHR expressed in the rat pituitary used as a positive control (n=4; one animal collected from each time point and pooled together) and oviduct (n=12; three animals collected during each time point). Group means with different superscript letters differ significantly (p≤0.05).
Cell-specific Localization of GnRHR in the Oviduct
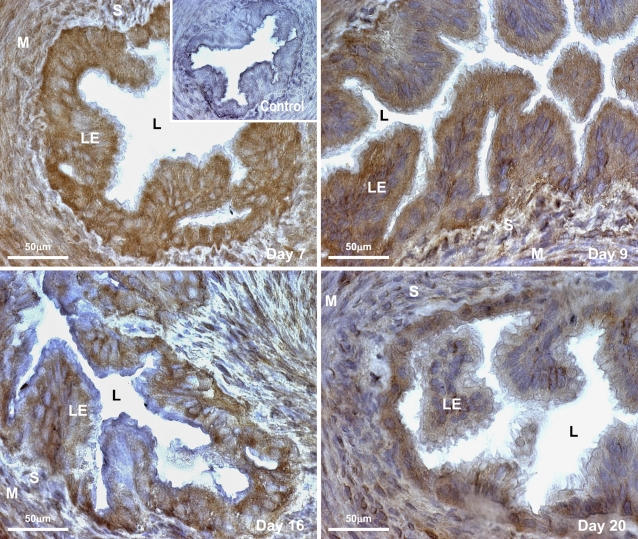
To visualize the exact localization of the GnRHR peptide in the rat oviduct during the postimplantation period of pregnancy, we subsequently examined expression of this receptor protein in the tubal or LE, stromal cells (S), and smooth muscle layer (M) in each part of the oviduct using DAB immunostaining (Figure 3). GnRHR was localized differentially in the cytoplasm of LE; S bounding the LE; and the M layers in the infundibulum, ampulla, and isthmus of the oviduct during each selected time point. Nuclei of the LE and stromal cells in all parts of the oviduct remained immunonegative. The number of GnRHR-immunopositive cells with densely stained cytoplasm was abundant in the LE throughout the oviduct during days 7 and 9 of pregnancy when compared to day 16. By day 20, the number of immunolabeled LE cells (as per eye observation) bounding the lumen of the oviduct had increased when compared to that of day 16 but was lower in comparison to days 7 and 9. Localized immunopositivity for GnRHR in the S and M layers of the oviduct was much less during each considered time point. Representative photomicrographs of the oviduct depict labeling for GnRHR in the ampullary region during each selected time point of pregnancy (data for GnRHR immunolabeling in the infundibulum and isthmus, being the same, have not been shown). LE, S, and M layers in the pre-absorbed control section shown as inset on day 7 photomicrograph or the negative control sections (data not shown) were immunonegative with lack of staining for GnRHR in all regions of the oviduct.
Figure 3.
Representative photomicrographs of oviductal sections (ampullary region) identifying cellular localization of GnRHR (visualized with DAB showing brown staining) in rats during different time points of pregnancy. Localized immunoreactivity for GnRHR is restricted to the cytoplasm of the luminal epithelium (LE) bounding the oviductal lumen (L) in all parts of the oviduct with comparatively less immunostaining in the stromal (S) cells and smooth muscle layer (M) of the oviduct during each selected time point. Inset represents a pre-absorbed control section of rat oviduct on day 7 exhibiting negative immunostaining for GnRHR in all the (LE, S, or M) layers.
Discussion
Interest in the functional significance of extrahypothalamic GnRH on regulation of reproductive functions in general has increased immensely in recent years due to the specific distribution of this decapeptide and its classical receptor in reproductive tissues (Wolfahrt et al. 1998; Hapgood et al. 2005; Schirman-Hildesheim et al. 2005). Using differential molecular approaches, the present study, for the first time, offers a unique detailed description of expression pattern and cellular localization of a classical GnRHR mRNA and protein in the rat oviduct during the postimplantation period of pregnancy, indicating the possible involvement of GnRH of oviductal origin (as shown in our previous study by Sengupta et al. 2007) in receptor-mediated autocrine/paracrine functions during pregnancy.
Although no significant difference is noted in the expression pattern of the receptor mRNA between the considered time points (days 7, 9, 16, and 20), real-time PCR studies demonstrate the transcription of GnRHR mRNA in the rat oviduct throughout the postimplantation period of pregnancy. WB analysis confirms the presence of the translated product (GnRHR protein) in the oviduct during each considered time point of pregnancy. Strong signal intensity of the receptor protein is noted during days 7, 9, and 20. A significant decrease in the signal intensity of GnRHR protein during day 16 of pregnancy, when compared to the other selected times points (days 7 and 20), is indicative of a decrease in the expression of the receptor protein by mid-pregnancy (day 16). As the expression of the ligand (GnRH) in the rat oviduct during day 16 is highest (Sengupta et al. 2007), low expression of its classical receptor GnRHR at this particular time point of pregnancy is indicative of the downregulation of the receptor expression by high-ligand concentration. Desensitization of cultured pituitary gonadotrophs as a consequence of downregulation of a number of pituitary GnRHR by high-ligand (GnRH) concentrations has been previously documented in the literature (Zilberstein et al. 1983; Naik et al. 1984; Garcia-Palencia et al. 2007). Similar downregulation of extrapituitary GnRHR expressed in cultured granulosa cells by high doses of GnRH in rats (Ranta et al. 1982) supports our present observation. Conversely, relatively higher expression of ligand and receptor protein (GnRH–GnRHR) by the tail end of pregnancy (day 20) as shown by the present study and previous report (Sengupta et al. 2007) emphasizes the ability of GnRH to exert a negative as well as positive (Cheon et al. 1999) effect on extrapituitary GnRHR mRNA/protein expression (Ranta et al. 1982). Our study also, for the first time, provides a hint that GnRHR protein, if not mRNA, is expressed in the rat oviduct at levels comparable to the pituitary expression of GnRHR during pregnancy, although daywise comparison between pituitary and oviductal GnRHR expression was not the purpose of the present investigation.
Immunolocalization of GnRHR protein was observed dominantly in the cytosol of LE with slight expression in stromal cells and the smooth muscle layers in all parts of the oviduct during each time point of pregnancy. Although classical GnRHR is reported to be a G-protein-coupled transmembrane receptor, its cytoplasmic expression in the LE, S, and M cells is believed to represent neosynthesized or internalized receptors (Hashizume et al. 2001; Choi et al. 2006). Decrease in the number of immunolabeled LE cells during day 16 supports the observed decrease in signal intensity of GnRHR protein during day 16, as shown by WB analysis. Expression of both the ligand (GnRH) (Sengupta et al. 2007) and its classical receptor GnRHR in the LE bounding the oviductal lumen throughout the postimplantation period of pregnancy is indicative of the involvement of GnRH of oviductal origin in receptor-mediated autocrine functions within the LE cells of the oviduct during pregnancy in rats. Differential expression of the receptor only, in the cytoplasm of the stromal cells and the smooth muscle layer, is indicative of the receptor-mediated paracrine role of oviductal GnRH synthesized exclusively by the LE (Sengupta et al. 2007) of the rat oviduct during the postimplantation period of pregnancy.
The oviduct in mammals expresses receptors for gonadal steroids like estrogen (Garcia-Palencia et al. 2007; Shao et al. 2007) and progesterone in several isoforms during cyclicity (Briton-Jones et al. 2005; Peralta et al. 2005) and early pregnancy (Anzaldua et al. 2007). The presence of the receptor alone provides an indication towards functional involvement of their respective ligand (estrogen/progesterone) in processes like sperm capacitation, fertilization, and early embryo cleavage (Briton-Jones et al. 2005) in the oviduct. Similarly, evidence for the synthesis and expression of GnRH receptor in the rat oviduct during the postimplantation period of pregnancy provided by the present investigation strengthens our previous argument (Sengupta et al. 2007) that oviductal GnRH synthesized and secreted exclusively by the LE throughout the postimplantation period of pregnancy in rats may have receptor-mediated autocrine/paracrine functions that may directly/indirectly help in maintenance of pregnancy. The exact functional link between oviductal GnRH and postimplantation embryonic development or parturition processes still remains an enigma and needs to be unraveled. The role of this oviductal decapeptide during pregnancy in non-human primates, its clinical significance in induction/termination of human pregnancy, and its pathophysiological effects are tempting future projects to be conducted in our laboratory.
Acknowledgments
This study was supported by NIH Grants RR-03034, HD-41749, and HD-52155.
Our sincere thanks to the animal care facility of Morehouse School of Medicine for housekeeping of experimental animals. We thank Dr. J. Patrickson for his expert advice in the IHC studies, Dr. R. Gonzalez for the use of software to perform densitometric analysis of signal intensity for Western blots, and Mr. P. Abramson for technical support.
References
- Abe H (1996) The mammalian oviductal epithelium: regional variations in cytological and functional aspects of the oviductal secretory cells. Histol Histopathol 11:743–746 [PubMed] [Google Scholar]
- Anzaldua SR, Camacho-Arroyo T, Reyna-Neyra A, Perez-Martinez M, Cerbon M (2007) Regional differences in expression of progesterone receptor in the oviduct and uterus of rabbit during early pregnancy. Comp Biochem Physiol A Mol Integr Physiol 147:685–690 [DOI] [PubMed] [Google Scholar]
- Briton-Jones C, Lok IH, Cheung CK, Po AL, Chiu TT, Haines C (2005) Ratio of mRNA expression of progesterone receptor isoforms AB is to B in the human oviduct mucosal cells during the ovulatory cycle. J Assist Reprod Genet 22:429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casan EM, Raga F, Bonilla-Musoles F, Polan ML (2000) Human oviductal gonadotropin-releasing hormone: possible implications in fertilization, early embryonic development and implantation. J Clin Endocrinol Metab 85:1377–1381 [DOI] [PubMed] [Google Scholar]
- Chegini N (1996) Oviductal derived growth factors and cytokines: implications in preimplantation. Semin Reprod Endocrinol 14:219–229 [DOI] [PubMed] [Google Scholar]
- Cheon M, Park D, Kim K, Park SD, Ryu K (1999) Homologous upregulation of GnRH receptor mRNA by continuous GnRH in cultured rat pituitary cells. Endocrine 11:49–55 [DOI] [PubMed] [Google Scholar]
- Choi JH, Gilks CB, Auersperg N, Leung PCK (2006) Immunolocalization of gonadotropin releasing hormone (GnRH)-I, GnRH-II and type I receptor during follicular development in the human ovary. J Clin Endocrinol Metab 91:4562–4570 [DOI] [PubMed] [Google Scholar]
- Garcia-Palencia P, Sanchez MA, Nieto A, Vilar MP, Gonzalez M, Veuga-Lopez A, Gonzalez-Bulnes A, et al. (2007) Sex steroid expression in the oviduct and uterus of sheep with estrous synchronized with progesterone or prostaglandin analogues. Anim Reprod Sci 97:25–35 [DOI] [PubMed] [Google Scholar]
- Han XB, Cao YQ, Zhuang LZ (1998) GnRH receptor and regulation the of its gene expression. Sheng Li Ke Xue Jin Zhang 29:198–202 [PubMed] [Google Scholar]
- Hapgood JP, Sadie H, Van Biljon W, Ronacher K (2005) Regulation of expression of mammalian gonadotropin-releasing hormone receptor genes. J Neuroendocrinol 17:619–638 [DOI] [PubMed] [Google Scholar]
- Hashizume T, Yang WH, Clay CM, Nett TM (2001) Internalization rates of murine and ovine gonadotropin-releasing hormone receptors. Biol Reprod 64:898–903 [DOI] [PubMed] [Google Scholar]
- Karande AA, Rajeshwari K, Schol DJ, Hilgers JHM (1995) Establishment of immunological probes to study human gonadotropin-releasing hormone receptors. Mol Cell Endocrinol 114:52–56 [DOI] [PubMed] [Google Scholar]
- Komiya H, Onuma T, Hiroi M, Araki Y (1996) In situ localization of messenger ribonucleic acid for an oviduct-specific glycoprotein during various hormonal conditions in the golden hamster. Biol Reprod 55:1107–1118 [DOI] [PubMed] [Google Scholar]
- Kraus S, Naor Z, Seger R (2001) Intracellular signaling pathways mediated by the gonadotropin-releasing hormone (GnRH) receptor. Arch Med Res 32:499–509 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275 [PubMed] [Google Scholar]
- Lyons RA, Saridogan E, Djahanbakhch O (2006) The reproductive significance of human fallopian tube cilia. Hum Reprod Update 12:363–372 [DOI] [PubMed] [Google Scholar]
- Millar RP (2005) GnRHs and GnRH receptors. Anim Reprod Sci 88:5–28 [DOI] [PubMed] [Google Scholar]
- Miller BT, Leondires M (2003) The fallopian tube in health and disease. In Seifer DB, Samuels P, Kniss DA, eds. The Physiologic Basis of Gynecology and Obstetrics. Baltimore, Lippincott Williams & Wilkins, 222–224
- Morishige WK, Pepe GJ, Rothchild I (1973) Serum luteinizing hormone, prolactin and progesterone levels during pregnancy in the rat. Endocrinology 92:1527–1530 [DOI] [PubMed] [Google Scholar]
- Naik SI, Young LS, Charlton HM, Clayton RN (1984) Pituitary gonadotropin-releasing hormone receptor regulation in mice. I. Males. Endocrinology 115:106–113 [DOI] [PubMed] [Google Scholar]
- Peralta LE, Olarte MR, Arganaraz M, Ciocca D, Miceli DC (2005) Progesterone receptors: their localization, binding activity and expression in the pig oviduct during follicular and luteal phases. Domest Anim Endocrinol 28:74–84 [DOI] [PubMed] [Google Scholar]
- Ranta T, Knecht M, Kody M, Catt KJ (1982) GnRH receptors in cultured rat granulose cells: mediation of the inhibitory and stimulatory actions of GnRH. Mol Cell Endocrinol 27:233–240 [DOI] [PubMed] [Google Scholar]
- Schirman-Hildesheim TD, Bar T, Ben-Aroya N, Koch Y (2005) Differential gonadotropin releasing hormone (GnRH) and GnRH receptor messenger ribonucleic acid expression patterns in different tissues of female rat across the estrous cycle. Endocrinology 146:3401–3408 [DOI] [PubMed] [Google Scholar]
- Sengupta A, Baker T, Chakrabarti N, Whittaker JA, Sridaran R (2007) Localization of immunoreactive gonadotropin-releasing hormone and relative expression of its mRNA in the oviduct during pregnancy in rats. J Histochem Cytochem 55:525–534 [DOI] [PubMed] [Google Scholar]
- Shao R, Weijdegard B, Fernandez-Rodriguez J, Egecioglu E, Zhu C, Andersson N, Thurin-Kjellberg A, et al. (2007) Ciliated epithelial-specific and regional-specific expression and regulation of the estrogen receptor-β2 in the fallopian tubes of immature rats: a possible mechanism for estrogen-mediated transport process in vivo. Am J Physiol Endocrinol Metab 293:E147–158 [DOI] [PubMed] [Google Scholar]
- Wolfahrt S, Kleine B, Rossmanith WG (1998) Detection of gonadotropin releasing hormone and its receptor mRNA in human placental trophoblasts using in-situ reverse transcription–polymerase chain reaction. Mol Hum Reprod 4:999–1006 [DOI] [PubMed] [Google Scholar]
- Zilberstein M, Zakut H, Naor Z (1983) Coincidence of down-regulation and desensitization in pituitary gonadotrophs stimulated by gonadotropin releasing hormone. Life Sci 32:663–669 [DOI] [PubMed] [Google Scholar]