Abstract
Objective
Determine the impact of a Prior Authorization Requirement (PAR) program on Medicaid pharmacy expenditures and utilization.
Data Source
Prescription claims for Nebraska Medicaid recipients who received a cyclooxygenase-2 (COX-2) inhibitor, a nonselective nonsteroidal antiinflammatory (NSAID) drug, or other pain relievers between July 2001 and June 2003.
Study Design and Data Collection/Extraction
This was a retrospective cross-sectional study with a 12-month pre-PAR implementation period and a 12-month post-PAR implementation period. Pharmacy transactions for COX-2 inhibitors, NSAIDs, other pain relievers, and gastroprotectants were identified by their National Drug Code (NDC) in a Microsoft SQL query. The PAR was designed to approve COX-2 inhibitor use only for recipients at high risk of GI side effects while restricting access to those patients at low to moderate risk of GI side effects.
Principal Findings
One year following implementation of the PAR, overall expenditures on COX-2 inhibitors for Nebraska Medicaid dropped 50 percent. The overall impact on pharmacy expenditures, including NSAIDs, pain relief medications, and gastroprotectants when necessary to relieve gastrointestinal (GI) side effects, for those recipients who switched from a COX-2 inhibitor to an NSAID or other pain relievers was a decline of approximately 35 percent.
Conclusion and Implications for State Policy
PAR for COX-2 inhibitors successfully reduced Medicaid prescription expenditures. Recipients at high risk for GI side effects appropriately received COX-2 inhibitors. Recipients at low to moderate risk for GI side effects who were switched to NSAIDs or other pain relievers had lower overall prescription expenditures. Further research is needed to determine the impact of PAR on overall health outcomes and costs. In this study, rather than take a “one size fits all” approach to prescription drug cost-saving strategies, Medicaid policy makers understood that patient variation required accurate identification of disease severity to determine when equally efficacious low-cost alternatives were appropriate.
Keywords: Prior authorization, Medicaid, COX-2 inhibitors, drug policy, pharmacy costs
In 2004, national health care spending rose 7.9 percent to $1.9 trillion (Smith et al. 2006). Expenditures on prescription drugs in noninstitutionalized settings grew from $51.0 billion in 1993 to $188.5 billion in 2004. The three main factors that drove the increase in prescription drug spending were utilization, changes in the types of drugs used (newer high-priced drugs replaced older less-expensive drugs partially due to aggressive promotion to both patients and physicians), and manufacturer price increases for existing drugs (Altman and Parks-Thomas 2002; Rosenthal et al. 2002; Kaiser Family Foundation 2005).
In 2004, 42 million Medicaid recipients had total health care expenditures that accounted for about 15.6 percent of all health care spending in the United States (Smith et al. 2006). A survey of 36 Medicaid programs in 2005 revealed the following cost-saving strategies for prescription drugs: 100 percent used prior authorization requirements (PAR), 92 percent required the use of generics, 81 percent charged the beneficiary limited copayments for prescription drugs; 68 percent used preferred drug lists, and 95 percent imposed limits on quantities dispensed (Kaiser Family Foundation 2005). In addition, 26 states sought supplemental rebates in 2004 (Kaiser Family Foundation 2004). Other savings strategies introduced by pharmacy benefit managers and adopted by Medicaid programs include tiered formularies and therapeutic interchange (Altman and Parks-Thomas 2002; Rosenthal et al. 2002; Fischer et al. 2004).
Medicaid PAR programs are designed to reduce the inappropriate or unnecessary use of prescription drugs. This is a popular cost-saving strategy because monetary savings can be achieved without patients being denied access to necessary drug therapy. PAR programs (1) encourage the use of less-costly alternative drugs that can be used for the same indications, (2) enforce step therapy, and (3) restrict situations where high cost therapy can be used (Phillips and Larson 1997). PAR programs reduce unnecessary utilization of prescription drugs by influencing the initial drug choice of prescribers at the time of prescribing. Prescribers must get permission to write prescriptions for specific drugs by providing clinical justification (Hamel and Epstein 2004). PAR can be used in a variety of circumstances. For example, prescription drugs selected for PAR typically are identified as part of a larger group of therapeutic choices, all with similar efficacy for the most common medical conditions they are used to treat. In other cases, PAR is used for high-cost drugs even when there is no evidence of therapies that have similar efficacy at a lower cost (Smalley et al. 1995; Momani, Madhavan, and Nau 2002).
Nonsteroidal antiinflammatory drugs (NSAIDs) are divided into two subcategories: (1) COX-2 specific inhibitors (referred to as COX-2 inhibitors for the remainder of this paper) such as celecoxib (Celebrex®), and (2) nonselective NSAIDs (referred to as NSAIDs for the remainder of this paper) such as ibuprofen (Motrin®). These drugs are indicated for the treatment of osteoarthritis, rheumatoid arthritis, and acute pain (Shaw et al. 2003). The efficacy of COX-2 inhibitors and NSAIDs for treating these conditions has been shown to be similar, with COX-2 inhibitors typically costing about $80 more per month of therapy as compared with NSAIDs (Bombardier et al. 2000; Fischer et al. 2004). The main disadvantage with NSAIDs is gastrointestinal (GI) adverse events that include GI discomfort, and more serious events such as ulceration and bleeding (Shaw et al. 2003). When COX-2 inhibitors were first introduced in the 1990s, they offered the potential for a decrease in these GI events (Shaw et al. 2003). However, more recent studies have found that COX-2 inhibitors do not reduce the risk of GI bleeding compared with NSAIDs as often as was originally believed (Stockl, Cyprien, and Chang 2005; Curtiss 2006). The effect of a PAR program for Celebrex® (a COX-2 inhibitor) on pharmacy and medical service utilization was studied in a Medicaid-managed care organization in Oregon (Hartung et al. 2004). Implementation of the PAR program resulted in a decline in the use of Celebrex® by 59 percent. There was no concurrent increase in use of other drug classes that could be substituted for Celebrex®. The impact of PAR programs in all 50 state Medicaid programs was previously evaluated, and in states where a PAR policy was initiated for COX-2 inhibitors, use and spending declined substantially (Fischer et al. 2004).
The objective was to determine the impact of PAR for COX-2 inhibitors on prescription expenditures and prescribing patterns in a Medicaid program. More specifically, the impact of the PAR for COX-2 inhibitors on total prescription costs for recipients who switched from a COX-2 inhibitor to an NSAID or other pain relievers was analyzed.
METHODS
Population
The population considered was all people continuously eligible for Nebraska Medicaid between July 1, 2001 and June 30, 2003. In 2001 there were 149,142 full-year eligibles, in 2002 there were 161,559 full-year eligibles, and in 2003 there were 140,689 full-year eligibles (Centers for Medicare and Medicaid Services 2004, 2005, 2006). For this study, we were interested only in those recipients who were continuously taking either a COX-2 inhibitor, an NSAID, or other pain relieves throughout the 2-year study period.
Description and Implementation of the PAR Program
On July 1, 2002 Nebraska Medicaid implemented a PAR program for the COX-2 inhibitors Celebrex® (Pfizer Inc., New York, NY), Vioxx® (Merck & Co., Whitehouse Station, NJ), and Bextra® (Pfizer Inc). Medicaid paid for COX-2 inhibitor prescriptions for beneficiaries meeting criteria for high GI event risk. High risk was defined as follows: (1) age 65 or older not taking any other NSAIDs or aspirin (these patients are not required to go through the PAR process and claims are automatically paid), (2) a known GI event (history or GI bleed/ulcer or active peptic ulcer), (3) current daily use of an oral corticosteroid (e.g., prednisone), (4) current use of anticoagulant (e.g., warfarin), and (5) a diagnosis of familial adenomatous polyposis (State of Nebraska Department of Health and Human Services 2005). The physicians or their designees were required to complete a prior-authorization request form available on the state website for those patients who fall into categories 2–5 above. The form was faxed to the Medicaid prior-authorization office where it was reviewed by a pharmacist who determined coverage based on these criteria established by an expert panel of physicians and pharmacists.
Recipients with low to moderate risk for serious GI events were not allowed to receive a COX-2 inhibitor under the PAR program. Alternatively, these low-to-moderate-risk recipients received NSAID therapy, salicylate therapy such as aspirin, or other pain relievers such as hydrocodone/acetaminophen combinations. Compared with COX-2 inhibitors, NSAIDs and salicylates have similar efficacy, lower cost, and presumably greater chance for GI side effects. GI side effects are commonly treated with gastroprotectant drugs such as proton pump inhibitors—PPIs (e.g., omeprazole or Prilosec®) and histamine-2 receptor antagonists—H2RAs (e.g., ranitidine or Zantac®). Compared with COX-2 inhibitors, other pain relievers have varying efficacy, may have higher or lower costs, and have low or unknown chance for GI side effects.
This study was focused on those recipients who were taking a COX-2 inhibitor and as a result of the PAR switched to (1) an NSAID or a salicylate and associated costs to treat or prevent GI events with gastroprotectant drug therapy, and (2) another type of pain reliever other than COX-2 inhibitors, NSAIDs, or salicylates.
Study Design, Inclusion Criteria, and Exclusion Criteria
This retrospective cross-sectional study had a 12-month pre-PAR implementation period and a 12-month post-PAR implementation period. Expenditures and number of prescription claims were analyzed for recipients who were (1) taking a COX-2 inhibitor in the 12 months before the PAR program; (2) switched to an NSAID, a salicylate, or other pain relievers because of the PAR program; and (3) remained on an NSAID or other pain relievers for the 12 months following implementation of the PAR. Criteria for inclusion in the analysis were as follows: (1) recipient switched from a COX-2 inhibitor to an NSAID or to other pain relievers as a result of the PAR and (2) recipient received an NSAID or another pain reliever for 12 months following implementation of the PAR. Recipients excluded were as follows: (1) those who were not taking a COX-2 inhibitor before July 1, 2002, (2) those who chose to pay out of pocket for a COX-2 inhibitor after July 1, 2002 (given the fact that these drugs cost $90 per month it was unlikely many Medicaid recipients who are indigent by definition fall into this exclusion category), and (3) those who decided not to use either a COX-2 inhibitor, an NSAID, or another pain reliever medication. Data were collected monthly and comparisons were carried out quarterly.
Identification of COX-2 Inhibitor, NSAID, and Gastroprotectant Use
All Medicaid pharmacy claims from July 1, 2001 through June 30, 2003 were screened using a Microsoft SQL query for either COX-2 inhibitor, NSAID, or for other pain reliever claims as defined by the generic code number (GCN; First Databank, San Bruno, CA) identifiers. Gastroprotectants were identified in the database as PPIs (e.g., Nexium®), H2RAs (e.g., famotidine), antacids (e.g., Maalox®), and others such as sucralfate and misoprostol.
Expenditures and utilization of COX-2 inhibitors and gastroprotectant drugs were determined monthly for the year before PAR implementation for those who switched. Recipients who switched from a COX-2 inhibitor to an NSAID or to another pain reliever were identified by determining those who were using a COX-2 inhibitor in May or June 2002 and switched to an NSAID or other pain relief medications in July or August 2002. Expenditures and utilization of COX-2 inhibitors were determined monthly for the year before PAR implementation. Then expenditures and utilization of gastroprotectant drugs were determined for those recipients who received a COX-2 inhibitor during a given month in the year before implementation of the PAR and who switched from a COX-2 inhibitor to an NSAID or salicylate pain reliever after PAR implementation.
Expenditures and utilization of NSAIDs, salicylates, other pain relievers, and gastroprotectant drugs were determined monthly for the year following PAR implementation for those who switched. Recipients who received an NSAID or other pain reliever in a given month of the year following implementation of the PAR and switched from a COX-2 inhibitor to an NSAID or other pain reliever in the summer of 2002 were identified. Expenditures and utilization of NSAIDs and other pain relievers were determined monthly for the year following the PAR implementation. Next, expenditures and utilization of gastroprotectant drugs (if any) were determined for those recipients who received an NSAID or salicylate pain reliever during a given month in the year following implementation of the PAR and switched from a COX-2 inhibitor to an NSAID or salicylate pain reliever in the summer of 2002.
Statistical Method
Utilization and expenditure trends for COX-2 inhibitors and NSAIDs for all Nebraska Medicaid recipients are reported using descriptive statistics. The primary outcome comparison of quarterly utilization and prescription expenditures before and after implementation of the COX-2 inhibitor PAR program was assessed using the Wilcoxon Signed Ranks Test (SPSS 13.0 for MicrosoftWindows). All statistical tests were considered statistically significant at p<.05.
RESULTS
Following implementation of the PAR program for COX-2 inhibitors, overall utilization (number of prescription claims) of COX-2 inhibitors decreased by over 50 percent (Table 1—top portion). At the same time, overall utilization of NSAIDs increased 20 percent, and utilization of other pain medications increased 12 percent. Overall expenditures on COX-2 inhibitors decreased 53 percent during the first quarter following PAR implementation and remained at that level for the remainder of the study. At the same time, overall expenditures on NSAIDs increased by 62 percent and expenditures on other pain medications increased 12 percent. However, the actual dollar amount increase for NSAIDs and other pain relievers combined was substantially less than the dollar amount decrease for COX-2 inhibitors.
Table 1.
Utilization and Expenditures for COX-2 Inhibitors, NSAIDs, Other Pain Relievers, and Gastroprotectants (PAR Implemented July 1, 2002)
| Overall | 2001Q3 | 2001Q4 | 2002Q1 | 2002Q2 | 2002Q3 | 2002Q4 | 2003Q1 | 2003Q2 |
|---|---|---|---|---|---|---|---|---|
| COX-2 inhibitors* | ||||||||
| Total expenditures | $1,372,643 | $1,488,836 | $1,565,460 | $1,678,913 | $792,227 | $842,935 | $800,534 | $790,118 |
| Total Rx claims | 19,314 | 19,875 | 19,736 | 20,382 | 9,794 | 10,200 | 9,499 | 9,232 |
| NSAIDs† | ||||||||
| Total expenditures | $364,148 | $343,814 | $321,809 | $352,922 | $516,424 | $557,984 | $550,500 | $572,687 |
| Total Rx claims | 18,708 | 19,546 | 19,864 | 19,282 | 25,129 | 25,677 | 24,327 | 23,207 |
| Pain relievers (other)‡ | ||||||||
| Total expenditures | $227,462 | $255,382 | $208,022 | $213,766 | $307,548 | $259,554 | $234,132 | $238,938 |
| Total Rx claims | 6,035 | 6,051 | 5,209 | 5,257 | 6,778 | 6,497 | 6,054 | 5,880 |
| Per/recipient/month data§ | ||||||||
| COX-2 inhibitors* | ||||||||
| Expenditure/recipient/month§ | $91.66 | $94.50 | $89.46 | $93.63 | 0 | 0 | 0 | 0 |
| NSAIDs† | ||||||||
| Expenditure/recipient/month§ | 0 | 0 | 0 | 0 | $23.33 | $24.19 | $22.32 | $22.12 |
| Pain relievers (other)‡ | ||||||||
| Expenditure/recipient/month§ | 0 | 0 | 0 | 0 | $2.92 | $4.28 | $7.46 | $7.80 |
| Gastroprotectants§ | ||||||||
| Expenditures/recipient/month | ||||||||
| PPIs | $20.99 | $21.70 | $19.76 | $18.98 | $44.88 | $36.85 | $7.04 | $10.56 |
| H2RAs | $1.12 | $1.09 | $1.18 | $.99 | $2.27 | $4.22 | $8.39 | $6.14 |
| Gastroprotectant (other) | $0.16 | $0.14 | $0.15 | $0.20 | $0.42 | $0.51 | $0.59 | $0.59 |
| OTC antacids | $0.13 | $0.11 | $0.12 | $0.09 | $0.27 | $0.26 | $0.26 | $0.24 |
| Total gastroprotectants | $22.40 | $23.04 | $21.21 | 20.26 | $47.84 | $41.84 | $16.28 | $17.53 |
| Claims/recipient/month | ||||||||
| PPIs | 0.17 | 0.18 | 0.15 | 0.14 | 0.31 | 0.29 | 0.06 | 0.08 |
| H2RAs | 0.06 | 0.06 | 0.05 | 0.04 | 0.12 | 0.20 | 0.37 | 0.33 |
| Gastroprotectants (other) | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| OTC antacids | 0.02 | 0.02 | 0.02 | 0.02 | 0.05 | 0.06 | 0.05 | 0.05 |
| Total expenditure¶ | ||||||||
| Expenditure/recipient/month∥ | $114.06 | $117.54 | $110.67 | $113.89 | $74.09 | $70.31 | $46.06 | $47.45 |
COX-2 inhibitors (Bextra®, Celebrex®, Vioxx®).
NSAIDs (diclofenac sodium, diclofenac potassium, diclofenac/misoprostol, etodolac, fenoprofen, flurbiprofen, ibuprofen, indomethacin, ketoprofen, ketorolac, meclofenamate, mefenamic acid, meloxicam, nabumetone, naproxen, oxaprozin, piroxicam, sulindac, tolmetin).
Other pain relievers includes narcotic analgesics (e.g., Percocet®), salycylate analgesics (e.g., aspirin), nonsalicylate, nonnarcotic analgesics (e.g., acetaminophen), antimigraine drugs (e.g., Imitrex®), other medications indicated for rheumatoid arthritis (Enbrel®, Humira®).
For those recipients who switched from a COX-2 inhibitor to an NSAID, or other pain reliever as a consequence of the PAR.
Total expenditure=Expenditure for COX-2 Inhibitor or nonselective NSAID or other pain reliever+gastroprotectant therapy.
Mean comparisons against 2002Q2 for the last four quarters of the study period statistically significant at p<.05.
PPIs, proton pump inhibitors (e.g., Prilosec®); H2RAs, histamine-2 receptor antagonists (e.g., ranitidine); OTC antacids, over-the-counter antacids (e.g., Maalox®).
For those patients who switched from a COX-2 inhibitor to an NSAID or salicylate pain reliever following implementation of the PAR, the number of claims per recipient per month for all gastroprotectants increased from 0.2 in the quarter before July 1, 2002 to 0.47 in the final quarter of the study period, an increase of 135 percent (Table 1). There were differences in utilization among each of the four therapeutic subclasses. The number of claims per recipient per month for PPIs was 0.14 in the quarter before July 1, 2002 and 0.08 in the final quarter of the study, a decrease of 43 percent. The number of claims per recipient per month for H2RAs was 0.04 in the quarter before July 1, 2002 and 0.33 in the final quarter of the study period, an increase of 725 percent. The number of claims per recipient per month for over-the-counter (OTC) antacids was 0.02 in the quarter before July 1, 2002 and 0.05 in the final quarter of the study, an increase of 150 percent. The number of claims per recipient per month for other antiulcer prescriptions remained the same at 0.01. Notably, on December 20, 2002, Nebraska Medicaid implemented a separate PAR program for PPIs. This significantly impacted utilization and expenditures of gastroprotectants for Medicaid recipients, including those taking COX-2 inhibitors and NSAIDs.
The amount spent per recipient per month on all gastroprotectants decreased from $20.26 in the quarter before July 1, 2002 to $17.53 in the final quarter of the study period, a decrease of 13 percent (Table 1). This was mainly due to the decline in the number of claims for PPIs, which resulted in a 44 percent decline in PPI expenditures. The amount spent on H2RAs increased by 520 percent, from $0.99 per recipient per month in the quarter prior July 1, 2002 to $6.14 in the final quarter of the study period. The amount spent on other antiulcer preparations increased by 195 percent, and the amount spent per recipient per month on OTC antacids increased by 167 percent. Increased spending for H2RAs, other gastroprotectants, and OTC antacid was more than offset by the decline in relatively more costly PPIs.
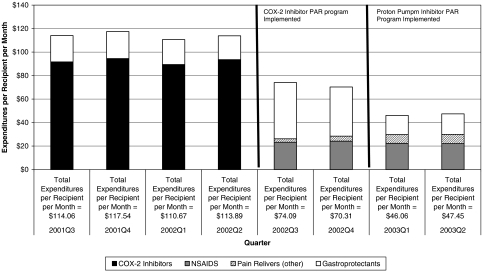
For patients who switched from a COX-2 inhibitor to an NSAID or other pain reliever following implementation of the PAR, expenditures on COX-2 therapy+gastroprotectant therapy were $113.89 per recipient per month in the quarter before July 1, 2002 (Figure 1). Expenditures on NSAID+gastroprotectant therapy or other pain reliever therapy were 35 percent less 6 months following implementation of the PAR for COX-2 inhibitors. By the final quarter of the study, expenditures on NSAID therapy+gastroprotectant therapy or other pain reliever therapy were $47.45 per recipient per month representing a decrease in expenditures of 58 percent.
Figure 1.
Prescription Expenditures per Recipient per Month: Those Taking COX-2 Inhibitors before July 1, 2002 and an NSAID or Other Pain Reliever after July 1, 2002
DISCUSSION AND IMPLICATIONS FOR STATE HEALTH POLICY
Prescription drug expenditures are a substantial portion of Medicaid budgets around the country. One of the tools used to reign in escalating drug expenditures is the PAR. In this cross-sectional observational study of Nebraska Medicaid pharmacy claims, the PAR program for COX-2 inhibitors resulted in an overall decline in pharmacy expenditures. As expected, there was a decline in utilization and expenditures for the targeted COX-2 inhibitors. Simultaneously, there was increased utilization for alternative yet substantially less-costly NSAIDs and other pain relievers. The increase in utilization and expenditures of gastroprotectants to treat side effects caused by NSAIDs and salicylates was modest and did not offset savings gained by switching recipients from COX-2 inhibitors to NSAIDs. The net effect on prescription expenditures was a decline of 35 percent after 6 months and 58 percent after one year.
The PAR policy for COX-2 inhibitors did not adversely influence access to prescription medications for those patients at high risk for GI side effects. Medicaid laws guarantee beneficiaries access to medically necessary Medicaid coverable drugs. The COX-2 inhibitor PAR program takes this into consideration by allowing recipients who are truly at a high risk for GI side effects (e.g., ulcers) to receive COX-2 inhibitor therapy as long as the recipient meets certain criteria (recipients age 65 or older received a COX-2 inhibitor without going through the prior authorization process), or the prescriber provides sufficient evidence, such as history of GI bleed or ulcer. Patients at lower to moderate risk for GI complications continue to have adequate access to efficacious but lower cost therapies such as NSAIDs and other pain relievers. The PAR program reduces unnecessary use of costlier COX-2 inhibitors among recipients with low-to-moderate risk for serious GI complications. This is a key point in understanding why the PAR for COX-2 inhibitors is an effective cost-savings strategy: NSAIDs and COX-2 inhibitors are equally efficacious, and COX-2 inhibitors have not been proven to prevent GI complications in low-to-moderate-risk patients as was originally believed (Bombardier et al. 2000; Juni, Rutjes, and Dieppe 2002). Following implementation of the COX-2 PAR, approximately 50 percent of patients attempting to fill a COX-2 inhibitor prescription were denied coverage because they did not meet high-risk criteria. Many of these patients were switched to less-costly and equally efficacious alternatives. Other studies of managed care populations have reported 68–73 percent did not meet the criteria for a high-risk GI event (Gleason et al. 2005).
A legitimate concern with a program aimed at limiting expenditures and utilization in one area is that expenditures and utilization may increase in other areas. In other words, how did the PAR program affect overall clinical outcomes. The main limitation of this study was that it only dealt with pharmacy expenditures and did not consider health care expenditures (the data were not available), such as physicians office visits, emergency department visits, and hospitalizations. A recent study in a managed care population showed that medical expenditures associated with a GI diagnosis did not increase in members who were denied a COX-2 inhibitor after a PAR was implemented (Gleason et al. 2005). It is tempting to say that these results can be extrapolated to the current study. However, the current study can only offer conclusions that are limited to pharmacy expenditures and utilization because medical costs information is lacking.
Some Medicaid recipients may have chosen to continue to pay for COX-2 inhibitors out of their own pocket after the PAR implementation. Although theoretically possible, this is not a very plausible scenario. Medicaid recipients must be indigent to qualify for state medical assistance. As mentioned previously, COX-2 treatments typically would cost a patient $90–$100 out-of-pocket during the period of this study. An indigent person typically does not have that amount of disposable income available, thus the need for these individuals to have state-covered health care that includes prescriptions medications.
The impact of Medicare Part D on overall Medicaid expenditures has been significant since the beginning of 2006. Debate continues about public versus private control over the benefit. For example, those advocating for public control and full disclosure would like to see the government negotiate rebates and discounts from the pharmaceutical industry rather than from the current system of many smaller private insurance entities negotiating with the pharmaceutical industry in a nontransparent environment. This study demonstrates how state policy has a significant impact on expenditures in a government-run transparent system. In Nebraska Medicaid, since implementation of the PAR and before 2006, approximately 80 percent of all patients taking COX-2 inhibitors were dual eligible for Medicare and Medicaid coverage. The result was Nebraska Medicaid saw a dramatic decline in expenditures on COX-2 inhibitors starting in 2006. This was expected because COX-2 inhibitors were covered without going through the PAR process for those who were 65 years of age and older because they were considered at high risk for GI side effects because of their age. In other words, there was no “barrier” to coverage for this group of recipients. A key issue to be considered is whether or not the private companies offering Medicare Part D plans are using criteria as specific as the Nebraska PAR for COX-2 inhibitors to distinguish high-risk recipients from low-to-moderate-risk recipients. Alternatively, Part D plans may choose whether or not to cover medications based on other considerations such as rebates and discounts that may not properly take patient specific variability into consideration. The advent of Medicare Part D clearly moved control over drug spending for dual eligibles from a transparent public system to one that is controlled by private industry, which for many reasons is likely to be less transparent. Private Part D plans may offer savings, but do these savings compromise patient care?
Even though this study does not provide conclusive results because it is not a randomized controlled trial and because medical expenditures were not analyzed, it provides credible inferences regarding the impact of a PAR program on prescription drug expenditures for Medicaid recipients. Vioxx® and Bextra® have been withdrawn from the U.S. market because of potentially life-threatening cardiovascular side effects. However, in Nebraska Medicaid the PAR for COX-2 inhibitors is still in place for Celebrex® and other potential COX-2 inhibitors that may be introduced to the market. All three COX-2 inhibitors were available during the study period. The primary objective of this study was achieved: to determine the impact of a PAR program on prescription expenditures and utilization. Implications from this study should compel state Medicaid policy makers to identify other therapeutic areas where there are equally efficacious low-cost alternatives.
CONCLUSION
PAR for COX-2 inhibitors was successful in reducing Medicaid prescription expenditures. Recipients at high- risk of GI side effects appropriately received COX-2 inhibitors. Recipients at low to moderate risk of GI side effects who were switched to NSAIDs or other pain relievers had lower expenditures, and expected increased expenditures on gastroprotectants to treat side effects that may have resulted from NSAIDs and salicylates replacing COX-2 inhibitors did not occur. Further research is needed to determine the impact of PAR on overall health outcomes and costs.
Acknowledgments
This project was supported by a grant from the Creighton University Health Futures Foundation.
The Institutional Review Board at Creighton University approved this project under Creighton University IRB Number 03-13035.
We would like to thank Ted Kasha for assistance with data analysis and members of the State of Nebraska Department of Health and Human Services for providing access to data and review of this manuscript before initial submission.
We are grateful to Amy Abbott, J. D. Bramble, Kimberly A. Galt, Karen A. Paschal, and Ann Rule for helpful and carefully considered comments and suggestions. We are also grateful to the editors and anonymous reviewers for their critical feedback.
Disclosures: The authors have no affiliation with or financial interest in any product mentioned in this manuscript.
Disclaimers: Dr. Siracuse is affiliated with Creighton University and Dr. Vuchetich is affiliated with Alegent Health. Data for this project were supplied by the State of Nebraska Department of Health and Human Services. The views expressed herein are the sole responsibility of the authors and do not necessarily reflect the views of Creighton University, Alegent Health, or those of the State of Nebraska Department of Health and Human Services.
REFERENCES
- Altman S H, Parks-Thomas C. Controlling Spending for Prescription Drugs. New England Journal of Medicine. 2002;346(11):855–6. doi: 10.1056/NEJM200203143461113. [DOI] [PubMed] [Google Scholar]
- Bombardier C, Laine L, Reicin A, Shapiro D, Burgos Vargas R, Davis B, Day R, Ferraz M B, Hawkey C J, Hochberg M C, Kvien T K, Schnitzer T J VIGOR-Study-Group. Comparison of Upper Gastrointestinal Toxicity of Rofecoxib and Naproxen in Patients with Rheumatoid Arthritis. VIGOR-Study-Group. New England Journal of Medicine. 2000;343(21) doi: 10.1056/NEJM200011233432103. 1520,8, 2 p following 1528. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services. “MSIS State Summary FY 2001”. 2004. [June 12, 2006]. Available at http://www.cms.hhs.gov/MedicaidDataSourcesGenInfo/02_MSISData.asp#TopOfPage.
- Centers for Medicare and Medicaid Services. “MSIS State Summary FY 2002”. 2005. [June 12, 2006]. Available at http://www.cms.hhs.gov/MedicaidDataSourcesGenInfo/02_MSISData.asp#TopOfPage. [PubMed]
- Centers for Medicare and Medicaid Services. “MSIS State Summary FY2003”. 2006. [June 12, 2006]. Available at http://www.cms.hhs.gov/MedicaidDataSourcesGenInfo/02_MSISData.asp#TopOfPage.
- Curtiss F R. Relative Value of the NSAIDs, Including COX-2 Inhibitors and Meloxicam. Journal of Managed Care Pharmacy. 2006;12(3):265–8. doi: 10.18553/jmcp.2006.12.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M A, Schneeweiss S, Avorn J, Solomon D H. Medicaid Prior-Authorization Programs and the Use of Cyclooxygenase-2 Inhibitors. New England Journal of Medicine. 2004;351(21):2187–94. doi: 10.1056/NEJMsa042770. [DOI] [PubMed] [Google Scholar]
- Gleason P P, Williams C, Hardy S, Hartwig S C, Lassen D. Medical and Pharmacy Expenditures after Implementation of a Cyclooxygenase-2 Inhibitor Prior Authorization Program. Pharmacotherapy. 2005;25(7):924–34. doi: 10.1592/phco.2005.25.7.924. [DOI] [PubMed] [Google Scholar]
- Hamel M B, Epstein A M. Prior-Authorization Programs for Controlling Drug Spending. New England Journal of Medicine. 2004;351(21):2156–8. doi: 10.1056/NEJMp048294. [DOI] [PubMed] [Google Scholar]
- Hartung D M, Touchette D R, Ketchum K L, Haxby D G, Goldberg B W. Effects of a Prior-Authorization Policy for Celecoxib on Medical Service and Prescription Drug Use in a Managed Care Medicaid Population. Clinical Therapeutics. 2004;26(9):1518–32. doi: 10.1016/j.clinthera.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Juni P, Rutjes A W, Dieppe P A. Are Selective COX 2 Inhibitors Superior to Traditional Non Steroidal Anti-Inflammatory Drugs? British Medical Journal Clinical Research Edition. 2002;324(7349):1287–8. doi: 10.1136/bmj.324.7349.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser Family Foundation. “Prescription Drug Trends”. 2004. [April 15, 2006]. Available at http://www.kff.org/rxdrugs/upload/Prescription-Drug-Trends-October-2004-UPDATE.pdf.
- Kaiser Family Foundation. “Prescription Drug Trends, November 2005”. 2005. [June 1, 2006]. Available at http://www.kff.org/insurance/upload/3057-04.pdf.
- Momani A A, Madhavan S S, Nau D P. Impact of NSAIDs Prior Authorization Policy on Patients' QoL. Annals of Pharmacotherapy. 2002;36(11):1686–91. doi: 10.1345/aph.1C008. [DOI] [PubMed] [Google Scholar]
- Phillips C R, Larson L N. Evaluating the Operational Performance and Financial Effects of a Drug Prior Authorization Program. Journal of Managed Care Pharmacy. 1997;3(6):699–706. [Google Scholar]
- Rosenthal M B, Berndt E R, Donohue J M, Frank R G, Epstein A M. Promotion of Prescription Drugs to Consumers. New England Journal of Medicine. 2002;346(7):498–505. doi: 10.1056/NEJMsa012075. [DOI] [PubMed] [Google Scholar]
- Shaw E, Stacy J, Arledge M D, Howell Smith D. Pharmacoeconomic Modeling of Prior-authorization Intervention for COX-2 Specific Inhibitors in a 3-Tier Copay Plan. Journal of Managed Care Pharmacy. 2003;9(4):327–34. doi: 10.18553/jmcp.2003.9.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley W E, Griffin M R, Fought R L, Sullivan L, Ray W A. Effect of a Prior-Authorization Requirement on the Use of Nonsteroidal Antiinflammatory Drugs by Medicaid Patients. New England Journal of Medicine. 1995;332(24):1612–17. doi: 10.1056/NEJM199506153322406. [DOI] [PubMed] [Google Scholar]
- Smith C, Cowan C, Heffler S, Catlin A. National Health Spending in 2004 Recent Slowdown Led by Prescription Drug Spending. Health Affairs. 2006;25(1):186–96. doi: 10.1377/hlthaff.25.1.186. [DOI] [PubMed] [Google Scholar]
- State of Nebraska Department of Health and Human Services. “COX-2 NSAIDs: Nebraska Medicaid Prior Authorization Process”. 2005. [June 12, 2006]. Available at http://www.hhs.state.ne.us/med/pharm/
- Stockl K, Cyprien L, Chang E Y. Gastrointestinal Bleeding Rates among Managed Care Patients Newly Started on COX-2 Inhibitors or Nonselective NSAIDs. Journal of Managed Care Pharmacy. 2005;11(7):550–8. doi: 10.18553/jmcp.2005.11.7.550. [DOI] [PMC free article] [PubMed] [Google Scholar]