Abstract
Objective
To estimate the proportion of seniors with dementia from three independent data sources and their agreement.
Data Sources
The longitudinal Asset and Health Dynamics among the Oldest Old (AHEAD) study (n=7,974), Medicare claims, and death certificate data.
Study Design
Estimates of the proportion of individuals with dementia from: (1) self- or proxy-reported cognitive status measures from surveys, (2) Medicare claims, and (3) death certificates. Agreement using Cohen's κ; multivariate logistic regression.
Principal Findings
The proportion varied substantially among the data sources. Agreement was poor (κ: 0.14–0.46 depending upon comparison assessed); the individuals identified had relatively modest overlap.
Conclusions
Estimates of dementia occurrence based on cognitive status measures from three independent data sources were not interchangeable. Further validation of these sources is needed. Caution should be used if policy is based on only one data source.
Keywords: Dementia, cognitive impairment, longitudinal health surveys, health surveys, Medicare claims data, death certificates, agreement
BACKGROUND
Accurate dementia prevalence estimates are important from public health, long-term care planning, and cost perspectives. Numerous estimates have been made, (Pfeffer, Afifi, and Chance 1987; Evans et al. 1989; O'Connor et al. 1989; Bachman et al. 1992; Canadian Study of Health and Aging 1994; Beard et al. 1995; Corrada, Brookmeyer, and Kawas 1995; White et al. 1996; Ferini-Strambi et al. 1997; U.S. GAO 1997; Hy and Keller 2000; Taylor, Sloan, and Doraiswamy 2004) but these have been based on findings from other countries or extrapolated from local U.S. samples. Although the exact prevalence is unknown, there is general agreement that Alzheimer's disease (AD) is the most common form of dementia (Cummings and Cole 2002) and that the prevalence of dementia is rising while levels of dementia risk factors, such as stroke, are declining (Ukraintseva et al. 2006). Several studies have reported higher age-adjusted rates in women and blacks (Heyman et al. 1991; Tang et al. 1998; Perkins et al. 2001; Tang et al. 2001; Taylor, Sloan, and Doraiswamy 2004). With the aging of the population and the increasing prevalence of dementia, improving national estimates of this disorder becomes an ever-important public health challenge.
Numerous studies have estimated prevalence from individual data sources such as regional epidemiological studies (Breitner et al. 1999), billing records (Newcomer et al. 1999; Taylor, Sloan, and Doraiswamy 2004), and death certificates (Ganguli and Rodriguez 1999). Such studies may not be representative, and while Medicare claims data have been used for estimation (Newcomer et al. 1999; Taylor, Sloan, and Doraiswamy 2004), uncertainty remains regarding their accuracy and completeness for identification of AD.
It would be useful if good estimates could be generated from large population-based databases. However, concerns exist regarding the accuracy of diagnosis because comprehensive and standardized clinical examinations of large numbers of elderly individuals are prohibitively expensive. There is thus a tradeoff between sample representativeness and diagnostic certainty.
This paper adds to the understanding of this tradeoff. It is, to our knowledge, the first to compare three independent sources of dementia data: cognitive measures from a longitudinal health survey, Medicare claims data, and death certificates. The objectives are to assess how the estimated proportion of elderly with dementia varies among these data sources, to determine overlap, and to evaluate potential predictors of agreement.
MATERIALS AND METHODS
Three Data Sources
Information about subjects in the analysis sample was gathered from: (1) self- or proxy-reported cognitive status screening measures from surveys administered in 1993 and 1995, (2) Medicare claims data (ICD-9), and (3) among the deceased, death certificate data from the National Death Index (NDI) (ICD-9/10).
Asset and Health Dynamics among the Oldest Old (AHEAD)
Our primary data set was the AHEAD component of the Health and Retirement Study (HRS) (Juster and Suzman 1995; Myers, Juster, and Suzman 1997). AHEAD is a nationally representative cohort study of health behaviors, disease and disability, medical care usage, and other topics. The baseline survey (1993; n = 7,974), included community-dwelling respondents aged ≥70 years and their spouses, regardless of age. If participants were unable to complete interviews, proxy respondents were interviewed. Follow-up interviews were conducted in 1995, 1998, 2000, and 2002 (average response rate, 87.6 percent). We used data from the two first surveys (1993 and 1995) to make the time period of our three data sources comparable. Our analysis sample was limited to subjects ≥65 years of age at baseline, with information on all covariates and at least one self- or proxy-completed cognitive score in 1993 or 1995 (n = 7,476).
Cognitive Measures in AHEAD
Dementia status was based on the respondents' performance in 1995, or if not completed in 1995 (e.g., due to death between the 1993 and 1995 surveys), in 1993, on the abbreviated version of the modified Telephone Interview for Cognitive Status (TICS-m) (Brandt, Spencer, and Folstein 1988; Welsh, Breitner, and Magruder-Habib 1993) a validated cognitive screening measure designed for use in telephone surveys (Folstein, Folstein, and McHugh 1975). A score of 8 or below on this 35-point scale (i.e., at least two standard deviations below the mean) was set as the level of impairment consistent with dementia (Herzog and Wallace 1997; Ofstedal, Fisher, and Hertzog 2005).
For proxy respondents, the short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) (Jorm 1994) was administered. Based on previous validation analyses, a score of 3.9 or greater on the five-point scale was set as the level of impairment consistent with dementia (Heeringa et al. 2006).
Medicare Claims Records
AHEAD participants were asked to release their Medicare records from the Centers for Medicare and Medicaid Services. For respondents who consented (80 percent), a set of restricted data files containing Medicare payment records were created. Claims were obtained for (average and maximum number of possible ICD-diagnoses per claim in parentheses): short-stay inpatient (4.6; 11), skilled nursing facility (4.6; 11), hospital outpatient (1.5; 10), physician/supplier (2.6; 4), home health (3.4; 10), and hospice services (1.3; 10). Exact dates of respondent enrollment in Medicare HMOs were available which is important because claims records are unavailable while a person was so enrolled. We used Medicare claims from January 1, 1992 to December 31, 1996, and data confidentiality was ensured (HRS Restricted Data 2006). The presence of at least one encounter with an ICD-9 code in the range outlined in Table 2 was used as a criterion for the presence of dementia and AD as identified in Medicare claims. Past work with Medicare claims for identifying dementia and AD shows that prevalence estimates do not vary greatly by requiring more than one mention of AD, so long as 2–3 years of claims are used; we used 5 years in this study (Taylor, Fillenbaum, and Ezell 2002).
Table 2.
Changing Estimated Proportions of Elderly with Dementia Depending on Source of Data, Choice of Cutpoint on Cognitive Screening Instrument, and Selection of Included Diagnoses
| AHEAD Survey | |
| Overall (TICS 8/9 and IQCODE 3.89/3.90) | 7.5% (561/7,476) |
| Self-report (TICS)* | |
| Cutpoint 7/8 | 3.2% (215/6,688) |
| Cutpoint 8/9 | 4.4% (293/6,688) |
| Cutpoint 9/10 | 6.3% (418/6,688) |
| Proxy report (IQCODE)* | |
| 3.79/3.80 | 36.6% (288/788) |
| 3.89/3.90 | 34.0% (268/788) |
| 3.99/4.00 | 32.9 % (259/788) |
| Medicare claims | |
| Diagnosis | |
| Any dementia-related diagnoses (ICD-9: 290, 290.0–4, 290.8–9, 294.1, 331, 331.0–2, 331.9, 797) | 13.0% (618/4,766) |
| Alzheimer disease | 3.6% (173/4,766) |
| Source | |
| Any source (outpatient, inpatient, physician, home health, hospice, skilled nursing facility) | 13.0% (618/4,766) |
| Inpatient+outpatient+physician claims | 11.6% (554/4,766) |
| Outpatient+physician claims | 10.2% (488/4,766) |
| Inpatient claims only | 4.1% (196/4,766) |
| NDI (death certificates) | |
| Any dementia-related diagnosis (ICD-9: 290, 290.0–4, 290.8–9, 294.1, 331, 331.0–2, 331.9, 797; ICD10: F00–03, G30, G31.0–1, R54) | 6.3% (165/2,622) |
| Alzheimer disease (ICD-9: 331.0; ICD-10: F00, G30) | 2.4% (63/2,622) |
Please note that a higher TICS score means less impaired while a higher IQCODE means more impaired.
Boldface indicates the most commonly used cutpoint.
TICS, Telephone Interview for Cognitive Status; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; NDI, National Death Index.
NDI
The NDI, which is based on death certificate data, provided date and cause of death (ICD-9 or ICD-10): if any of the codes listed in Table 2 were present, the respondent was said to have dementia or AD. To check for these codes, files were submitted from the Health and Retirement Survey to the National Center for Health Statistics (NCHS) for participants assumed to have died before 2000. Using a probabilistic matching procedure, NCHS made available deceased cases that were considered “reliable” matches (NDI 2000 early release 2006).
Data Analyses
We identified subjects with AD or any dementia from Medicare claims and NDI, and those with a TICS or IQCODE score consistent with dementia from AHEAD (see Table 2 for specific ICD-9/ICD-10 codes). The effect of changing cut-points for the TICS and IQCODE was also assessed.
Among the deceased subjects, the concordance for dementia in the three data sets was displayed graphically using a Venn diagram. For those still alive in 2000, the concordance among survey data and claims data only was displayed. Agreement on dementia among the three data sources was assessed using Cohen's κ (Fleiss 1981). Sensitivity analyses were done using a narrower “time window”: AHEAD 1993 survey; Medicare claims 1992–1994; NDI 1993–1994. For all subjects, agreement between survey and claims data was modeled using logistic regression (outcome: identification as having dementia by survey and claims versus not being identified by both), adjusting for age, gender, race, education, and decedent status. Identification as having dementia by all three sources (versus not being identified by all three) was modeled for deceased subjects. Analyses were performed using SAS version 9.1.3.
RESULTS
Figure 1 shows the time points and periods from which the three data sets were derived. The majority of the study participants were female, but the higher mortality rates among men resulted in a greater proportion of males among those for whom death certificates were available (Table 1). The proportions of black and white were similar to those in the overall U.S. population, and, as expected, deceased participants were older and followed for fewer years than participants still alive at the end of the study period.
Figure 1.
Time Frame for Data Collection
Table 1.
Characteristics of the Three Study Samples
| Variables | AHEAD Baseline Survey (n = 7,974)* | Subjects with Medicare Claims (n = 4,766) | NDI (Death Certificates) (n = 2,622) |
|---|---|---|---|
| Gender | |||
| Female | 63.3% | 64.0% | 54.8% |
| Male | 36.7% | 36.0% | 45.2% |
| Race | |||
| White | 85.6% | 86.7% | 84.3% |
| Black/other | 14.4% | 13.3% | 15.7% |
| Age (mean ± SD) | 76.6 ± 6.1 | 77.1 ± 6.2 | 79.8 ± 6.7 |
| Years of education (mean ± SD) | 10.9 ± 3.6 | 10.7 ± 3.8 | 10.3 ± 3.8 |
| Years followed (mean ± SD) | 6.7 ± 2.7 | 6.6 ± 2.7 | 3.9 ± 1.9 |
Only the 7,476 of these with at least one cognitive score are included in subsequent analyses.
AHEAD, Asset and Health Dynamics among the Oldest Old; NDI, National Death Index.
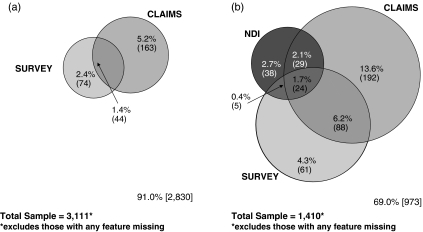
Table 2 shows the proportion of individuals with presumed dementia, and how this proportion changes when data source, ICD-9/10 diagnoses and cognitive scale cut points were modified. The overall proportion with dementia in the AHEAD survey was 7.5 percent using previously reported cut points for the TICS scale (7/8 self-report) and IQCODE (3.89/3.90 proxy). The correlation of being identified in the survey as having cognition consistent with dementia was 0.42 (p<.001) for those respondents answering both surveys, either in person or via a proxy. Using all sources of Medicare claims for the entire period, 13.0 percent of the subjects had any dementia-related diagnosis, and 3.6 percent had a specific diagnosis of AD. From the death certificates, 6.3 percent were identified as having dementia, and 2.4 percent as having AD. Figure 2 further illustrates that not only were the estimated proportions with dementia in the three data sources of different magnitudes, to a large extent the sets identified showed modest overlap.
Figure 2.
(a) Living Asset and Health Dynamics among the Oldest Old (AHEAD) Participants with Medicare Claims: Estimated Proportions with Dementia. (b) Deceased AHEAD Participants with Death Certificates and Medicare Claims: Estimated Proportions with Dementia
For the subjects still alive, Cohen's κ of agreement between claims data and survey data was 0.23 (95 percent CI 0.17–0.30). Among the deceased, between claims and survey data, κ was 0.33 (95 percent CI 0.27–0.39), between claims and death certificates 0.14 (95 percent CI 0.10–0.19), and between surveys and death certificates 0.14 (0.09–0.19). We also assessed agreement using a shorter time frame. When assessing the earlier period (1993 survey, claims 1992–1994, and died in 1993–1994), κ was 0.13 (95 percent CI 0.08–0.18) between claims and survey for those who were alive at study end, and much higher for those who were dead; 0.33 (95 percent CI 0.10–0.55). When assessing the later period (1995 survey, claims 1994–1996, deceased in 1995 or 1996) κ was 0.36 (95 percent CI 0.30–0.41) for claims and the survey for those who were alive and study end, and even higher for those who were deceased, 0.41 (95 percent CI 0.27–0.56). In the multivariable models (Table 3), we found that younger individuals and those with higher education were more likely to be assessed as having dementia by two or more data sources. Persons who died were less likely to be found to have dementia in both survey data and Medicare claims, presumably due to shorter follow-up periods.
Table 3.
Effect of Selected Variables on Agreement Regarding Dementia Diagnosis Defined by Different Methods
| Respondent Identified as Having Dementia in: | ||||
|---|---|---|---|---|
| Variable | Survey Data* and Medicare Claims† Only | Survey Data and NDI Death Certificate Data‡ Only | Medicare Claims Data and NDI Death Certificate Data Only | All Three Sources |
| N | 4,774 | 2,426 | 3,778 | 1,502 |
| Age (per year) | 0.94 (0.93–0.96) | 0.92 (0.91–0.94) | 0.89 (0.88–0.91) | 0.93 (0.91–0.95) |
| Female | Reference | Reference | Reference | Reference |
| Male | 1.06 (0.87–1.28) | 1.23 (0.96–1.56) | 0.67 (0.54–0.83) | 0.91 (0.72–1.15) |
| Black | Reference | Reference | Reference | Reference |
| White | 1.26 (0.97–1.64) | 1.58 (1.17–2.12) | 1.02 (0.75–1.39) | 1.22 (0.87–1.71) |
| Education (per year) | 1.05 (1.02–1.07) | 1.08 (1.05–1.11) | 1.03 (1.00–1.06) | 1.03 (1.00–1.06) |
| Deceased | 0.46 (0.39–0.58) | NA | NA | NA |
TICS≤8 or IQCODE≥3.9 in 1995. In 1993, only the self-reported cognition (TICS) was available. The proxy cognition code (e.g., IQCODE) was not available for the 1993 survey.
At least one Medicare claim with an ICD-9 code (listed in Table 2) indicating dementia or AD. Medicare claims were available from 1992 to 1996.
Death certificate should include at least one ICD-9 or ICD-10 code (listed in Table 2) indicating dementia or AD. NDI data were used from 1993 to 1996.
TICS, Telephone Interview for Cognitive Status; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; NDI, National Death Index; AD, Alzheimer's disease.
DISCUSSION
To our knowledge, this is the first study to compare the occurrence of dementia among three independent data sources relating to the same subjects. The estimated proportion of AHEAD respondents with dementia varies substantially depending on which data source is used. The modest overlap of cases identified shows that the sources not only differ in sensitivity, but they often identify different cases, leading to levels of statistical agreement in the “poor” range (Fleiss 1981). Recent work comparing dementia assessment in survey and Medicare claims also found agreement to be poor to fair (Pressley et al. 2003). A study assessing Medicare claims diagnosis of dementia among a cohort of persons with autopsy-proven dementia found that ∼80 percent of cases could be so identified; given the severity of dementia in that cohort, that study may represent the high water mark of claims sensitivity, but the specificity could not be estimated (Taylor, Fillenbaum, and Ezell 2002). Agreement in our study was higher among younger, white, and more educated respondents, findings similar to a recent study comparing self-report and validated evidence of several chronic diseases among elderly women (Simpson et al. 2004). The highest levels of agreement found in our study were among deceased subjects when using a shorter period of follow-up. Agreement is most likely higher in such subjects because of more advanced stage of dementia (as shown by death) and the fact that survey measures are, on average, closer to death because of the shorter period of follow-up.
What are the likely reasons that these data sources provide such difference prevalence estimates for such a serious and salient condition as dementia? Not surprisingly, the self-report survey responses identified relatively few persons as having cognitive impairment consistent with dementia. The high rate of cognitive impairment among the survey respondents with proxy reports compared with those with self-report reflects the underlying reason for proxy interviews being done. While reliance on proxy respondents may underestimate the occurrence of dementia (Teresi et al. 1995), the proxy may be more sensitive to subtle cognitive changes on a daily basis and be more willing to report them than the subjects themselves who have mild dementia but were able to complete the interview, but who may fear being labeled with the term dementia. Fear of labeling may be present not only in surveys, but also in claims. However, Taylor, Sloan, and Doraiswamy (2004) found increasing prevalence of dementia in Medicare claims across time that is not likely due to a true increase in disease occurrence. They posited that the secular trend was due to increased recognition of the disease among physicians who are noting the diagnosis on claims over time. Further, because hospital admissions are reimbursed using prospective payment, persons treated for an acute condition, but who have dementia comorbidity, may not be coded as such because it is unlikely to affect reimbursement.
A similar concern can be raised about death certificate data: underreporting of dementia on death certificates is likely if physicians only consider the immediate cause of death. In a comparison of clinical survey data and death certificate data from subjects from the Canadian Study of Health and Aging with confirmed clinical diagnosis of dementia before death, less than half had a dementia related diagnosis anywhere on the death certificate (Østbye, Hill, and Steenhuis 1999).
Similar studies of disease prevalence and agreement among different data sources have been conducted for diabetes (Hebert et al. 1999), kidney disease (Kern et al. 2006), cancer (Koroukian, Cooper, and Rimm 2003), asthma (Rawson and Malcolm 1995), and a series of other chronic diseases (Peabody et al. 2004). While such studies also have reported underestimates of disease prevalence and poor concordance among data sets, the agreement usually was higher than in similar studies of dementia. Agreement studies in the area of dementia, more than for most other chronic diseases, are also plagued by the lack of clear diagnostic categories; even studies of well-defined cases of dementia (Erkinjuntti et al. 1997) have shown large variation in the prevalence of dementia depending on the diagnostic classification scheme used (e.g., ICD-9, DSM-III, CERAD).
The primary limitation of our study is the lack of a gold standard diagnosis of dementia. The use of the telephone-administered cognitive instrument to detect dementia clearly is associated with some misclassification.
Another limitation is missing information from different data sources for a variety of reasons. One in five AHEAD respondents did not consent to having Medicare claims records linked to their survey responses so this information was unavailable. Further, because all persons in our sample had not died by the end of the study period, death certificate data were not available for all study subjects. While this merely represents the reality of cohort studies (all persons do not die by the end of the study period), it nevertheless means that all subjects do not have the same opportunity to be found to have dementia, decreasing agreement. While the time periods for the three data sources were not fully congruent (see Figure 1), our sensitivity analyses with a narrower time window provided similar results. The proportions reported may be considered period prevalences (the total number of persons known to have had the disease or attribute at any time during a specified period) (Last 2001). The number of years of follow-up, having three independent sources of dementia assessment, and the national representativeness of the database, still constitute strengths of the study.
In conclusion, there is a great deal of interest for clinical, planning, and policy reasons to have accurate assessments of dementia prevalence, estimated in representative samples. Our study shows that repeated cognitive screening data from surveys, Medicare claims data, and decedent data among those who have died, are not interchangeable. Therefore, significant caution must be used if health planning or policy decisions are to be based on only one of these data sources because each likely will be associated with high numbers of “false negatives.” At the same time, including all individuals appearing in any one data source may include many “false positives” and lead to overestimates. None of the three data sets investigated represent standardized, comprehensive clinical examinations aimed to diagnose dementia. A supplement study to HRS/AHEAD, the Aging, Demographics, and Memory Study (ADAMS), currently underway, aims to clinically assess a subset of individuals age 70 and older, those with suspected impairment (based on TICS/IQCODE scores) as well as a number of controls (Langa et al. 2005), and will be very helpful to contrast the findings from a true “gold standard” assessment with the information from the surveys, Medicare, and NDI. Such information can be used to validate our results as well as results from other studies relying on population surveys, administrative data, or death certificates, and thus more accurately forecast service needs among individuals with dementia and their families.
Acknowledgments
This work was supported by the National Institute of Nursing Research (R-01 008763). Thanks to Elizabeth Efird, B.A., for helpful editing of this manuscript.
REFERENCES
- Bachman DL, Wolf PA, Linn R, Knoefel JE, Cobb J, Belanger A, Dagostino RB, White LR. Prevalence of Dementia and Probable Senile Dementia of the Alzheimer Type in the Framingham Study. Neurology. 1992;42:115–9. doi: 10.1212/wnl.42.1.115. [DOI] [PubMed] [Google Scholar]
- Beard CM, Kokmen E, O'Brien PC, Kurland LT. The Prevalence of Dementia Is Changing over Time in Rochester, Minnesota. Neurology. 1995;45:75–9. doi: 10.1212/wnl.45.1.75. [DOI] [PubMed] [Google Scholar]
- Brandt J, Spencer M, Folstein MF. The Telephone Interview for Cognitive Status. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1988;7:111–7. [Google Scholar]
- Breitner JC, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, Tschantz JT, Plassman BL, Meyer MR, Skoog I, Khachaturian A. APOE-epsilon 4 Count Predict when Prevalence of Ab Increases, Then Declines: The Cache County Study. Neurology. 1999;53(2):321–31. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- Canadian Study of Health and Aging Working Group. Canadian Study of Health and Aging: Study Methods and Prevalence of Dementia. Canadian Medical Association Journal. 1994;150:899–913. [PMC free article] [PubMed] [Google Scholar]
- Corrada M, Brookmeyer R, Kawas C. Sources of Variability in Prevalence Rates of Alzheimer's Disease. International Journal of Epidemiology. 1995;24:1000–5. doi: 10.1093/ije/24.5.1000. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Cole G. Alzheimer Disease. Journal of the American Medical Association. 2002;287:2335–8. doi: 10.1001/jama.287.18.2335. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T, Østbye T, Steenhuis R, Hachinski V. The Effect of Different Diagnostic Criteria on the Prevalence of Dementia. New England Journal of Medicine. 1997;337:1667–74. doi: 10.1056/NEJM199712043372306. [DOI] [PubMed] [Google Scholar]
- Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO. Prevalence of Alzheimer's Disease in a Community Population of Older Persons: Higher Than Previously Reported. Journal of the American Medical Association. 1989;262:2551–6. [PubMed] [Google Scholar]
- Ferini-Strambi L, Marcone A, Garancini P, Danelon F, Zamboni M, Massussi P, Tedesi B, Smirne S. Dementing Disorders in North Italy: Prevalence Study in Vescovato, Cremona Province. European Journal of Epidemiology. 1997;13:301–4. doi: 10.1023/a:1007340727385. [DOI] [PubMed] [Google Scholar]
- Fleiss JL. Statistical Methods for Rates and Proportions. New York: John Wiley and Sons; 1981. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini Mental State. A Practical Method for Grading the Mental Status of Patients for the Clinician. Journal Psychiatric Research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Rodriguez EG. Reporting of Dementia on Death Certificates: A Community Study. Journal of the American Geriatrics Society. 1999;47:842–9. doi: 10.1111/j.1532-5415.1999.tb03842.x. [DOI] [PubMed] [Google Scholar]
- Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM. Identifying Persons with Diabetes Using Medicare Claims Data. American Journal of Medical Quality. 1999;14(6):270–7. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- Heeringa S, Fisher GG, Rodgers WL, Ofstedal MB. Technical Documentation for ADAMS. HRS/AHEAD Documentation Report. Ann Arbor, MI: Survey Research Center, Institute for Social Research, University of Michigan; 2006. [Google Scholar]
- Herzog AR, Wallace RB. Measures of Cognitive Functioning in the AHEAD Study. Journal of Gerontology Series B: Psychological Sciences and Social Sciences. 1997;52:37–48. doi: 10.1093/geronb/52b.special_issue.37. [DOI] [PubMed] [Google Scholar]
- Heyman A, Fillenbaum G, Prosnitz B, Raiford K, Burchett B, Clark C. Estimated Prevalence of Dementia among Elderly Black and White Community Residents. Archives of Neurology. 1991;48:594–8. doi: 10.1001/archneur.1991.00530180046016. [DOI] [PubMed] [Google Scholar]
- “HRS Restricted Data: Medicare Claims and Summary Data”. [2006 July 10]. Available at http://hrsonline.isr.umich.edu/rda/medicare.htm.
- Hy LX, Keller DM. Prevalence of AD among Whites: A Summary by Level of Severity. Neurology. 2000;55:198–204. doi: 10.1212/wnl.55.2.198. [DOI] [PubMed] [Google Scholar]
- Jorm AF. A Short Form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Development and Cross-Validation. Psychological Medicine. 1994;24:145–53. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- Juster F, Suzman R. An Overview of the Health and Retirement Study. Journal of Human Resources. 1995;30(5):S7–56. [Google Scholar]
- Kern EFO, Maney M, Miller DR, Tseng C, Tiwari A, Rajan M, Aron D, Pogach L. Failure of ICD-9-CM Codes to Identify Patients with Comorbid Chronic Kidney Disease in Diabetes. Health Services Research. 2006;41(2):564–80. doi: 10.1111/j.1475-6773.2005.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koroukian SM, Cooper GS, Rimm AA. Ability of Medicaid Claims Data to Identify Incident Cases of Breast Cancer in the Ohio Medicaid Population. Health Services Research. 2003;38(3):947–60. doi: 10.1111/1475-6773.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa KM, Plassman BL, Wallace RB, Herzog AR, Heeringa SG, Ofstedal MB, Burke JR, Fisher GG, Fultz NN, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Weir DR, Willis RS. The Aging, Demographics, and Memory Study: Study Design and Methods. Neuroepidemiology. 2005;25:181–91. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- Last JM. A Dictionary of Epidemiology. Oxford: Oxford University Press; 2001. [Google Scholar]
- Myers GC, Juster FT, Suzman RM. Asset and Health Dynamics among the Oldest Old (AHEAD): Initial Results from the Longitudinal Study. Introduction. Journal of Gerontology Series B: Psychological Sciences and Social Sciences. 1997;52:v–viii. [PubMed] [Google Scholar]
- “National Death Index 2000 Early Release.”. [2006 July 10]; Available at http://hrsonline.isr.umich.edu/meta/years/iy6.php?iyear=1017. [Google Scholar]
- Newcomer R, Clay T, Luxenberg JS, Miller RH. Misclassification and Selection Bias When Identifying Alzheimer's Disease Solely from Medicare Claims Records. Journal of the American Geriatrics Society. 1999;47:215–9. doi: 10.1111/j.1532-5415.1999.tb04580.x. [DOI] [PubMed] [Google Scholar]
- O'Connor DW, Pollitt PA, Hyde JB, Fellows JL, Miller ND, Brook CPB, Reiss BB, Roth M. The Prevalence of Dementia as Measured by the Cambridge Mental Disorders of the Elderly Examination. Acta Psychiatrica Scandinavica. 1989;79:190–8. doi: 10.1111/j.1600-0447.1989.tb08587.x. [DOI] [PubMed] [Google Scholar]
- Ofstedal MB, Fisher GG, Herzog AR. Documentation of Cognitive Functioning Measures in the Health and Retirement Study. HRS Documentation Report DR-006. Ann Arbor, MI: Survey Research Center, University of Michigan; 2005. [Google Scholar]
- Østbye T, Hill G, Steenhuis R. Mortality in Elderly Canadians with and without Dementia: A 5-Year Follow-up. Neurology. 1999;53(3):521–6. doi: 10.1212/wnl.53.3.521. [DOI] [PubMed] [Google Scholar]
- Peabody JW, Luck J, Jain S, Bertenthal D, Glassman P. Assessing the Accuracy of Administrative Data in Health Information Systems. Medical Care. 2004;42(11):1066–72. doi: 10.1097/00005650-200411000-00005. [DOI] [PubMed] [Google Scholar]
- Perkins P, Annegers JF, Doody RS, Cooke N, Aday L, Vernon SW. Incidence and Prevalence of Dementia in a Multiethnic Cohort of Municipal Retirees. Neurobiology of Aging. 2001;22:169–75. doi: 10.1212/wnl.49.1.44. [DOI] [PubMed] [Google Scholar]
- Pfeffer RI, Afifi AA, Chance JM. Prevalence of Alzheimer's Disease in a Retirement Community. American Journal of Epidemiology. 1987;125:420–36. doi: 10.1093/oxfordjournals.aje.a114548. [DOI] [PubMed] [Google Scholar]
- Pressley JC, Trott C, Tang M, Durkin M, Stern Y. Dementia in Community-Dwelling Elderly Patients: A Comparison of Survey Data, Medicare Claims, Cognitive Screening, Reported Symptoms, and Activity Limitations. Journal of Clinical Epidemiology. 2003;56(9):896–905. doi: 10.1016/s0895-4356(03)00133-1. [DOI] [PubMed] [Google Scholar]
- Rawson NS, Malcolm E. Validity of the Recording of Ischaemic Heart Disease and Chronic Obstructive Pulmonary Disease in the Saskatchewan Health Care Datafiles. Statistics in Medicine. 1995;14(24):2627–43. doi: 10.1002/sim.4780142404. [DOI] [PubMed] [Google Scholar]
- Simpson CF, Boyd CM, Carlson MC, Griswold ME, Guralnik JM, Fried LP. Agreement between Self-Report of Disease Diagnoses and Medical Record Validation in Disabled Older Women: Factors That Modify Agreement. Journal of the American Geriatrics Society. 2004;52(1):123–7. doi: 10.1111/j.1532-5415.2004.52021.x. [DOI] [PubMed] [Google Scholar]
- Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, Mayeux R. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in Northern Manhattan. Neurology. 2001;56:50–6. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- Tang MX, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, Andrews H, Feng L, Tycko B, Mayeux R. The APOE e-4 Allele and the Risk of Alzheimer Disease among African Americans, Whites, and Hispanics. Journal of the American Medical Association. 1998;279:751–5. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- Taylor DH, Jr, Fillenbaum GG, Ezell ME. The Accuracy of Medicare Claims Data in Identifying Alzheimer's Disease. Journal of Clinical Epidemiology. 2002;55:929–37. doi: 10.1016/s0895-4356(02)00452-3. [DOI] [PubMed] [Google Scholar]
- Taylor DH, Jr, Sloan FA, Doraiswamy M. Marked Increase in Alzheimer's Disease Identified in Medicare Claims Records between 1991–1999. Journal of Gerontology Series A: Biological Sciences and Medical Sciences. 2004;59A(7):762–6. doi: 10.1093/gerona/59.7.m762. [DOI] [PubMed] [Google Scholar]
- Teresi JA, Golden RR, Cross P, Gurland B, Kleinman M, Wilder D. Item Bias in Cognitive Screening Measures: Comparisons of Elderly White, Afro-American, Hispanic and High and Low Education Subgroups. Journal of Clinical Epidemiology. 1995;48:473–83. doi: 10.1016/0895-4356(94)00159-n. [DOI] [PubMed] [Google Scholar]
- Ukraintseva S, Sloan F, Arbeev K, Yashin A. Increasing Rates of Dementia at Time of Declining Mortality from Stroke. Stroke. 2006;37:1155–9. doi: 10.1161/01.STR.0000217971.88034.e9. [DOI] [PubMed] [Google Scholar]
- U.S. General Accounting Office. Alzheimer's Disease: Estimates of Prevalence in the United States. Washington, DC: U.S. General Accounting Office (GAO/HEHE 97–16); 1997. [Google Scholar]
- Welsh KA, Breitner JCS, Magruder-Habib KM. Detection of Dementia in the Elderly Using Telephone Screening of Cognitive Status. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1993;6:103–10. [Google Scholar]
- White L, Petrovitch H, Ross GW, Masaki KH, Abbott RD, Teng EL, Rodriguez BL, Blanchette PL, Havlik RJ, Wergowske G, Chiu D, Foley DJ, Murdaugh C, Curb JD. Prevalence of Dementia in Older Japanese-American Men in Hawaii: The Honolulu-Asia Aging Study. Journal of the American Medical Association. 1996;276:955–60. [PubMed] [Google Scholar]