Because most children are unable to provide informed consent, some guidelines exclude them from participation in clinical research. The German guidelines of 1931, perhaps the first systematic guidelines for clinical research, prohibit non-therapeutic research with children and research that “in any way endangers” children.”1, 2 The Nuremberg Code’s stipulation that consent is “essential” to ethical research seems to prohibit all research with children.3
Excluding children from research would definitively address the potential to exploit them. Yet, this approach also has the potential to cripple society’s ability to ensure medical interventions are safe and effective for children.4 Indeed, estimates that approximately 70% of medications have not been tested in children, even for basic safety and efficacy, underscore the costs of declining to conduct research with children.5, 6
More recent guidelines attempt to protect children without blocking appropriate research by allowing pediatric research that offers a ‘prospect’ of direct benefit, and pediatric research that poses minimal risk7, 8 or, at most, a minor increase over minimal risk.9, 10 A good deal has been written on the minimal risk standard, and how it might be improved.11, 12, 13, 14 Few articles have evaluated whether the prospect of direct benefit standard protects children without blocking appropriate research.
THE U.S. REGULATIONS
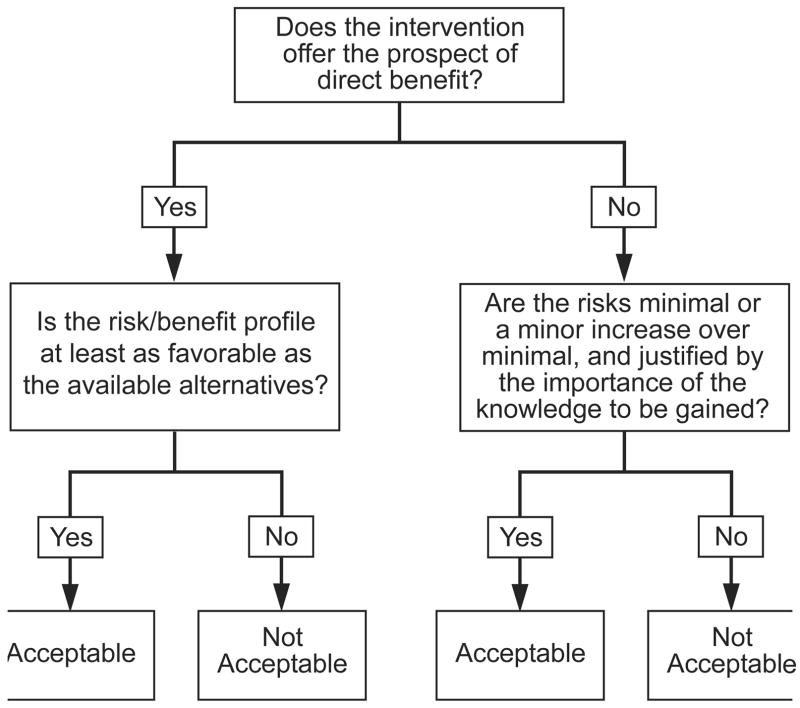
U.S. regulations allow institutional review boards (IRBs) to approve pediatric research in three categories: minimal risk, prospect of direct benefit, and minor increase over minimal risk.9, 10 To implement these regulations, IRBs first evaluate whether a given pediatric research intervention, for instance, an experimental medication, poses no more than ‘minimal’ risk, defined as the risks encountered in daily life or during the performance of routine examinations or tests (46.102). If the intervention poses greater than minimal risk, IRBs next evaluate whether it offers children a ‘prospect’ of direct benefit (diagram 1).
Under U.S. regulations, IRBs may approve pediatric research interventions as prospect of direct benefit only when they satisfy two additional conditions: 1) the risks of the intervention are “justified by the anticipated benefit to the subjects”; and 2) “the relation of the anticipated benefit to the risk is at least as favorable” as that of available alternatives (45CFR46.405). In contrast, IRBs may approve pediatric research interventions which pose more than minimal risk, and do not offer a prospect of direct benefit, when the risks are no greater than a minor ‘increase’ over minimal (45CFR46.406).
PROBLEMS WITH THE PROSPECT OF BENEFIT STANDARD
Despite wide acceptance, the prospect of direct benefit standard raises three concerns. First, there is no accepted definition of what constitutes a ‘prospect’ of direct benefit. This lack of clarity may lead IRBs to approve, as prospect of direct benefit, interventions that offer any potential for direct benefit, independent of the risks involved. Second, the requirement that IRBs may approve prospect of direct benefit interventions only when the potential for direct benefit justifies the risks conflates the ethical standards appropriate for clinical research with the ethical standards appropriate to clinical care. Clinical interventions should offer a potential for benefit which justifies their risks. Research interventions, in contrast, can be acceptable when the risks exceed the potential for direct benefit, provided the net risks are not excessive.
Third, this standard allows children to be exposed to greater net risks when undergoing research interventions that do not offer a prospect of direct benefit. Under the U.S. regulations, IRBs may approve pediatric research interventions which pose greater than minimal risk, and do not offer a prospect of direct benefit, when the risks are no greater than a minor increase over minimal. In this case, IRBs may approve pediatric research interventions which pose some net risks, that is, risks which are not justified by the intervention’s potential for direct benefit. Conversely, IRBs may approve pediatric research interventions which pose greater than minimal risk and offer a prospect of direct benefit only when the intervention’s potential for direct benefit justifies its risks. IRBs are not allowed to approve research interventions that offer a prospect of direct benefit when the risks exceed the prospect of direct benefit, even when the net risks are minimal.
In phase 0 clinical trials, patients are given non-therapeutic doses of an experimental treatment so investigators can evaluate different assays that might be used in later efficacy testing of the treatment.15 Because these trials offer no prospect of direct benefit, the U.S. regulations allow IRBs to approve them in children, provided the net risks are no greater than a minor increase over minimal. In contrast, the interventions tested in phase 2 and 3 studies typically offer participants a prospect of direct benefit. Hence, the prospect of direct benefit standard prohibits IRBs from allowing children to participate in these studies when they pose net risks, no matter how minimal the net risks might be.
What might justify allowing pediatric patients to face some net risks in research that offers no prospect of direct benefit, but not allowing them to face the same or even lower net risks in research that offers a prospect of direct benefit? The importance of protecting children from excessive risks does not seem to support this difference in evaluations. It is difficult to imagine reasonable parents considering it acceptable for investigators to expose their children to some net risks for research purposes, but only when the investigators do not offer the children a prospect of direct benefit.
THE ETHICAL SIGNIFICANCE OF CLINICAL EQUIPOISE
Proponents might try to defend the use of two different risk standards by arguing that prospect of direct benefit interventions must satisfy the requirement of clinical equipoise. Purely research interventions do not need to satisfy the requirement of clinical equipoise. Hence, they may pose some net risks.
Clinical equipoise involves an assessment of whether, given all the available evidence, there is sufficient reason to believe that the risk-benefit profile of an intervention is at least as favorable as the risk-benefit profile of the available alternatives.16 If the risk-benefit profile is at least as favorable, the intervention “satisfies’ clinical equipoise. If the risk-benefit profile of the intervention is less favorable by any margin than the risk-benefit profile of available alternatives, it “fails” to satisfy clinical equipoise.
Clinical equipoise is important for scientific reasons. In most cases, research is justified only when the intervention being evaluated might be at least as good as existing clinical options. Indeed, one may wonder whether there ever could be good reason to spend the time and money, and expose research participants to the risks and burdens of research to evaluate an intervention which, the evidence suggests, is less good than already available options.
The possibility that a research intervention will be at least as good as the available options represents the appropriate default for clinical trials. This implies that the evaluation of whether a research intervention satisfies clinical equipoise is important for ethical reasons as well. Nonetheless, it can make sense to test an intervention which, the evidence suggests, has a less favorable risk-benefit ratio in several cases, in particular, when the inferior intervention costs significantly less and is only slightly more risky or less efficacious.
Comparative studies of cheaper treatments are important to determining whether the efficacy of the superior treatment is sufficient in children to justify its higher cost. The fact that studies of inferior treatments can be appropriate in some cases, especially when the inferior treatment is only slightly more risky or less efficacious, reveals that clinical equipoise is not a strict ethical requirement for all clinical research with children.
Studies in adults find that proton pump inhibitors are somewhat more effective than histamine-2 antagonists for gastroesophageal reflux disease, but cost significantly more. This finding raises the question of whether proton pump inhibitors offer children a decrease in nausea that is substantial enough to justify their increased costs. To evaluate this possibility, investigators might propose to conduct a comparative trial of the two interventions in children. Unfortunately, the prospect of direct benefit standard prohibits IRBs from approving comparative studies in children that include an inferior treatment when existing data suggests that it poses greater than minimal risk.
Such studies are prohibited even when there is compelling evidence that the inferior treatment poses only minimally increased risks compared to the more effective treatment, and offers the potential for enormous cost savings. In this way, the prospect of direct benefit standard can inadvertently block comparative trials that pose minimal net risks, and are needed to provide evidence for important resource allocation decisions. The need for comparative studies of this kind is likely to increase significantly in the future, underscoring the inadequacy of the prospect of direct benefit standard, and the importance of finding better ways to protect pediatric research participants.17, 18
THE NET RISKS TEST
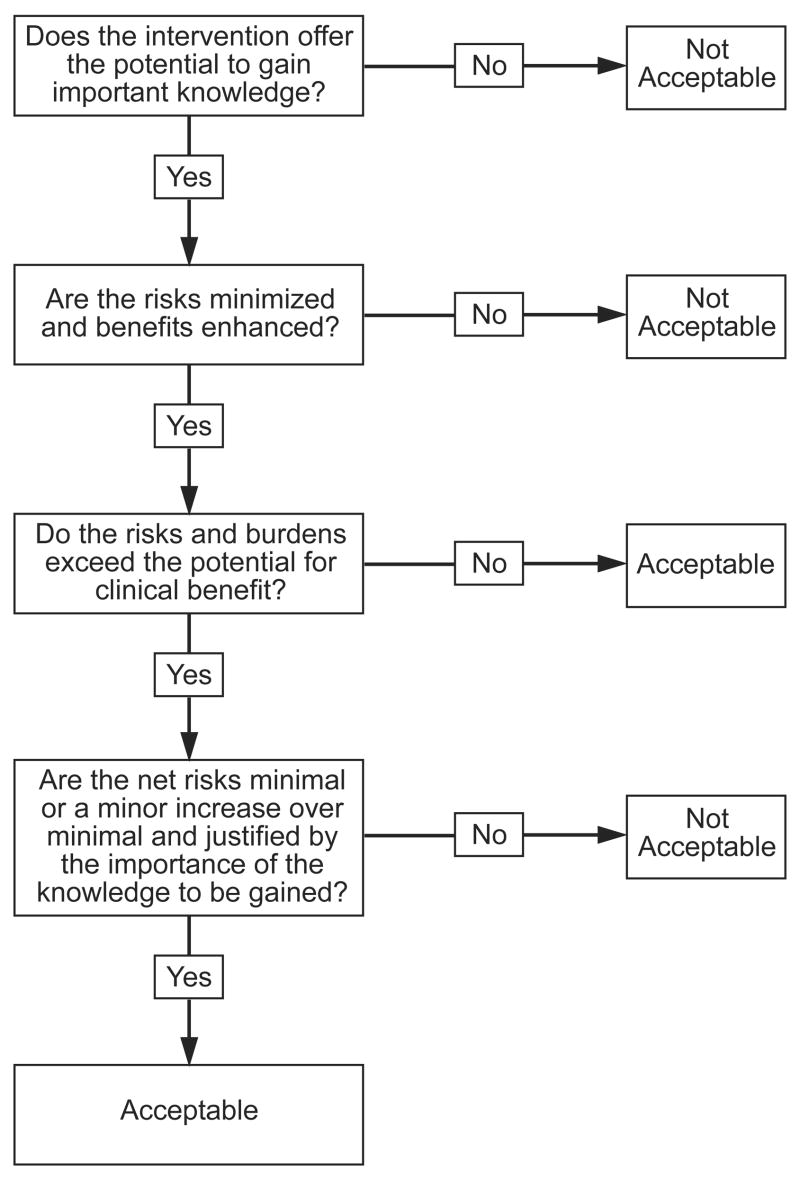
IRBs should ensure that all interventions included in a given study are needed for research purposes, or are clinically indicated. Next, IRBs should minimize the risks and burdens of all the interventions, consistent with sound scientific design, and enhance their benefits. Once these assessments are completed, IRBs should assess the ethical acceptability of the remaining risks and burdens to participants. In particular, IRBs need a method to ensure that research interventions do not pose excessive net risks to children. The ‘net risks’ test, divided into the following three steps, provides one possible approach (diagram 2).
The IRB should determine whether the potential for clinical benefit of undergoing each intervention justifies its risks and burdens by assessing whether the risk-benefit profile of the intervention is at least as favorable for participants as the available alternatives, including not undergoing the intervention at all. If the risk-benefit profile is at least as favorable, the intervention poses no net risks. If the risk-benefit profile is less favorable than one or more available alternatives, including not undergoing the intervention, it poses net risks. The magnitude of the net risks is determined by the extent to which the intervention presents increased risks and/or decreased potential benefits compared to available alternatives.
A study might provide an experimental treatment with a risk-benefit profile equivalent to standard of care, followed by a research PET scan. The experimental treatment poses no net risks because its risk-benefit profile is as favorable for participants as the available alternatives. The PET scan, in contrast, poses net research risks because it offers a less favorable risk-benefit profile than the alternative of not undergoing a PET scan.
The IRB next should ensure that the net risks of each intervention are minimal or a minor increase over minimal, and are justified by the social value of the knowledge to be gained by the intervention’s inclusion in the study. For example, the IRB should assess whether the risks of the PET scan are minimal or a minor increase over minimal, and justified by the information to be obtained from it.
Limiting IRB assessment to the risk-benefit profile of individual interventions ignores the possibility that “research may involve several different procedures that may involve minimal risk or burden individually, but that may present more than minimal risk when considered collectively.”19 The finding that an MRI and a blood draw each pose minimal risks fails to assess whether inclusion of a series of these procedures in a single study poses greater than minimal risk. To address this possibility, IRBs should ensure that the cumulative net risks of a given study are not excessive.
EVALUATING THE NET RISKS TEST
The net risks test has the virtue of applying a single risk standard to all research interventions: does the intervention pose excessive net risks? In practice, this evaluation can be performed in two steps: 1) does the intervention’s potential for clinical benefit justify its risks and burdens? 2) If not, are the ‘net’ risks minimal or a minor increase over minimal? The net risks test is similar to the assessment used to determine whether an approved intervention is clinically indicated. Both assessments require clinicians to estimate the risk-benefit profile of a given intervention, and then compare its risk-benefit profile to that of available alternatives, including not undergoing the intervention at all. This similarity suggests that the net risks test should be more familiar to clinicians than the prospect of benefit standard.
In the research setting, there often are few data available to make risk-benefit assessments, implying that IRBs will have to use their judgement when applying the net risks test. To determine whether the risks of a given research intervention exceed its potential for clinical benefit, IRBs can assess whether, in the judgement of a knowledgeable clinician, undergoing the intervention would conflict with participating children’s clinical interests. When it does, the intervention poses net risks. When there are not sufficient data, IRBs should err on the side of caution and categorize interventions as posing net risks.
CONCLUSION
U.S. regulations direct IRBs to categorize pediatric research interventions that pose greater than minimal risk as offering a ‘prospect’ of direct benefit or not. Unfortunately, this approach to protecting children inadvertently applies a higher risk standard to interventions that offer a prospect of direct benefit and may block important research that is needed to provide health care for all children.
The net risks test avoids these problems by applying the same standard to all pediatric research interventions: does the intervention pose excessive net risks? The advantages of the net risks test provide reason for new regulations to consider adopting it rather than the prospect of benefit standard. For regulations that already include the prospect of benefit standard, guidance should be sought from regulatory authorities on whether the net risks tests can be used within existing regulations, or whether its use will require modification of the regulations.
Figure 1.
Figure 2.
Footnotes
This work was completed as part of the author’s official duties as an employee of the NIH Clinical Center. The opinions expressed are the author’s own. They do not represent any position or policy of the National Institutes of Health, Public Health Service, or Department of Health and Human Services. The author has no financial conflicts of interest related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Regulations on new therapy and human experimentation. Reprinted in: International Digest of Health Legislation. 1980;31:408–11. [PubMed] [Google Scholar]
- 2.Vollmann J, Winau R. Informed consent in human experimentation before the Nuremberg code. BMJ. 1996;313:1445–49. doi: 10.1136/bmj.313.7070.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Nuremberg Code. JAMA. 1996;276:1691. [PubMed] [Google Scholar]
- 4.Kauffman R. E. Clinical trials in children: problems and pitfalls. Paediatr Drugs. 2000;2:411–18. doi: 10.2165/00128072-200002060-00001. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell PHY, Murphy SB, Butow PH, Craig JC. Clinical trials in children. Lancet. 2004;364:803–11. doi: 10.1016/S0140-6736(04)16942-0. [DOI] [PubMed] [Google Scholar]
- 6.Roberts R, Rodriquez W, Murphy D, Crescenzi T. Pediatric drug labeling: improving the safety and efficacy of pediatric therapies. JAMA. 2003;290:905–11. doi: 10.1001/jama.290.7.905. [DOI] [PubMed] [Google Scholar]
- 7.Berlin I, Gorelick D. The French law on ‘protection of persons undergoing biomedical research;’ implications for the U. S J Law Med Ethics. 2002;31:434–41. doi: 10.1111/j.1748-720x.2003.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 8.International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Good clinical practice: consolidated guidance. Published in Federal Register. 1997 May 9;62:25692. [Google Scholar]
- 9.U.S Department of Health and Human Services. Code of Federal Regulations. 45 CFR 46, subpart D.
- 10.U.S Food and Drug Administration. Code of Federal Regulations. Title 21, 50.25. Vol 1, revised April 1, 2005.
- 11.Kopelman LM. Minimal risk as an international ethical standard in research. J Med Philos. 2004;29:351–78. doi: 10.1080/03605310490500545. [DOI] [PubMed] [Google Scholar]
- 12.Ross LF, Nelson RM. Pediatric research and the federal minimal risk standard. JAMA. 2006;295:759–60. doi: 10.1001/jama.295.7.759-a. [DOI] [PubMed] [Google Scholar]
- 13.Resnik DB. Eliminating the daily risks standard from the definition of minimal risk. J Med Ethics. 2005;31:35–8. doi: 10.1136/jme.2004.010470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wendler D, Glantz L. A new standard for assessing the risks of pediatric research: pro and con. J Pediatr. 2007;150:579–82. doi: 10.1016/j.jpeds.2007.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kummar S, et al. Compressing drug development timelines in oncology using phase ‘0’ trials. Nat Rev Cancer. 2007;7:131–39. doi: 10.1038/nrc2066. [DOI] [PubMed] [Google Scholar]
- 16.Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317:141–45. doi: 10.1056/NEJM198707163170304. [DOI] [PubMed] [Google Scholar]
- 17.Eddy DM. Health system reform. Will controlling costs require rationing services? JAMA. 1994;272:324–28. doi: 10.1001/jama.272.4.324. [DOI] [PubMed] [Google Scholar]
- 18.Daniels N. Just Health Care. New York, N.Y.: Cambridge University Press; 1995. [Google Scholar]
- 19.Field MJ, Berman RE, editors. Institute of Medicine. Ethical conduct of clinical research involving children. The National Academies Press; Washington, D.C.: 2005. [PubMed] [Google Scholar]