Abstract
Luteinizing hormone (LH), produced in the anterior lobe of the pituitary, is a member of the hypothalamic-pituitary-gonad axis that is required for production of the sex hormones estradiol, progesterone, and testosterone. Perturbations in levels of hormones associated with this axis can result in defects in sexual development and maturity. LH bears unique N-linked carbohydrate units that terminate with a sulfated N-acetylgalactosamine structure (GalNAc-4-SO4) that mediates its clearance from the blood. To determine the significance of this terminal structure, we ablated the gene encoding the sulfotransferase responsible for sulfate addition to GalNAc on LH, GalNAc-4-sulfotransferase-1 (GalNAc-4-ST1) in mice. Mice lacking GalNAc-4-ST1 exhibited increased levels of circulating LH. In male mice, this resulted in elevated levels of testosterone and precocious maturation of testis and seminal vesicles. Female mice lacking GalNAc-4-ST1 demonstrated elevated estrogen levels and exhibited precocious sexual maturation and increased fecundity. Female mice remained in estrus for prolonged periods and produced almost 50% more litters per mouse than wild-type mice over the same period of time. Thus, sulfate modification of the terminal glycosylation of LH plays a central role in regulating the hypothalamic-pituitary-gonad axis in vivo.
Introduction
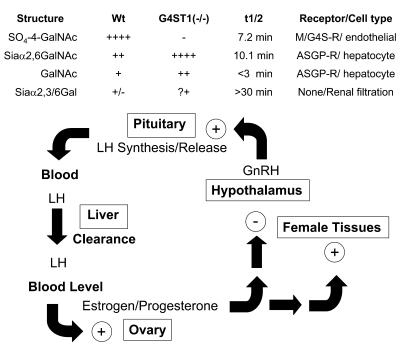
The hypothalamic-pituitary-gonad axis (HPG axis) (see Figure 1) regulates the production of estradiol and progesterone by the ovary and testosterone (T) by the testis (1). These steroid hormones act on a variety of female and male tissues. Precise regulation of the production of steroid hormones is essential for initiating changes associated with development of secondary sexual characteristics, follicle development, ovulation, implantation, and numerous other functions that are characteristic of females and males of each vertebrate species (2, 3). The hypothalamus acts as a pulse generator, synthesizing and releasing gonadotropin-releasing hormone (GnRH), which in turn stimulates the production and pulsatile secretion of the gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) into the blood by cells in the anterior lobe of the pituitary (4–6). Following their release, LH and FSH are transported to the ovary or the testis where they bind to their respective 7-transmembrane G protein–coupled receptors, resulting in adenylate cyclase activation and cAMP production (7). The synthesis of estradiol, progesterone, and T increases in response to the elevation in cAMP levels. In addition to acting on female and male tissues, the steroid hormones exert negative feedback on the hypothalamus and pituitary to reduce the synthesis and release of GnRH and LH. Perturbations in this delicately balanced process can result in precocious or delayed puberty, infertility, and other changes that are associated with elevated or reduced levels of the steroid hormones (8–11).
Figure 1. Schematic of HPG axis.
The LH synthesized by WT mice bears N-linked oligosaccharides that terminate predominantly with SO4-4-GalNAc. Following GnRH-stimulated release into the blood, LH is rapidly removed from the blood by the Man/GalNAc-4-SO4-R expressed by endothelial cells in the liver, resulting in a half-life of 7.2 minutes in the blood. The amount of LH that reaches the LH receptor in the ovary and, as a result, the amount of estrogen/progesterone produced is determined by the half-life of LH. In the absence of GalNAc-4-4-ST1, the N-linked oligosaccharides on LH synthesized in the pituitary are predominantly modified with terminal Siaα2,6GalNAc. LH-bearing terminal Siaα2,6GalNAc is cleared by the ASGP-R expressed by hepatocytes, resulting in a longer half-life of 10.1 minutes. The longer half-life results in higher levels of LH in the blood and increased production of estrogen/progesterone by the ovary in GalNAc-4-ST1–/– mice as compared with WT mice. Thus, the rate of LH clearance and the concentration that LH attains in the blood are determined by the structures of its carbohydrate moieties. The strength of the signal produced by the same amount of LH released is determined by the structure of its terminal sugars. The impact of altered half-life appears to be superimposed on the other feedback mechanisms that normally regulate estrogen/progesterone levels. The same effects are seen in males with respect to T production.
The α and β subunits of the glycoprotein hormones LH, FSH, and thyroid-stimulating hormone form a protein core that is surrounded by a shell of carbohydrate (12). The Asn-linked carbohydrates on the glycoprotein hormones are important for the folding and assembly of these proteins. The carbohydrates also have a major impact on the physical chemical properties of the hormones and may modulate binding to and activation of the hormone receptors (13–15). Parsons and Pierce (16, 17) were the first to note that the oligosaccharides on LH have an unusual composition. We demonstrated that LH bears N-linked oligosaccharides terminating with the sequence SO4-4-GalNAcβ1,4-acetylglucosamine-β1,2mannose (SO4-4-GalNAcβ1,4GlcNAcβ1,2Man) (15, 18–20). The presence of this structure on LH reflects the action of a protein-specific β1,4GalNAcT that recognizes a peptide sequence in the hormone, followed by a GalNAc-4-sulfotransferase (GalNAc-4-ST) that subsequently modifies the terminal β1,4-linked GalNAc with sulfate (21–25). We have shown that the peptide recognition determinant, protein-specific β1,4GalNAcT, and GalNAc-4-ST have all been conserved throughout vertebrate evolution. As a result, the pituitary glycoprotein hormones of all vertebrates bear N-linked carbohydrates terminating with the sequence SO4-4-GalNAcβ1,4GlcNAcβ1,2Man (12, 14).
The terminal GalNAc-4-SO4 is recognized by a highly abundant endocytic receptor located in hepatic endothelial cells, the Man/GalNAc-4-SO4 receptor, which mediates the clearance of secreted LH from the blood (26–29). We hypothesized that clearance by the Man/GalNAc-4-SO4 receptor is needed to produce the episodic rise and fall seen in circulating LH levels and/or to maintain the appropriate concentration of LH in the blood.
We (30, 31) and others (32) have cloned 2 homologous GalNAc-4-STs, GalNAc-4-ST1 (also known as GalNAc-4-ST8 and CHST8) and GalNAc-4-ST2 (also known as GalNAc-4-ST9 and CHST9), that are able to transfer SO4 to terminal β1,4-linked GalNAc. Our recent studies have demonstrated that GalNAc-4-ST1 is the predominant GalNAc-4-ST expressed in human and mouse pituitary (33). We have now defined the importance of the unique sulfated carbohydrate structures on LH by genetically ablating GalNAc-4-ST1 to produce GalNAc-4-ST1–/– mice. We report that altering the structure of the N-linked carbohydrates on LH has a significant impact on regulation of the HPG axis, resulting in elevated levels of LH and steroid hormones. As a result, female and male GalNAc-4-ST1–/– mice display accelerated development of their reproductive organs and are more fecund than WT mice. The changes seen in GalNAc-4-ST1–/– mice indicate that the structure of the carbohydrate on LH provides what we believe is a novel form of regulation that determines the strength of the signal produced by LH in vivo.
Results
Ablation of GalNAc-4-ST1.
We previously established that GalNAc-4-ST1 is the predominant GalNAc-4-ST expressed in the pituitary based on the steady state levels of transcript and on the resistance of GalNAc-4-ST activity present in extracts of pituitary to loss of activity by incubation at 37°C (30, 31, 33).The carboxyterminal 306 amino acids of GalNAc-4-ST1 contain the catalytic domain and are encoded by a single exon, exon 4 (31). A 14-kb fragment of DNA containing exons 3 and 4 flanked by loxP sites followed by PGK-neo and an additional loxP site was introduced into the genome of mouse ES cells by homologous recombination (34). Exon 3, exon 4, and PGK-neo were removed from the genome by transient expression of Cre recombinase in ES cells and subcloning. The removal of exon 3, exon 4, and PGK-neo was confirmed by Southern blot analysis (Figure 2). Mice heterozygous for the null allele of GalNAc-4-ST1 were bred with C57BL/6 mice for 5 generations to generate GalNAc-4-ST1–/– mice on a C57BL/6 background. Mice homozygous for ablation of GalNAc-4-ST1, GalNAc-4-ST1–/–, were identified by PCR and by Southern blot analysis.
Figure 2. Generation of GalNAc-4-ST1–/– mice.
(A) A targeting construct containing exons 3 and 4 of mouse GalNAc-4-ST1 flanked by loxP sites followed by PGK-neo also flanked by loxP sites was constructed as illustrated. 5′ and 3′ probes to regions outside of the area used to prepare the targeting construct were used to identify homologous recombinants in ES cells before and after treatment with Cre recombinase. (B) DNA from live progeny of heterozygous matings were genotyped by Southern blotting following EcoRI digestion. The WT product is 11.1 kb and the KO product is 10.3 kb. Examples of WT (+/–) and KO (–/–) progeny are shown.
GalNAc-4-ST1 transcripts are not detected by RT-PCR in pituitaries from GalNAc-4-ST1–/– mice (data not shown). Furthermore, the GalNAc-4-ST activity in extracts of pituitaries of GalNAc-4-ST1–/– mice is less than 5% of that present in extracts of pituitaries from WT mice (data not shown). GalNAc-4-ST1–/– mice are nonetheless viable, fertile, and without gross morphologic or behavioral abnormalities.
Serum LH is elevated in GalNAc-4-ST1–/– male mice.
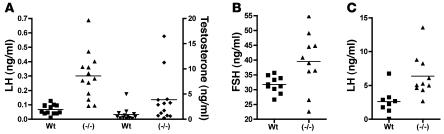
Circulating levels of LH, FSH, and T were determined for male WT and GalNAc-4-ST1–/– mice (Figure 3). The LH levels were elevated in GalNAc-4-ST1–/– male mice, with a mean value that was 4.4-fold greater than that in WT mice (P = 0.0001) (Figure 3A). The T levels were also elevated in GalNAc-4-ST1–/– mice, with a mean value that was 4.2-fold that in WT mice (P = 0.04), although nearly half of the males had values that fell within the normal range for WT mice (Figure 3A). The levels of FSH were more variable in GalNAc-4-ST1–/– mice than in WT mice but nonetheless had a mean value that was 24% greater than for WT mice (P = 0.026) (Figure 3B), suggesting that in mice FSH is also to some extent modified with GalNAc-4-SO4.
Figure 3. LH, T, and FSH levels in WT and GalNAc-4-ST1–/– male mice.
Serum was collected from 8- to 10-week-old male WT and GalNAc-4-ST1–/– mice. Levels of LH, T, and FSH were determined by radioimmunoassay. In each case, the difference in mean values was significant. (A) P = 0.0001, LH (squares, triangles); P = 0.04, T (inverted triangles, diamonds). (B) P = 0.026, FSH. (C) P = 0.009, LH 5 days following castration.
The HPG axis determines the circulating levels of LH and T. LH drives T production by Leydig cells in the testis while T in turn reduces LH synthesis and release. Castration results in maximal stimulation of LH synthesis and release from the pituitary. Following castration, the mean level for circulating LH (Figure 3C) increased 21-fold in GalNAc-4-ST1–/– mice and 38-fold in WT mice. Nonetheless, the mean level of LH remained 2.5-fold greater in GalNAc-4-ST1–/– mice than in WT mice (P = 0.009). Thus, even under conditions where synthesis and release of LH were no longer regulated by the HPG axis through T feedback, the levels of LH were elevated in GalNAc-4-ST1–/– mice. The elevated levels of LH seen in the GalNAc-4-ST1–/– mice as compared with WT mice following castration could not be accounted for by differences in the rate of LH synthesis or release from the pituitaries of WT and KO mice.
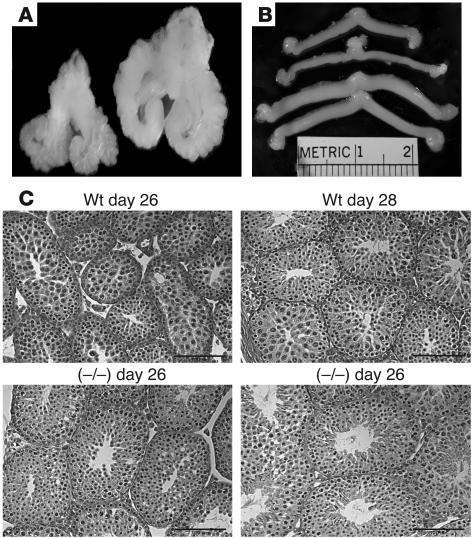
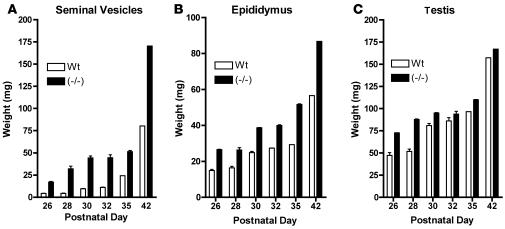
The elevated levels of LH, FSH, and T in male GalNAc-4-ST1–/– mice had physiologic consequences for tissues that are responsive to T. The males exhibited extensive barbering (trimming of whiskers inflicted on one another), which may indicate more aggressive behavior. The size of the seminal vesicles is determined by the levels of T and provides a biological indicator of T levels in vivo. The seminal vesicles of mature GalNAc-4-ST1–/– mice had a mean weight that was twice that of seminal vesicles in WT mice (Figures 4A and 5A). The difference in size was observed as early as postnatal day 26. Similarly, the epididymides were larger in sexually immature GalNAc-4-ST1–/– mice than in WT mice, and this difference persisted in adult mice (Figure 5B). The testes of GalNAc-4-ST1–/– mice were larger than those of WT mice on postnatal days 26 and 28; however, this difference did not persist in the adults (Figure 5C). Sperm were first identified in tubules of the GalNAc-4-ST1–/– testis on postnatal day 28 (Figure 4C), whereas they first appeared in tubules of WT mice on postnatal day 30. Thus, the testes of male GalNAc-4-ST1–/– mice reached maturity roughly 2 days earlier than did those of WT mice. The increased size of the seminal vesicles indicated that the GalNAc-4-ST1–/– mice produced significantly more T than WT mice by postnatal day 26 and continuing into adulthood. Histologic examination of testes from adult GalNAc-4-ST1–/– mice did not reveal any abnormalities.
Figure 4. Comparison of seminal vesicles and uteri from WT and GalNAc-4-ST1–/– mice.
(A) The seminal vesicles, prostate, and bladder were dissected from WT (left) and GalNAc-4-ST1–/– (right) male adult mice. The seminal vesicles are significantly enlarged. (B) The uterus and ovaries were dissected from WT (upper 2) and GalNAc-4-ST1–/– (lower 2) female adult mice. The uteri are enlarged in GalNAc-4-ST1–/– mice as compared with the WT mice. (C) Histologic sections of testis from postnatal day 26 and 28, WT and GalNAc-4-ST1–/–. Sperm can be seen in the section from GalNAc-4-ST1–/– mice on day 28 but not in the section from WT mice on day 28. Scale bars: 1 μm.
Figure 5. Comparison of weights for seminal vesicles, epididymides, and testes of WT and GalNAc-4-ST1–/– mice during postnatal development.
(A) Seminal vesicles, (B) epididymides, and (C) testes were carefully dissected from WT and GalNAc-4-ST1–/– male mice on the postnatal days indicated and weighed. Tissues were taken from 8, 18, 16, 14, 10, and 10 WT and 16, 19, 14, 22, 10, and 5 GalNAc-4-ST1–/– mice on postnatal days 26, 28, 30, 32, 35, and 42, respectively.
Alterations in regulation of the HPG axis of female GalNAc-4-ST1–/– mice.
Female GalNAc-4-ST1–/– mice also displayed changes that indicated alterations in regulation of the HPG axis. GalNAc-4-ST1–/– mice produced litters at a faster rate than WT mice. Over a 2-month period using cages containing 1 male and 2 female mice for WT (7 cages) and GalNAc-4-ST1–/– (6 cages) mice, WT mice produced 1.1 litters per female whereas GalNAc-4-ST1–/– mice produced 1.8 litters per female. Thus, GalNAc-4-ST1–/– mice were more fecund than WT C57BL/6 mice. The GalNAc-4-ST1–/– mice also produced litters that were larger than those of WT mice. Moreover, GalNAc-4-ST1–/– females showed more effective mothering; in contrast with WT C57BL/6 mice, GalNAc-4-ST1–/– females rarely cannibalized their young. The tendency of WT C57BL/6 mice to cannibalize their young makes precise quantitation of litter size at birth difficult in the WT mice. The ovaries of adult GalNAc-4-ST1–/– mice have mean cross-sectional areas that are twice as large as those of WT mice and are more nodular than those of WT mice. The ovaries from GalNAc-4-ST1–/– mice have more corpora lutea when examined microscopically but otherwise appear to be normal (data not shown). The increased fecundity and the appearance of the ovaries both suggest the estrus cycle in GalNAc-4-ST1–/– mice was altered.
The estrus cycle was characterized for 9 WT and 12 GalNAc-4-ST1–/– mice by daily examination of vaginal swabs for 19 consecutive days. The estrus cycle, 4 to 6 days long in mice, consists of proestrus, estrus, metestrus, and diestrus. Estrogen and LH begin to rise in proestrus, and ovulation occurs in estrus; eggs move from the ovary to the uterus during metestrus, and if pregnancy does not occur, the unfertilized eggs are eliminated and the corpora lutea involute during diestrus. Over a period of 19 days, WT mice typically complete 4 cycles (Figure 6A). In contrast, the estrus cycle of GalNAc-4-ST1–/– mice was abnormal. GalNAc-4-ST1–/– mice remained in estrus for prolonged periods (Figure 6A). On average, only 1.5 completed cycles could be identified over a 19-day period in GalNAc-4-ST1–/– mice (Figure 6B).
Figure 6. GalNAc-4-ST1–/– female mice display alterations in their estrus cycle and postnatal maturation.
Stages of the estrus cycle were determined for WT and GalNAc-4-ST1–/– mice by daily vaginal lavage for 19 consecutive days. (A) Examples of estrus cycles in WT and GalNAc-4-ST1–/– mice. P, proestrus; E, estrus; D1, diestrus1; D2, diestrus 2. (B) The number of cycles observed over 19 days was lower in 12 KO mice as compared with 9 WT mice; ***P < 0.0001. (C) Two groups of 15 WT and 15 GalNAc-4-ST1–/– female mice were examined daily beginning on postnatal day 25 for opening of the vagina, an indicator of having attained sexual maturity. The cumulative number of mice that attained maturity for each postnatal day is shown. WT mice, gray bars; KO mice, black bars.
We examined female GalNAc-4-ST1–/– mice to determine whether, like male mice, they reach sexual maturity at an earlier age than WT mice. The time course for vaginal opening, an indication of sexual maturation, was compared for 15 GalNAc-4-ST1–/– and 15 WT mice (Figure 6C). GalNAc-4-ST1–/– mice reached sexual maturity 5 days earlier than WT mice.
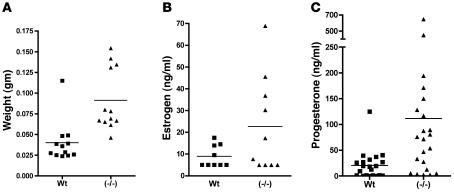
Precocious sexual maturation, alterations in the estrus cycle, and the increase in the number of corpora lutea in the ovaries of GalNAc-4-ST1–/– mice each indicated that serum LH levels and, as a result, estrogen and progesterone levels were elevated in GalNAc-4-ST1–/– mice. We examined the morphologic appearance of uteri from 12 WT and 12 GalNAc-4-ST1–/– mice. The uteri from GalNAc-4-ST1–/– mice were swollen and highly vascularized as compared with those of WT mice (Figure 4B), resulting in a mean weight that was 2.3 times that of uteri from WT mice (P = 0.0007) (Figure 7A). Since the size of the uterus is dependent on estrogen levels, the increased size indicates that the GalNAc-4-ST1–/– mice had elevated levels of estrogen as compared with WT mice. Extracts were prepared from the ovaries of the same GalNAc-4-ST1–/– and WT mice and levels of estrogen and progesterone determined as summarized in Figure 7, B and C. The mean value for estrogen was 2.5-fold greater in ovaries from GalNAc-4-ST1–/– mice than WT mice; however, in both groups, 5 mice were just at or below the level of estrogen detectability. As a result, the difference in estrogen levels did not attain statistical significance. Nonetheless, the increased size of the uteri in all of the GalNAc-4-ST1–/– mice indicates that the levels of estrogen were higher in GalNAc-4-ST1–/– than WT mice, even in those animals that fell below the level of detection. GalNAc-4-ST1–/– mice also displayed significantly greater variation in their levels of progesterone than WT mice (Figure 7C). The 5.3-fold greater mean value for progesterone in GalNAc-4-ST1–/– mice was significantly greater (P = 0.0118) than that of WT mice, consistent with the presence of more corpora lutea in the ovaries of the GalNAc-4-ST1–/– mice and/or the production of more progesterone per corpus luteum.
Figure 7. Uterine weights, estrogen levels, and progesterone levels are increased in GalNAc-4-ST1–/– mice.
(A) The uteri from 10 WT and 10 GalNAc-4-ST1–/– adult female mice were collected and weighed. Means differed by 2.3-fold; P = 0.0007. The ovaries from WT and GalNAc-4-ST1–/– adult female mice were collected and extracts prepared by sonnication. (B) Estrogen level mean values differed by 2.5-fold; P = 0.0885. (C) Progesterone level mean values differed by 5.3-fold; P = 0.0118. The levels of estrogen and progesterone were determined by RIA.
Ablation of GalNAc-4-ST1 alters the structure of the carbohydrates on LH and increases its circulatory half-life.
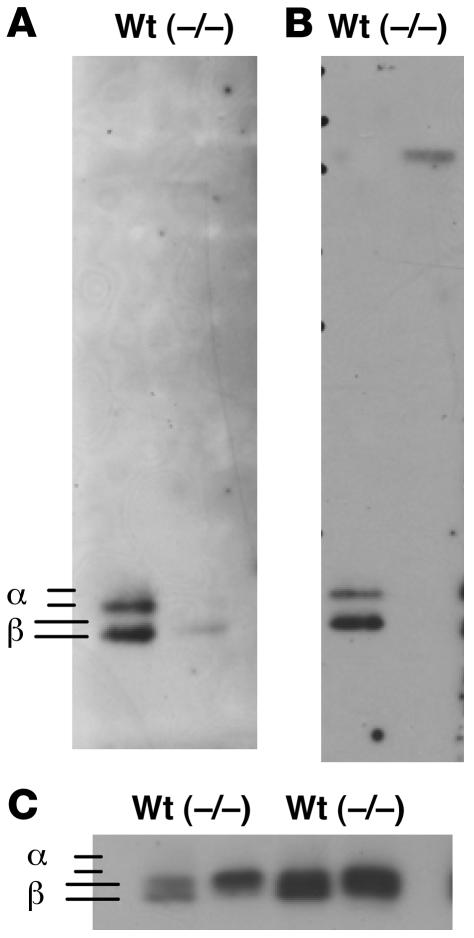
In order to establish whether the carbohydrate moieties on LH in GalNAc-4-ST1–/– mice were deficient in terminal GalNAc-4-SO4, we prepared extracts from pituitaries of WT and GalNAc-4-ST1–/– male mice that had or had not been castrated. Western blots were performed with antibodies specific for the LHβ subunit (Figure 8C) or the common α subunit (data not shown), respectively. The amount of LHβ and α was similar in pituitaries of WT and GalNAc-4-ST1–/– mice; however, in WT mice 2 distinct forms of LHβ (Figure 8C) and α (not shown) were detected, whereas only a single form was detected in the GalNAc-4-ST1–/– mice.
Figure 8. GalNAc-4-ST1–/– mice do not add sulfate to N-linked carbohydrates on the glycoprotein hormones.
Extracts prepared from 5 WT and 5 GalNAc-4-ST1–/– mice that had not been castrated (A and C, left) or that had been castrated (B and C, right) were separated by SDS-PAGE and electrophoretically transferred to PVDF membranes for Western blot analysis. (A) Cys-Fc (chimeric protein specific for terminal GalNAc-4-SO4). (B) Mab6.3 (monoclonal antibody specific for SO4-4-GalNAcβ1,4GlcNAcβ1,2Man-). (C) Rabbit anti-LHβ. The location of the bands identified as the α subunit (not shown) and the LHβ subunit (C) in separate blots are indicated.
The same samples were examined by Western blotting with 2 additional probes: (a) a chimeric protein consisting of the cysteine-rich domain of the Man/GalNAc-4-SO4 receptor that binds terminal GalNAc-4-SO4 and the constant region of human IgG1 (Cys-Fc) (28) (Figure 8A); and (b) a monoclonal antibody that specifically binds terminal β1,4-linked GalNAc-4-SO4, but not GalNAc-3-SO4 Mab6.3 (35, 36) (Figure 8B). In both instances, the major blotted species detected by these carbohydrate-specific probes comigrated with the glycoprotein hormone β and α subunits, indicating that the glycoprotein hormones are the predominant glycoproteins in the pituitary of WT mice that bear terminal GalNAc-4-SO4. GalNAc-4-ST1–/– mice do not have bands that react with either Cys-Fc (Figure 8A) or Mab6.3 (Figure 8B) at the locations of the β and α subunits, indicating that ablation of GalNAc-4-ST1 abolishes the addition of sulfate to the oligosaccharides on LH. Removal of terminal SO4 from LH oligosaccharides results in slower migration during separation by SDS-PAGE (35, 36). The faster migrating species of LHβ seen in the WT, corresponding to the sulfated form of LHβ, is not present in the pituitary extract from GalNAc-4-ST1–/– mice, further supporting the conclusion that the sulfate addition is abolished in GalNAc-4-ST1–/– mice.
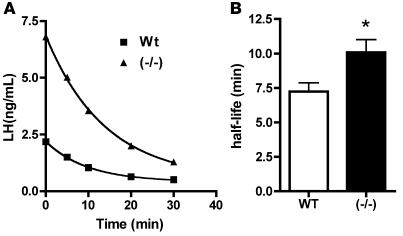
We examined the clearance of endogenous LH from the blood of WT and GalNAc-4-ST1–/– mice by injecting acyline, a potent GnRH antagonist (37, 38), to acutely block the stimulated secretion of LH into the blood and taking samples for analysis of serum LH levels over time (Figure 9). Since the levels of serum LH in WT male mice were not sufficient to permit LH determination following acyline treatment, the analysis of clearance in WT and GalNAc-4-ST1–/– mice was carried out using animals that had been castrated. Examples of clearance curves for WT and GalNAc-4-ST1–/– mice are shown in Figure 9A. Serum LH levels were elevated in castrated GalNAc-4-ST1–/– mice as compared with WT mice and rapidly declined following acyline injection (Figure 9A). In both instances, the best fit was obtained with an equation for 1-phase exponential decay. In the case of the data shown in Figure 9A, LH was cleared with a half-life of 9.65 minutes in GalNAc-4-ST1–/– and 6.82 minutes in WT mice. In spite of the large differences in initial serum levels of LH and the span of the exponential decay curves, LH levels plateaued at similar values of 0.53 and 0.41 ng/ml in GalNAc-4-ST1–/– and WT mice, respectively. Comparison of 17 WT and 14 GalNAc-4-ST1–/– mice yielded half-lives of 7.2 and 10.1 minutes, respectively (Figure 9B). This difference in half-life is significant, with a P value of 0.01.
Figure 9. Clearance of endogenous LH in WT and GalNAc-4-ST1–/– mice.
WT and GalNAc-4-ST1–/– 36-week-old mice were surgically castrated or ovariectomized 5 days prior to the clearance studies. Serum was taken from the WT (n = 8) and GalNAc-4-ST1–/– (n = 8) castrated male mice and from the WT (n = 9) and GalNAc-4-ST1–/– (n = 6) ovariectomized female mice at the initiation of the clearance study. Acyline (10 μg/mouse) was administered intravenously, and blood was withdrawn at 5, 10, 20, and 30 minutes following introduction of acyline. (A) Comparison of LH clearance by individual castrated WT (squares) and castrated GalNAc-4-ST1–/– (triangles) mice following acyline injection. Half-lives for a total of 17 WT and 14 GalNAc-4-ST1–/– mice were determined using GraphPad Prism 4.0. (B) LH removed from the blood of WT mice had a half-life of 7.2 minutes (SEM = 0.6), and LH removed from the blood of GalNAc-4-ST1–/– mice had a half-life of 10.1 minutes (SEM = 0.9). The difference in half-lives is significant: *P = 0.01.
The clearance of LH in GalNAc-4-ST1–/– mice was slower than in WT mice but was still sufficiently rapid to indicate that it was receptor mediated. In the absence of GalNAc-4-ST1, it is possible that the oligosaccharides on LH could terminate with either GalNAcβ1,4GlcNAc or with Siaαβ2,6GalNAcβ1,4GlcNAc. These carbohydrate structures would be recognized by another carbohydrate-specific receptor system that is also located in the liver, the asialoglycoprotein receptor (ASGP-R) (39–41). Pituitary extracts from WT and GalNAc-4-ST1–/– mice were passed over either immobilized Wisteria floribunda agglutinin (WFA), a lectin that binds terminal β1,4-linked GalNAc (42, 43), or immobilized Sambucus nigra agglutinin-1 (SNA-1), a lectin that binds terminal Siaα2,6Gal and Siaα2,6GalNAc (44, 45), in order to ascertain whether either of these structures was present on LH from WT or GalNAc-4-ST1–/– mice. Western blot analysis using anti-LHβ was used to assess the distribution of LH in the unbound and elution fractions, as shown in Figure 10.
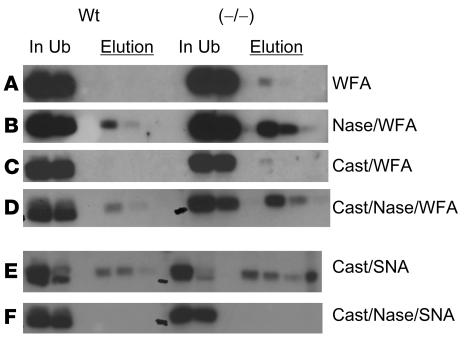
Figure 10. LH from GalNAc-4-ST1–/– mice has increased levels of Siaα2,6GalNAc.
Extracts were prepared from 5 WT and 5 GalNAc-4-ST1–/– mice that had not been castrated (A and B) or had been castrated (cast) (C–F). Identical aliquots of each extract were incubated with immobilized WFA or SNA-1, which binds structures terminating with GalNAc or Siaα2,6GalNAc, respectively. Terminal sialic acid was removed by digestion with neuraminidase as indicated prior to incubation with WFA or SNA-1 (B, D, and F). Equal aliquots of the starting material (In), the unbound fraction (Ub), and the fractions selectively eluted with GalNAc or lactose (Elution) were analyzed by SDS-PAGE and Western blotting with anti-LHβ. (A) Binding of pituitary extracts by WFA-agarose. (B) Binding of pituitary extracts by WFA-agarose following neuraminidase digestion. (C) Binding of pituitary extracts from castrated mice by WFA-agarose. (D) Binding of pituitary extracts from castrated mice by WFA-agarose following neuraminidase digestion. (E) Binding by SNA-1–agarose. (F) Binding by SNA-1–agarose following digestion with neuraminidase.
LH in pituitary extracts from WT and GalNAc-4-ST1–/– mice was not bound by immobilized WFA (Figure 10A), indicating that there is little terminal β1,4-linked GalNAc on the LH from either WT or GalNAc-4-ST1–/– mice. Following digestion with neuraminidase from Arthrobacter ureafaciens to remove terminal sialic acid, a small amount of the LH from WT pituitaries was bound by WFA (Figure 10B), indicating that a fraction of the oligosaccharides on murine LH (mLH) bore terminal Siaα2,6GalNAc; however, the fraction of LH that was bound after neuraminidase digestion was much greater for mLH from GalNAc-4-ST1–/– mice (Figure 10B). Similar results were obtained following castration (Figure 10, C and D).
Two closely migrating bands of LHβ were present in pituitary extracts from WT mice (Figure 10E). The slower-moving species of LHβ was no longer present in the unbound fraction following incubation with immobilized SNA-1, which binds Siaα2,6GalNAc, but was instead in the fractions eluted with lactose. In contrast, LHβ in pituitary extracts from GalNAc-4-ST1–/– mice migrated almost exclusively at the position of the slower-moving band seen in WT mice, was quantitatively removed from the unbound fraction following incubation with SNA-1, and was eluted with lactose (Figure 10E). Digestion with neuraminidase from A. ureafaciens to remove terminal sialic acid abolished binding by SNA-1, confirming that the SNA-1 binding reflected the presence of terminal α2,6-linked Sia (Figure 10F). Thus, in the absence of SO4 addition, a major fraction of the oligosaccharides on LH from GalNAc-4-ST1–/– mice terminated with Siaα2,6GalNAc. In contrast, while oligosaccharides terminating with Siaα2,6GalNAc were present on LH from WT mice, these structures were much less abundant than on LH from GalNAc-4-ST1–/– mice.
Discussion
The physiologic changes that we have observed in GalNAc-4-ST1–/– mice demonstrate that the presence of terminal GalNAc-4-SO4 on LH N-linked oligosaccharides plays a critical role in regulating levels of circulating LH in vivo and, as a result, levels of the steroid hormones T, estrogen, and progesterone. In the absence of GalNAc-4-ST1, male mice have elevated levels of serum LH and T, display precocious sexual development, and have enlarged seminal vesicles as compared with WT mice. Female GalNAc-4-ST1–/– mice have elevated levels of LH, estrogen, and progesterone, display precocious sexual development, have enlarged uteri, have an abnormal estrus cycle, and are more fecund than WT mice.
LH was the first glycoprotein hormone that we demonstrated to bear N-linked carbohydrate moieties that terminate with the sequence SO4-4-GalNAcβ1,4GlcNAc (20). GalNAc is added to the oligosaccharides on LH by a protein-specific β1,4GalNAcT (21, 22, 24, 25). In the absence of the protein-specific β1,4GalNAcT, the same oligosaccharides are modified with β1,4-linked Gal. Terminal β1,4-linked GalNAc can subsequently be modified by the addition of either SO4 by GalNAc-4-ST1 or α2,6-linked Sia by α2,6-sialyltransferase. Should terminal Gal be present rather than GalNAc, it can be further modified with either α2,3- or α2,6-linked Sia but not SO4 (12, 15, 23). In WT mice the major fraction of LH bears structures that terminate with the sequence SO4-4-GalNAcβ1,4GlcNAc-, while a smaller fraction bears oligosaccharides that terminate with the sequence Siaα2,6GalNAcβ1,4GlcNAc-. In contrast, in GalNAc-4-ST1–/– mice, virtually all of the N-linked structures on LH terminate with Siaα2,6GalNAcβ1,4GlcNAc-, and structures that terminate with SO4-4-GalNAcβ1,4GlcNAc are absent.
The pattern of terminal glycosylation of LH could potentially have an impact on activation of the LH receptor. However, in previous studies, we found that the presence of terminal Sia on recombinant bovine LH increased the amount of LH required to activate the LH receptor (36). The presence of structures terminating with Sia on LH from GalNAc-4-ST1–/– mice would be expected to decrease rather than increase the potency of the hormone.
We have proposed that structures of the N-linked oligosaccharides on LH are critical in vivo because they are recognized by endocytic receptors that determine the half-life of the secreted hormone in the blood (see Figure 10). Glycoproteins bearing structures that terminate with SO4-4-GalNAcβ1,4GlcNAc are cleared from the circulation by the Man/GalNAc-4-SO4-R located in hepatic endothelial cells (27, 34), whereas glycoproteins bearing structures that terminate with Siaα2,6GalNAcβ1,4GlcNAc are cleared from the blood by the ASGP-R, located in hepatic parenchymal cells (39, 40, 46). LH-bearing terminal Siaα2,3Galβ1,4GlcNAc is not removed from the blood by these receptors but is instead removed by filtration in the kidney, resulting in a half-life that is on the order of 30 minutes (35). A change in the circulatory half-life of LH resulting from a change in its pattern of terminal glycosylation could account for the phenotype we observe in GalNAc-4-ST1–/– mice.
Previous studies of half-life have relied on the injection of LH purified from various exogenous sources. In the current experiments, we have examined the half-life of endogenous LH in WT and GalNAc-4-ST1–/– mice by acutely blocking the release of pituitary LH with the GnRH antagonist acyline. The exponential decay curve obtained in WT mice is most consistent with clearance by a single receptor system with a half-life of 7.2 minutes. The curve obtained in GalNAc-4-ST1–/– mice also indicates clearance by a single receptor system but with a half-life of 10.1 minutes. Thus, in WT mice, LH predominantly bears structures that terminate with SO4-4-GalNAcβ1,4GlcNAc and is cleared by the Man/GalNAc-4-SO4-R, whereas in GalNAc-4-ST1–/– mice, LH predominantly bears structures that terminate with Siaα2,6GalNAcβ1,4GlcNAc and is cleared by the ASGP-R. The amount of either terminal GalNAc or Siaα2,3/6Galβ1,4GlcNAc on LH from GalNAc-4-ST1–/– mice is not sufficient to discern either a component that clears with a half-life of less than 3 minutes or a half-life of more than 30 minutes. The increased circulatory half-life of LH provides an explanation for the elevated serum levels of LH in GalNAc-4-ST1–/– mice in the face of similar rates of synthesis and secretion in castrated mice.
There are indications that the pattern of terminal glycosylation on LH may be modulated by end organs such as the ovary in vivo. For example, levels of the protein-specific β1,4GalNAcT in pituitary rise and fall in response to estrogen levels in parallel with the levels of LH (47). Furthermore, we have observed that the levels of β1,4GalNAcT3, GalNAc-4-ST1, and α2,6-sialyltransferase mRNA in the pituitary are modulated by estradiol (our unpublished observations). Changes in the levels of these transferases could alter the proportion of oligosaccharides terminating with SO4-4-GalNAcβ1,4GlcNAc-, and Siaα2,6GalNAcβ1,4GlcNAc-, which are present on LH and thus increase or decrease the rate of LH clearance. Altering the rate of LH clearance provides a mechanism for modulating the amount of steroid hormone produced by the HPG axis in response to the same signal. The pattern of terminal glycosylation may determine the strength of the signal generated by the same amount of LH across the entire HPG axis to produce the steroid hormones estrogen, progesterone, and T, as illustrated schematically in Figure 10. The importance of this complex system is supported by its uniqueness and by its conservation throughout vertebrate evolution. The specificity of the transferases, the specificity of the receptors, the response to estradiol, and the novelty of the carbohydrate structure itself indicate that this system is specifically geared to modulate the signal strength of the HPG axis, leading to steroid hormone production. Since human LH, like murine LH, has N-linked oligosaccharides that terminate with SO4-4-GalNAcβ1,4GlcNAc-, changes in the pattern of terminal glycosylation or clearance by the Man/GalNAc-4-SO4-R have the potential to increase or decrease the potency of the hormone in vivo. Such changes could have an impact on human fertility or the onset of puberty.
There are a number of glycoproteins synthesized in other tissues that are also selectively modified with terminal GalNAc-4-SO4, for example, the LDL-receptor homolog SorLA/LR11 expressed in the kidney and the brain (48) and tenascin-R expressed in the brain (49). It is possible that additional differences between WT and GalNAc-4-ST1–/– mice will be identified upon further examination; however, none of the other glycoproteins identified thus far as bearing structures terminating with SO4-4-GalNAcβ1,4GlcNAc would be expected to have an impact on steroid hormone production or reproduction.
The outcome of increased LH levels resulting from altered terminal glycosylation of LH in the GalNAc-4-ST1–/– mice is thus a strongly enhanced reproductive drive. LH levels and steroid hormone levels are elevated in both male and female GalNAc-4-ST1–/– mice. Furthermore, both male and female mice display precocious and/or exaggerated sexual development. This suggests that an increase in the strength of the signal produced by the HPG axis in GalNAc-4-ST1–/– mice results in steroid hormone production sufficiently enhanced for sexual development to proceed at a significantly earlier time, whereas a lesser signal strength in WT mice delays their sexual maturation until several days later. It is possible that the structure of the carbohydrates on LH is normally modulated at the time of sexual maturation in order to produce more steroid hormone in response to signals from the HPG axis.
LH elevation in the GalNAc-4-ST1–/– mice yields a distinctive phenotype of enhanced reproduction that stands in marked contrast to prior models of elevated LH where high levels of exogenous LH or human chorionic gonadotropin (hCG) have been produced by transgenic means. Expression of α and/or β subunits under control of the LHα subunit, ubiquitin, or metallothionein-1 promoters has yielded 15-fold or greater elevation of LH levels that is not consistently found in both sexes. Although some female mice have shown fertility or precocious puberty, the predominant pattern in these models has been extensive reproductive defects such as infrequent ovulation, disrupted estrus cycles, hemorrhagic polycystic ovaries, ovarian granulosa/theca cell tumors, and infertility in the face of elevated estrogen and T levels, the latter with enlarged seminal vesicles (50–56). The across-the-board enhancement of reproductive potential in both male and female GalNAc-4-ST1–/– mice indicates that this model is uniquely informative regarding the mechanisms of reproductive control.
The Man/GalNAc-4-SO4-R and the ASGP-R are major carbohydrate- specific endocytic receptors that are expressed by hepatic endothelial and parenchymal cells, respectively. Due to their abundance and rapid rates of internalization, both receptors have the capacity to remove large amounts of glycoproteins bearing carbohydrates that are recognized from the blood. The concentration of glycoprotein hormones such as LH in the blood is precisely regulated and must reflect the rate of synthesis and secretion as well as the rate of removal from the circulation. The Man/GalNAc-4-SO4-R and the ASGP-R are ideally suited to regulating the circulating levels of LH by determining the rate of clearance of different glycoforms being synthesized in the pituitary.
Regulation of the fluctuating levels of circulating LH is essential for the complex process of steroidogenesis. Our studies demonstrate that the unique carbohydrate structures present on LH play a critical role in maintaining the levels of LH seen in the blood. Altering the combination of specific glycoforms on LH changes its rate of removal from the blood and has significant physiologic consequences. Increased T in male mice results in early sexual maturation, more aggressive behavior in the form of barbering, and increased size of secondary sex organs such as seminal vesicles. Increased estrogen and progesterone in female mice results in early sexual maturation and increased reproductive capability due to more frequent litters as well as better mothering. Thus, the carbohydrate structures on LH are essential for regulation of the HPG axis and represent a major driving force for reproductive capability.
Methods
Ablation of GalNAc-4-ST1.
The carboxyterminal 306 amino acids of GalNAc-4-ST1 are encoded by exon 4 of the GalNAc-4-ST1 gene (31), CHST-8, gene 68948. A 129/SvJ ES BAC library (Incyte) was screened with a probe derived from the region encoding the catalytic domain of mouse GalNAc-4ST1. A 14-kb Xba fragment containing exon 3 and exon 4 of the GalNAc-4-ST1 gene was isolated from the identified BAC clone and subcloned into pZero (Invitrogen). A loxP site was introduced in 5′ of exon 2 by inverse PCR amplification using KlenTaq Long and Accurate DNA Polymerase (KTLA polymerase) (57–59). (Figure 2). A cassette containing the neomycin phosphotransferase cDNA driven by the phosphoglycerate kinase promoter (PGK-neo) flanked by loxP sites was inserted immediately 3′ to exon 4. The targeting construct was introduced into RW-4 ES cells by electroporation using established protocols developed in the Siteman Cancer Center Murine Embryonic Stem Cell Core (Washington University) and available online at http://escore.im.wustl.edu. Clones were selected for growth in the presence of G418. ES clones that had undergone homologous recombination were identified by Southern blot using 5′ and 3′ probes external to the 14-kb fragment. An ES cell clone that had undergone homologous recombination was transfected with Pturbo-CRE (GenBank Accession Number AF334827) and subcloned by dilution. ES cell clones were screened by Southern analysis for the removal of exon 3, exon 4, and the PGK-neo cassette. Positive clones were injected into C57BL/6J blastocysts. Chimeric males were mated with C57BL/6J females to generate mice heterozygous for ablation of exon 3 and exon 4 of the GalNAc-4-ST1 gene, GalNAc-4-ST1+/–. GalNAc-4-ST1+/– mice (Figure 1B) were backcrossed with C57BL/6J mice for 5 generations to generate GalNAc-4-ST1–/– mice on a C57BL/6J background. All experiments involving mice were approved by the Washington University School of Medicine Animal Studies Committee.
Serum collection.
Mice were anesthetized with ketamine (87 mg/kg) and zylazine (13 mg/kg) for terminal blood collection by cardiac puncture. Blood was allowed to clot for 90 minutes at 25°C and then sedimented for 15 minutes at 25°C in a centrifuge. The serum was removed and stored at –80°C until analysis.
Clearance of endogenous LH.
Mice were anesthetized with ketamine and zylazine. A baseline, time zero, sample of 150 μl of blood was drawn. For each mouse, 10 μg of acyline (courtesy of P. Michael Conn, Oregon Health and Science University, Portland, Oregon, USA) was injected intravenously. Blood (150 μl) was withdrawn at the times indicated. After each withdrawal, 200 μl of warm saline was injected into the peritoneum.
Hormone assays.
Quantitation of LH, FSH, T, estrogen, and progesterone was performed by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (Charlottesville, Virginia, USA).
Castration.
Male mice were castrated 5 days prior to collection of serum for analysis of hormone levels. The mice were anesthetized with ketamine and zylazine. A 2-cm ventral midline incision was made in the scrotum. The tunica was pierced, and the testis was pushed out by gentle pressure. The spermatic artery was cauterized and the testis removed. The epididymis, deferential vessels, and ductus were replaced in the tunica. The incision was closed with wound clips.
Staging of estrus cycle.
Estrus cycle length and regularity were assessed by taking daily vaginal smears from female mice at the same time for 20 consecutive days. Dried smears were stained with Wright stain and examined microscopically. The stage of the estrus cycle was determined according to the criteria of Allen (60).
Tissue extraction.
Mouse pituitaries were removed and frozen at –80°C until extraction. Pituitaries from 5 mice were pooled and extracted with 150 μl of ice cold T-PER tissue protein extraction reagent (Pierce) containing Complete Protease Inhibitor EDTA Free (CPI-EDTA Free) (Roche Molecular Biochemicals). The pituitaries were sonicated at 4°C in a Mettler Ultrasonic water bath for 45 minutes with occasional vortexing followed by 2 freeze-thaw cycles with sonication for 10 minutes between each cycle. The samples were sedimented at 12,000 g for 15 minutes and supernatants taken for analysis.
Neuraminidase digestion.
Samples were adjusted to a final concentration of 50 mM sodium acetate, pH 5.0, and incubated at 37°C overnight with or without the addition of neuraminidase from A. ureafaciens (Roche Chemical).
Lectin binding.
WFA and SNA-1 immobilized on agarose (EY Laboratories Inc.) were washed with cold T-PER containing CPI-EDTA Free and packed into Handee Mini-Spin columns (Pierce). Samples were incubated with the immobilized lectins for 3 hours at 25°C with gentle agitation. Unbound material was collected and passed over the agarose beads 3 additional times. The column was then washed with 10 column volumes of cold T-PER containing CPI-EDTA Free. Bound glycoproteins were eluted by incubating with T-PER containing CPI-EDTA Free, 0.01% bovine serum albumin, and 50 mM GalNAc for WFA-agarose or 100 mM lactose for SNA-1–agarose. Remaining material not eluted with the free sugars was recovered by heating the beads in SDS-PAGE loading buffer. The starting material, unbound fractions, and bound fractions were analyzed by Western blotting.
Western blot analyses.
Samples were analyzed by SDS-PAGE using NuPage 12% Bis-Tris gels with MOPS buffers (Invitrogen). Following electrophoretic transfer to PVDF membranes (Millipore), the membranes were blocked with 1% casein (Hammersten grade; BDH Chemicals) and then analyzed using Super Signal West Femto Maximum Sensitivity Substrate (Pierce) for detection of HRP-labeled probes. HRP-Cys-Fc was prepared by conjugating HRP to Cys-Fc using the EZ-link Activated Peroxidase Antibody Labeling Kit (Pierce). Incubations with probes were carried out for 24–72 hours at 4°C. Rabbit antibody to bovine LHβ was prepared as described previously (61). Antibody to α was a gift from I. Boime (Washington University). Bound rabbit antibodies were detected with HRP goat-anti-rabbit IgG (Pierce). Monoclonal antibody Mab6.3 was prepared by immunization with SO4-4-GalNAcβ1,4GlcNAcβ1,2Man-BSA (27, 62) and detected using HRP goat anti-mouse IgM (Pierce). Mab6.3 reacts with SO4-4-GalNAcβ1,4GlcNAcβ1,2Man-BSA but not with SO4-3-GalNAcβ1,4GlcNAcβ1,2Man-BSA.
Statistics.
Statistical analyses and curve fitting were performed using GraphPad Prism 4.0. Half-lives from clearance study data were determined using the formula for one-phase exponential decay for nonlinear regression analyses. Significance was determined as indicated using the unpaired t test with the options set to 2 tailed and a confidence interval of 95%.
Acknowledgments
This work was supported by NIH grant R01-DK41738 to J.U. Baenziger. Hormone assays were performed by the University of Virginia Center for Research in Reproduction Ligand and Analysis Core, which is supported by the National Institute of Child Health and Human Development (Specialized Cooperative Centers Program in Reproduction and Infertility Research) grant U54-HD28934. Acyline was generously provided by P. Michael Conn, Oregon Health and Science University. Antibody to the α subunit was provided by I. Boime, Washington University. The Embryonic Stem Cell Core at the Siteman Cancer Center assisted with ES cell propagation and transfection. We thank Nancy L. Baenziger, Stuart Kornfeld, and Prema Narayan for their thoughtful comments.
Footnotes
Nonstandard abbreviations used: ASGP-R, asialoglycoprotein receptor; FSH, follicle-stimulating hormone; GalNAc, N-acetylgalactosamine; GalNAc-4-ST, GalNAc-4-sulfotransferase; GlcNAc, N-acetylglucosamine; GnRH, gonadotropin-releasing hormone; HPG, hypothalamic-pituitary-gonad; LH, luteinizing hormone; Man, mannose; Sia, sialic acid; SNA-1, Sambucus nigra agglutinin-1; T, testosterone; WFA, Wisteria floribunda agglutinin.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:1815–1824 (2008). doi:10.1172/JCI32467
References
- 1.Ying S.Y. Inhibins, activins, and follistatins: gonadal proteins modulating the secretion of follicle-stimulating hormone. Endocr. Rev. 1988;9:267–293. doi: 10.1210/edrv-9-2-267. [DOI] [PubMed] [Google Scholar]
- 2. Strauss, J.F., 3rd, and Penning, T.M. 1999. Synthesis of the sex steroid hormones: molecular and structural biology with applications to clinical practise. InMolecular biology in reproductive medicine. B.C.J.M. Fauser, A.J. Rutherford, J.F. Strauss, 3rd, and A. Van Steirteghem, editors. The Parthenon Publishing Group. Carnforth, United Kingdom. 201–232. [Google Scholar]
- 3. Bousfield, G.R., Perry, W.M., and Ward, D.N. 1994. Gonadotropins. Chemistry and biosynthesis. InThe physiology of reproduction. E. Knobil, and J.D. Neill, editors. Raven Press. New York, New York, USA. 1749–1792. [Google Scholar]
- 4.Clarke I.J., Cummins J.T. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111:1737–1739. doi: 10.1210/endo-111-5-1737. [DOI] [PubMed] [Google Scholar]
- 5.Belchetz P.E., Plant T.M., Nakai Y., Keogh E.J., Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202:631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- 6.Marshall J.C., Kelch R.P. Low dose pulsatile gonadotropin-releasing hormone in anorexia nervosa: a model of human pubertal development. J. Clin. Endocrinol. Metab. 1979;49:712–718. doi: 10.1210/jcem-49-5-712. [DOI] [PubMed] [Google Scholar]
- 7.Ascoli M., Fanelli F., Segaloff D.L. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr. Rev. 2002;23:141–174. doi: 10.1210/er.23.2.141. [DOI] [PubMed] [Google Scholar]
- 8.Achermann J.C., Ozisik G., Meeks J.J., Jameson J.L. Genetic causes of human reproductive disease. J. Clin. Endocrinol. Metab. 2002;87:2447–2454. doi: 10.1210/jc.87.6.2447. [DOI] [PubMed] [Google Scholar]
- 9.Achermann J.C., Jameson J.L. Fertility and infertility: genetic contributions from the hypothalamic-pituitary-gonadal axis. Mol. Endocrinol. 1999;13:812–818. doi: 10.1210/me.13.6.812. [DOI] [PubMed] [Google Scholar]
- 10.Seminara S.B., Crowley W.F., Jr. Perspective: the importance of genetic defects in humans in elucidating the complexities of the hypothalamic-pituitary-gonadal axis. Endocrinology. 2001;142:2173–2177. doi: 10.1210/en.142.6.2173. [DOI] [PubMed] [Google Scholar]
- 11.Themmen A.P.N., Huhtaniemi I.T. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr. Rev. 2000;21:551–583. doi: 10.1210/er.21.5.551. [DOI] [PubMed] [Google Scholar]
- 12.Manzella S.M., Hooper L.V., Baenziger J.U. Oligosaccharides containing β1,4-linked N-acetylgalactosamine, a paradigm for protein-specific glycosylation. J. Biol. Chem. 1996;271:12117–12120. doi: 10.1074/jbc.271.21.12117. [DOI] [PubMed] [Google Scholar]
- 13.Combarnous Y. Molecular basis of the specificity of binding of glycoprotein hormones to their receptors. Endocr. Rev. 1992;13:670–691. doi: 10.1210/er.13.4.670. [DOI] [PubMed] [Google Scholar]
- 14.Manzella S.M., et al. Evolutionary conservation of the sulfated oligosaccharides on vertebrate glycoprotein hormones that control circulatory half-life. J. Biol. Chem. 1995;270:21665–21671. doi: 10.1074/jbc.270.37.21665. [DOI] [PubMed] [Google Scholar]
- 15.Baenziger J.U., Green E.D. Pituitary glycoprotein hormone oligosaccharides: structure, synthesis and function of the asparagine-linked oligosaccharides on lutropin, follitropin and thyrotropin. Biochim. Biophys. Acta. 1988;947:287–306. doi: 10.1016/0304-4157(88)90012-3. [DOI] [PubMed] [Google Scholar]
- 16.Pierce J.G., Parsons T.F. Glycoprotein hormones: structure and function. Annu. Rev. Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- 17.Parsons T.F., Pierce J.G. Oligosaccharide moieties of glycoprotein hormones: bovine lutropin resists enzymatic deglycosylation because of terminal O-sulfated N-acetylhexosamines. Proc. Natl. Acad. Sci. U. S. A. 1980;77:7089–7093. doi: 10.1073/pnas.77.12.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green E.D., Baenziger J.U. Asparagine-linked oligosaccharides on lutropin, follitropin, and thyrotropin: II. Distributions of sulfated and sialylated oligosaccharides on bovine, ovine, and human glycoprotein hormones. J. Biol. Chem. 1988;263:36–44. [PubMed] [Google Scholar]
- 19.Green E.D., Baenziger J.U. Asparagine-linked oligosaccharides on lutropin, follitropin, and thyrotropin: I. Structural elucidation of the sulfated and sialylated oligosaccharides on bovine, ovine, and human pituitary glycoprotein hormones. J. Biol. Chem. 1988;263:25–35. [PubMed] [Google Scholar]
- 20.Green E.D., Van Halbeek H., Boime I., Baenziger J.U. Structural elucidation of the disulfated oligosaccharide from bovine lutropin. J. Biol. Chem. 1985;260:15623–15630. [PubMed] [Google Scholar]
- 21.Mengeling B.J., Manzella S.M., Baenziger J.U. A cluster of basic amino acids within an a-helix is essential for a-subunit recognition by the glycoprotein hormone N-acetylgalactosaminyltransferase. Proc. Natl. Acad. Sci. U. S. A. 1995;92:502–506. doi: 10.1073/pnas.92.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith P.L., Baenziger J.U. Molecular basis of recognition by the glycoprotein hormone- specific N-acetylgalactosamine-transferase. . Proc. Natl. Acad. Sci. U. S. A. 1992;89:329–333. doi: 10.1073/pnas.89.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baenziger, J.U., and Green, E.D. 1991. Structure, synthesis, and function of the asparagine-linked oligosaccharides on pituitary glycoprotein hormones. InBiology of carbohydrates, volume 3. V. Ginsberg and P.W. Robbins, editors. JAI Press Ltd. London, United Kingdom. 1–46. [Google Scholar]
- 24.Smith P.L., Baenziger J.U. Recognition by the glycoprotein hormone-specific N-acetylgalactosaminetransferase is independent of hormone native conformation. . Proc. Natl. Acad. Sci. U. S. A. 1990;87:7275–7279. doi: 10.1073/pnas.87.18.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith P.L., Baenziger J.U. A pituitary N-acetylgalactosamine transferase that specifically recognizes glycoprotein hormones. Science. 1988;242:930–933. doi: 10.1126/science.2460923. [DOI] [PubMed] [Google Scholar]
- 26.Fiete D., Baenziger J.U. Isolation of the SO4-4-GalNAcβ1,4GlcNAcβ1,2Manα-specific receptor from rat liver. . J. Biol. Chem. 1997;272:14629–14637. doi: 10.1074/jbc.272.23.14629. [DOI] [PubMed] [Google Scholar]
- 27.Fiete D., Srivastava V., Hindsgaul O., Baenziger J.U. A hepatic reticuloendothelial cell receptor specific for SO4-4GalNAcβ1,4GlcNAcβ1,2Manα that mediates rapid clearance of lutropin. . Cell. 1991;67:1103–1110. doi: 10.1016/0092-8674(91)90287-9. [DOI] [PubMed] [Google Scholar]
- 28.Fiete D.J., Beranek M.C., Baenziger J.U. A cysteine-rich domain of the “mannose” receptor mediates GalNAc-4-SO4 binding. . Proc. Natl. Acad. Sci. U. S. A. 1998;95:2089–2093. doi: 10.1073/pnas.95.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiete D., Beranek M.C., Baenziger J.U. The macrophage/endothelial cell mannose receptor cDNA encodes a protein that binds oligosaccharides terminating with SO4-4-GalNAcβ1,4GlcNAcβ or Man at independent sites. . Proc. Natl. Acad. Sci. U. S. A. 1997;94:11256–11261. doi: 10.1073/pnas.94.21.11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang H.G., Evers M.R., Xia G., Baenziger J.U., Schachner M. Molecular cloning and expression of an N-acetylgalactosamine-4-O-sulfotransferase that transfers sulfate to terminal and non-terminal beta 1,4-linked N-acetylgalactosamine. J. Biol. Chem. 2001;276:10861–10869. doi: 10.1074/jbc.M011560200. [DOI] [PubMed] [Google Scholar]
- 31.Xia G., Evers M.R., Kang H.-G., Schachner M., Baenziger J.U. Molecular cloning and expression of the pituitary glycoprotein hormone N-acetylgalactosamine-4-O-sulfotransferase. J. Biol. Chem. 2000;275:38402–38409. doi: 10.1074/jbc.M007821200. [DOI] [PubMed] [Google Scholar]
- 32.Hiraoka N., Misra A., Belot F., Hindsgaul O., Fukuda M. Molecular cloning and expression of two distinct human N-acetylgalactosamine 4-O-sulfotransferases that transfer sulfate to GalNAcβ14GlcNAcβ1R in both N- and O-glycans. Glycobiology. 2001;11:495–504. doi: 10.1093/glycob/11.6.495. [DOI] [PubMed] [Google Scholar]
- 33.Boregowda R.K., Mi Y., Bu H., Baenziger J.U. Differential expression and enzymatic properties of GalNAc-4-sulfotransferase-1 and GalNAc-4-sulfotransferase-2. Glycobiology. 2005;15:1349–1358. doi: 10.1093/glycob/cwj024. [DOI] [PubMed] [Google Scholar]
- 34.Mi Y.-L., Shapiro S.D., Baenziger J.U. Regulation of lutropin circulatory half-life by the mannose/N-acetylgalactosamine-4-SO4-receptor is critical for implantation in vivo. . J. Clin. Invest. 2002;109:269–276. doi: 10.1172/JCI13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baenziger J.U., Kumar S., Brodbeck R.M., Smith P.L., Beranek M.C. Circulatory half-life but not interaction with the lutropin/chorionic gonadotropin receptor is modulated by sulfation of bovine lutropin oligosaccharides. Proc. Natl. Acad. Sci. U. S. A. 1992;89:334–338. doi: 10.1073/pnas.89.1.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith P.L., Kaetzel D., Nilson J., Baenziger J.U. The sialylated oligosaccharides of recombinant bovine lutropin modulate hormone bioactivity. J. Biol. Chem. 1990;265:874–881. [PubMed] [Google Scholar]
- 37.Rivier J.E., et al. Gonadotropin-releasing hormone antagonists: novel members of the azaline B family. J. Med. Chem. 1995;38:2649–2662. doi: 10.1021/jm00014a017. [DOI] [PubMed] [Google Scholar]
- 38.Herbst K.L., Anawalt B.D., Amory J.K., Bremner W.J. Acyline: the first study in humans of a potent, new gonadotropin-releasing hormone antagonist. J. Clin. Endocrinol. Metab. 2002;87:3215–3220. doi: 10.1210/jc.87.7.3215. [DOI] [PubMed] [Google Scholar]
- 39.Park E.I., Manzella S.M., Baenziger J.U. Rapid clearance of sialylated glycoproteins by the asialoglycoprotein receptor. J. Biol. Chem. 2003;278:4597–4602. doi: 10.1074/jbc.M210612200. [DOI] [PubMed] [Google Scholar]
- 40.Park E.I., Mi Y., Unverzagt C., Gabius H.J., Baenziger J.U. The asialoglycoprotein receptor clears glycoconjugates terminating with sialic acid alpha 2,6GalNAc. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17125–17129. doi: 10.1073/pnas.0508537102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashwell G., Harford J. Carbohydrate-specific receptors of the liver. Annu. Rev. Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- 42.Mengeling B.J., et al. A microplate assay for analysis of solution phase glycosyltransferase reactions: determination of kinetic constants. Anal. Biochem. 1991;199:286–292. doi: 10.1016/0003-2697(91)90103-Z. [DOI] [PubMed] [Google Scholar]
- 43.Torres B.R., McCrumb D.K., Smith D.F. Glycolipid-lectin interactions: reactivity of lectins from helix pomatia, wisteria floribunda, and dolichos biflorus with glycolipids containing N-acetylgalactosamine. Arch. Biochem. Biophys. 1988;262:1–11. doi: 10.1016/0003-9861(88)90161-0. [DOI] [PubMed] [Google Scholar]
- 44.Van Damme E.J., Barre A., Rouge P., Van Leuven F., Peumans W.J. The NeuAc(alpha-2,6)-Gal/GalNAc-binding lectin from elderberry (Sambucus nigra) bark, a type-2 ribosome-inactivating protein with an unusual specificity and structure. Eur. J. Biochem. 1996;235:128–137. doi: 10.1111/j.1432-1033.1996.00128.x. [DOI] [PubMed] [Google Scholar]
- 45.Shibuya N., et al. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2-6)Gal/GalNAc sequence. J. Biol. Chem. 1987;262:1596–1601. [PubMed] [Google Scholar]
- 46.Park E.I., Baenziger J.U. Closely related mammals have distinct asialoglycoprotein receptor carbohydrate specificities. J. Biol. Chem. 2004;279:40954–40959. doi: 10.1074/jbc.M406647200. [DOI] [PubMed] [Google Scholar]
- 47.Dharmesh S.M., Baenziger J.U. Estrogen modulates expression of the glycosyltransferases that synthesize sulfated oligosaccharides on lutropin. Proc. Natl. Acad. Sci. U. S. A. 1993;90:11127–11131. doi: 10.1073/pnas.90.23.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fiete D., Mi Y., Oats E.L., Beranek M.C., Baenziger J.U. N-linked oligosaccharides on the low density lipoprotein receptor homolog SorLA/LR11 are modified with terminal GalNAc-4-SO4 in kidney and brain. J. Biol. Chem. 2007;282:1873–1881. doi: 10.1074/jbc.M606455200. [DOI] [PubMed] [Google Scholar]
- 49.Woodworth A., Fiete D., Baenziger J.U. Tenascin-R in the cerebellum is modified with N-linked oligosaccharides terminating with GalNAc-4-SO4 [abstract]. . Glycobiology. 2001;11:45. [Google Scholar]
- 50.Kero J., et al. Elevated luteinizing hormone induces expression of its receptor and promotes steroidogenesis in the adrenal cortex. J. Clin. Invest. 2000;105:633–641. doi: 10.1172/JCI7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Risma K.A., Hirshfield A.N., Nilson J.H. Elevated luteinizing hormone in prepubertal transgenic mice causes hyperandrogenemia, precocious puberty, and substantial ovarian pathology. Endocrinology. 1997;138:3540–3547. doi: 10.1210/en.138.8.3540. [DOI] [PubMed] [Google Scholar]
- 52.Risma K.A., et al. Targeted overexpression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proc. Natl. Acad. Sci. U. S. A. 1995;92:1322–1326. doi: 10.1073/pnas.92.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matzuk M.M., DeMayo F.J., Hadsell L.A., Kumar T.R. Overexpression of human chorionic gonadotropin causes multiple reproductive defects in transgenic mice. Biol. Reprod. 2003;69:338–346. doi: 10.1095/biolreprod.102.013953. [DOI] [PubMed] [Google Scholar]
- 54.Ahtiainen P., et al. Phenotypic characterisation of mice with exaggerated and missing LH/hCG action. Mol. Cell. Endocrinol. 2007;260–262:255–263. doi: 10.1016/j.mce.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 55.Rulli S.B., et al. Elevated steroidogenesis, defective reproductive organs, and infertility in transgenic male mice overexpressing human chorionic gonadotropin. Endocrinology. 2003;144:4980–4990. doi: 10.1210/en.2003-0403. [DOI] [PubMed] [Google Scholar]
- 56.Rulli S.B., et al. Reproductive disturbances, pituitary lactotrope adenomas, and mammary gland tumors in transgenic female mice producing high levels of human chorionic gonadotropin. Endocrinology. 2002;143:4084–4095. doi: 10.1210/en.2002-220490. [DOI] [PubMed] [Google Scholar]
- 57.Cheng S., Fockler C., Barnes W.M., Higuchi R. Effective amplification of long targets from cloned inserts and human genomic DNA. Proc. Natl. Acad. Sci. U. S. A. 1994;91:5695–5699. doi: 10.1073/pnas.91.12.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barnes W.M. PCR amplification of up to 35-kb DNA with high fidelity and high yield from lambda bacteriophage templates. Proc. Natl. Acad. Sci. U. S. A. 1994;91:2216–2220. doi: 10.1073/pnas.91.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Barnes, W.M. 2003. InPCR primer: a laboratory manual. C.W.a.D. Dieffenbach, G.S., editor. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, New York, USA. 441–449. [Google Scholar]
- 60.Allen E. The oestrus cycle in the mouse. Am. J. Anat. 1922;30:297–371. doi: 10.1002/aja.1000300303. [DOI] [Google Scholar]
- 61.Green E.D., Baenziger J.U., Boime I. Cell-free sulfation of human and bovine pituitary hormones: comparison of the sulfated oligosaccharides of lutropin, follitropin, and thyrotropin. J. Biol. Chem. 1985;260:15631–15638. [PubMed] [Google Scholar]
- 62.Hindsgaul V., Hindsgaul O., Baenziger J.U. Synthesis of oligosaccharide structures unique to pituitary glycoprotein hormones. Can. J. Chem. 1987;65:1645–1652. [Google Scholar]