Abstract
Increased albuminuria is associated with obesity and diabetes and is a risk factor for cardiovascular and renal disease. However, the link between early albuminuria and adiposity remains unclear. To determine whether adiponectin, an adipocyte-derived hormone, is a communication signal between adipocytes and the kidney, we performed studies in a cohort of patients at high risk for diabetes and kidney disease as well as in adiponectin-knockout (Ad–/–) mice. Albuminuria had a negative correlation with plasma adiponectin in obese patients, and Ad–/– mice exhibited increased albuminuria and fusion of podocyte foot processes. In cultured podocytes, adiponectin administration was associated with increased activity of AMPK, and both adiponectin and AMPK activation reduced podocyte permeability to albumin and podocyte dysfunction, as evidenced by zona occludens–1 translocation to the membrane. These effects seemed to be caused by reduction of oxidative stress, as adiponectin and AMPK activation both reduced protein levels of the NADPH oxidase Nox4 in podocytes. Ad–/– mice treated with adiponectin exhibited normalization of albuminuria, improvement of podocyte foot process effacement, increased glomerular AMPK activation, and reduced urinary and glomerular markers of oxidant stress. These results suggest that adiponectin is a key regulator of albuminuria, likely acting through the AMPK pathway to modulate oxidant stress in podocytes.
Introduction
Chronic kidney disease and albuminuria have recently been recognized as among the most significant clinical risk factors for cardiovascular disease (CVD), hospitalization, and all-cause mortality (1–5). Microalbuminuria (albumin/creatinine ratio [ACR], 30–300 μg/mg), resulting from leakage of albumin across the glomerular podocyte filtration barrier into the urine, is considered a marker for dysfunction of the systemic vasculature and indicates a heightened risk of CVD (1, 2, 6). Recently, albuminuria in the so-called high normal range (ACR, 10–30 μg/mg) has been identified as a risk factor for CVD (7). Renal dysfunction may contribute to overall CVD by promoting vascular thickening and vascular calcification (8) as well as by activating inflammatory pathways (9). It has also been recognized that insulin resistance is closely associated with oxidant stress, early decline in renal function, and albuminuria (1, 10). Despite the close relationships demonstrated to exist between CVD and kidney dysfunction, the mechanism linking these entities has not yet been elucidated.
Adiponectin, a 30-kDa circulating plasma protein primarily secreted by adipocytes, has been recently recognized to be a key predictive factor for cardiovascular mortality in patients with renal dysfunction (9, 11). Adiponectin is found at relatively high total levels in the bloodstream of human subjects, ranging in concentration from 5 to 30 μg/ml. Adiponectin is secreted from adipose tissue and circulates in multimeric forms ranging from trimers to high–molecular weight oligomers containing 12- to 18-mers (12). Interestingly, a recombinant fragment of adiponectin containing the C-terminal globular head domain interacts with cellular adiponectin receptors and mimics many of the actions of the full-length, oligomeric form of the protein (12). The globular domain can be generated by cleavage from the full-length protein by neutrophil elastase and other proteases (13, 14), although it remains controversial whether the globular fragment of adiponectin is generated in situ or circulates in vivo. Both forms of adiponectin exert largely beneficial effects to improve insulin sensitivity and decrease the adverse effects of inflammatory mediators in vascular cells (12, 15). The globular and full-length forms of adiponectin (gAd and fAd, respectively) both bind to 2 adiponectin receptors (AdipoR1 and AdipoR2) (16) and signal via stimulation of 5′-AMP activated protein kinase (AMPK) and potentially other intracellular pathways (16, 17). Protective effects of adiponectin may involve reduction of oxidant stress, possibly by inhibition of NADPH oxidases, as our group has shown in endothelial cells (17) and in myocardial tissue (18). Plasma adiponectin levels are reduced with increasing visceral obesity and tightly correlate with insulin resistance and development of type 2 diabetes mellitus (19). Interestingly, in patients with type 1 diabetes, a SNP in the adiponectin promoter showed linkage with diabetic nephropathy (20), and low plasma adiponectin levels were predictive of the development of coronary artery calcification (21, 22), which suggests an important role for adiponectin in development of kidney and macrovascular disease with hyperglycemia.
In the African-American (AA) population, low plasma adiponectin levels have been reported in obese subjects and may be predictive of the development of type 2 diabetes (23, 24). Of note, both diabetes (25) and AA ethnicity are strong predisposing risk factors for progressive kidney disease (26). Although both adiponectin levels and albuminuria are associated with CVD and kidney dysfunction, previous studies attempting to link adiponectin with albuminuria have been inconclusive (27–29). To our knowledge, a causative role for adiponectin in the development of albuminuria in its early stages has not previously been demonstrated.
In the present study, we found that circulating adiponectin levels had a strong negative correlation with the degree of albuminuria in nondiabetic obese AA subjects. In the adiponectin knockout (Ad–/–) mouse, urinary levels of albumin and hydrogen peroxide were increased, and podocyte foot process effacement was evident. In cell culture studies with podocytes, adiponectin potently decreased permeability to albumin and induced translocation of zona occludens–1 (ZO-1) to the plasma membrane, largely via an AMPK-dependent pathway. In addition, adiponectin reduced the renal predominant NADPH oxidase Nox4 (30) in podocytes. Treatment of Ad–/– mice with exogenous adiponectin decreased urine albumin and urinary hydrogen peroxide in association with a marked improvement in podocyte morphology, increased glomerular AMPK activity, and reduced glomerular Nox4. Diabetic Ad–/– mice also had marked increases in albuminuria that were reduced by exogenous adiponectin. Our data thus provide what we believe to be the first evidence that podocytes are a direct target of adipocyte action.
Results
Low adiponectin levels correlate with albuminuria in obese AAs.
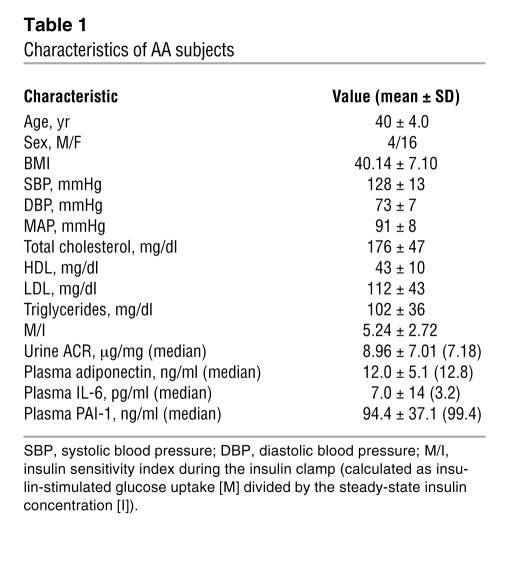
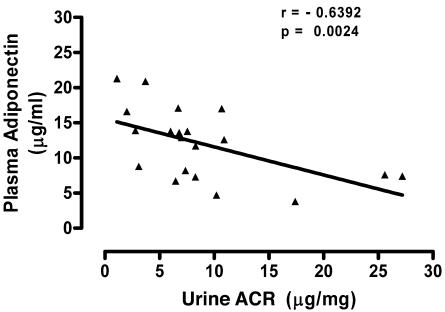
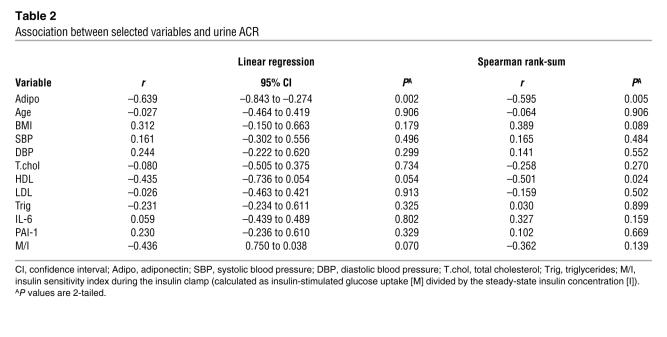
A total of 20 obese AA subjects who were on no medications for lipid or glycemic control were examined for relationships between ACR and adipocytokines. Subject characteristics are presented in Table 1. Serum creatinine was normal (≤1.4 mg/dl) and ACR was below 30 μg/mg in all subjects. In this sample of obese subjects, there was a statistically significant negative correlation between plasma adiponectin concentration and urinary albumin excretion, expressed as log-transformed urine ACR (r = –0.639, P < 0.01; Figure 1 and Table 2). Of the other variables (Table 2), there was a significant correlation between ACR and HDL by the Spearman rank-sum test (r = –0.501; P = 0.024), but the correlation did not reach significance by Pearson linear regression (P = 0.054). No significant correlations between ACR and plasma levels of IL-6 or plasminogen activator inhibitor–1 (PAI-1) were found. There were no significant correlations between ACR and other clinical parameters including BMI, insulin sensitivity, blood pressure, total cholesterol, LDL, and triglycerides.
Table 1 .
Characteristics of AA subjects
Figure 1. Negative correlation between albuminuria and plasma adiponectin levels in obese AAs.
Data show regression between adiponectin levels and urine ACRs. Confidence intervals, Spearman’s correlation coefficient, and P values for all variables tested are listed in Table 2.
Table 2 .
Association between selected variables and urine ACR
Increased albuminuria and oxidant stress in Ad–/– mice.
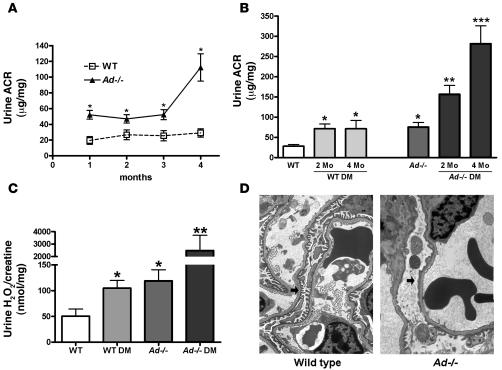
In order to determine whether adiponectin may be a causative factor for albuminuria, we examined the Ad–/– mouse. This mouse has been previously reported to have normal glucose tolerance and insulin sensitivity when fed normal rodent chow (31). The Ad–/– mice had normal levels of blood pressure, blood glucose, insulin, and lipids as well as normal body weight (data not shown). The degree of albuminuria in the Ad–/– mice increased more than 2-fold between 1 and 3 months of age and increased further at 4 months of age compared with age- and sex-matched WT mice (106.2 ± 12.9 versus 31.3 ± 1.8 μg albumin/mg creatinine; P < 0.01; Figure 2A). Mice with diabetes were also examined. Diabetes induced with multiple low doses of streptozotocin in WT C57BL/6 mice only modestly increased albuminuria, even 4 months after induction (Figure 2B). The degree of hyperglycemia and body weight was similar in WT and Ad–/– diabetic mice at 4 months of diabetes (blood glucose, 495 ± 128 versus 522 ± 135 mg/dl; body weight, 27.2 ± 2.7 versus 27.3 ± 2.3 g). However, induction of type 1 diabetes in Ad–/– mice led to a significant increase in albuminuria within 2 months of diabetes induction and exhibited a progressive increase at 4 months after diabetes induction (Figure 2B).
Figure 2. Ad–/– mice exhibit increased albuminuria, oxidant stress, and podocyte dysfunction.
(A) Urine ACR in Ad–/– mice significantly increased compared with corresponding age-matched WT mice at 1, 2, 3, and 4 months of age (n = 10 per group). *P < 0.01 versus corresponding age-matched WT. (B) WT and Ad–/– mice were made diabetic with low-dose streptozotocin, and urine ACR was measured before and at 2 and 4 months of diabetes. Albuminuria was significantly increased in Ad–/– mice with diabetes compared with corresponding WT diabetic groups (n = 5–10 per group). DM, diabetes mellitus. *P < 0.05 versus WT control; **P < 0.05 versus WT DM at 2 mo; ***P < 0.05 versus WT DM at 4 mo. (C) Urinary hydrogen peroxide/creatinine levels significantly increased in Ad–/– mice with and without diabetes (n = 10 per group). *P < 0.05 versus WT control; **P < 0.05 versus WT DM at 2 mo. Values are mean ± SEM. (D) Podocyte foot processes were segmentally effaced in Ad–/– mouse kidneys by EM. Arrows denote areas of normal foot processes in WT kidneys and areas of foot process effacement in Ad–/– glomeruli. Images are representative of 10 EM images per kidney from 2 mice per group. Original magnification, ×5,000.
Because oxidant stress is a close accompaniment to the development of cardiovascular complications in states of adiponectin deficiency and may be regulated by adiponectin (17, 18), urinary levels of hydrogen peroxide were measured. Urinary hydrogen peroxide was chosen as a measure of oxidant stress because it is relatively stable, is present at high concentrations in the urine, and reflects both systemic and renal oxidant stress (32, 33). Urinary hydrogen peroxide levels increased in Ad–/– mice (Figure 2C). With the additional stress of hyperglycemia, there was a marked increase in urinary levels of hydrogen peroxide (Figure 2C).
Direct effects of adiponectin on podocytes.
To determine how adiponectin may contribute to albuminuria, we examined EM sections of the glomeruli from WT and Ad–/– mice at 3 months of age. Podocyte foot processes were segmentally fused in the Ad–/– glomeruli (Figure 2D). Glomerular basement membrane thickness, endothelial cells, and mesangial cells were similar in appearance to those of normal WT mice. Thus adiponectin deficiency is associated with podocyte dysfunction.
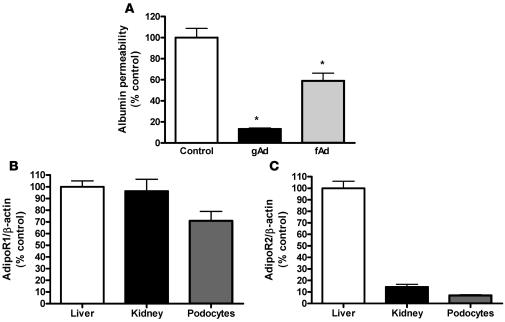
To determine whether adiponectin had direct effects on podocyte function, a permeability assay was used to measure albumin permeability across a differentiated podocyte cell monolayer in vitro. Compared with the degree of albumin permeability across podocytes cultured on porous membranes in serum-free conditions without adiponectin, permeability was significantly reduced with the addition of gAd or fAd (Figure 3A). These data indicate a direct action of adiponectin on podocytes independent of the systemic and/or metabolic effects of adiponectin. As determined by real-time PCR, AdipoR1 of kidney and podocytes was expressed to a similar degree as in liver, but AdipoR2 was much reduced in kidney and podocytes compared with liver (Figure 3, B and C). The reduction of AdipoR2 protein in kidney and podocytes compared with liver was demonstrated by immunoblotting (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI32691DS1).
Figure 3. Adiponectin inhibits permeability across a podocyte monolayer.
(A) Permeability of albumin across a podocyte monolayer was reduced by gAd or fAd at 3 μg/ml (n = 3 per group). Cells were treated as described in Methods, and permeability was assessed by albumin concentration across podocyte monolayer. Values (mean ± SEM) are presented as percent of control. *P < 0.01 versus control. (B and C) Expression of AdipoR1 (B) and AdipoR2 (C) by real-time PCR in WT mouse liver, kidney, and differentiated podocytes. Values (mean ± SEM) are presented relative to β-actin and expressed as 100% in mouse liver.
Adiponectin stimulates AMPK in podocytes.
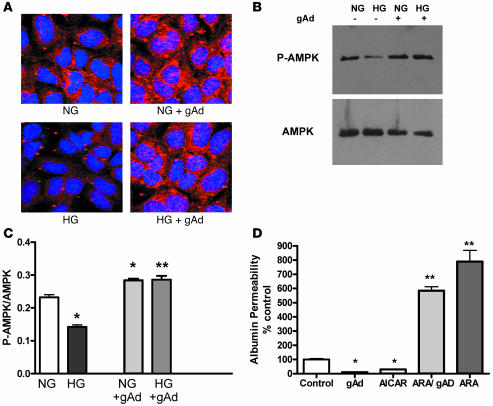
Among the pathways implicated in adiponectin action, the AMPK pathway appears to have a major role for adiponectin’s activity in liver and skeletal muscle as well as its protective effect in cardiomyocytes (34). The baseline AMPK activity in podocytes cultured in normal glucose increased with addition of adiponectin (Figure 4, A–C). AMPKα phosphorylation was further reduced by high glucose exposure, but the reduction was prevented by adiponectin (Figure 4, A–C). A functional role for AMPK was demonstrated by a specific activator of AMPK, 5-aminoimidazole-4-carboxamide-1-β-d-ribonucleoside (AICAR), which reduced permeability of podocytes to albumin, similar to the effect of adiponectin (Figure 4D). Furthermore, a specific inhibitor of AMPK, adenine 9-β-d-arabinofuranoside (ARA), increased permeability to albumin, either alone or in the presence of adiponectin (Figure 4D).
Figure 4. AMPK activity is increased by adiponectin and regulates podocyte permeability.
(A and B) Treatment of podocytes with 3 μg/ml gAd for 24 h increased AMPK activity in podocytes cultured in normal glucose (NG; 5.5 mM d-glucose) and high glucose (HG; 25 mM d-glucose), as demonstrated by confocal microscopy (A) and immunoblotting (B). AMPK activity was assessed with antibodies specific for the p-AMPKα subunit. Total AMPKα was measured with antibody for AMPKα as a loading control (B). Images are representative confocal photographs and immunoblots from 5 separate experiments. (C) Quantitation of P-AMPKα/AMPK from immunoblots in B (n = 5). Values are mean ± SEM. *P < 0.05 versus normal glucose; **P < 0.05 versus high glucose alone. (D) Albumin permeability was decreased by the AMPK activator AICAR (1 mM) and increased by the AMPK inhibitor ARA (n = 5 per group). The effect of adiponectin to reduce permeability was also blocked by ARA. Cells were treated as described in Figure 3A and Methods. Values (mean ± SEM) are presented as percent of control. *P < 0.01 versus control; **P < 0.001 versus gAd alone.
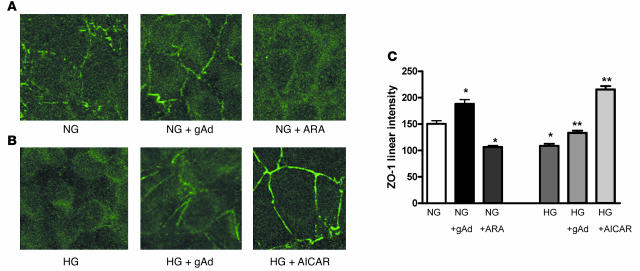
To determine whether podocyte proteins associated with podocyte dysfunction are affected by adiponectin, we used ZO-1, a tight junction protein that is highly expressed adjacent to the insertion of the slit diaphragm of the foot process (35). ZO-1 links slit diaphragm proteins through its PDZ (PSD-95/disc-large/ZO-1) domains to the actin cytoskeleton and is translocated away from the plasma membrane to the cytosol in response to high glucose exposure (36) and to puromycin (37). As shown by confocal analysis, ZO-1 was markedly redistributed to the cytosol with high glucose exposure or with AMPK inhibition by ARA (Figure 5). Treatment of podocytes with adiponectin or AICAR restored the linear membrane localization of ZO-1 (Figure 5, A–C).
Figure 5. ZO-1 localization is regulated by adiponectin and AMPK in podocytes.
(A) Immunofluorescence microscopy demonstrated linear ZO-1 staining along the cell membranes of podocytes with normal glucose exposure, which was further enhanced by treatment with gAd and markedly reduced with inhibition of AMPK by ARA. (B) High glucose exposure–induced reduction of linear staining of ZO-1 was attenuated by adiponectin and increased with AMPK activation by AICAR. (C) Semiquantitation of the data from 30 cells per coverslip for each condition in A and B. Experiments were repeated 5 times and expressed as mean ± SEM of linear staining per condition. *P < 0.05 versus normal glucose; **P < 0.05 versus high glucose.
Adiponectin replacement restores normoalbuminuria.
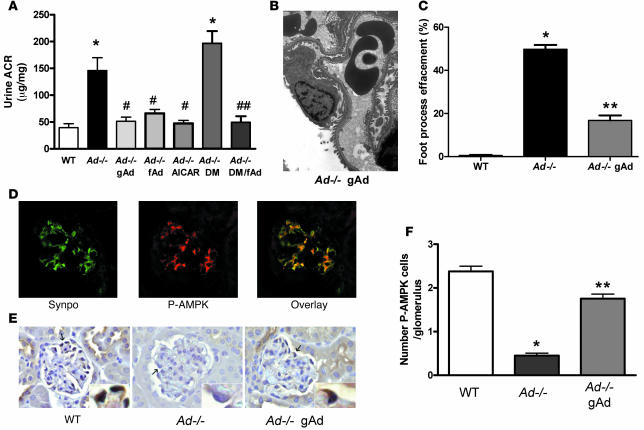
To determine whether adiponectin replacement is sufficient to treat increased urinary levels of albumin, gAd or fAd was administered to Ad–/– mice at 4 months of age, after the onset of increased albuminuria and foot process effacement. Adiponectin administration normalized albuminuria in Ad–/– mice (Figure 6A). To determine whether exogenous adiponectin affects albuminuria in the clinically relevant condition of diabetes, fAd was administered to mice 2 months after diabetes induction. As shown in Figure 6A, the treated Ad–/– diabetic mice had a marked reduction in albuminuria after 10 days of adiponectin treatment.
Figure 6. Adiponectin restores normoalbuminuria and increases AMPK activity.
(A) Ad–/– mice at 4 months of age were treated with saline, gAd, fAd, or AICAR, and urine ACR was measured. In addition, Ad–/– diabetic mice (2 months of diabetes) were treated with fAd. gAd, fAd, and AICAR treatment significantly decreased the urine ACR to the control values seen in WT mice (n = 7–10 per group). *P < 0.05 versus WT; #P < 0.05 versus Ad–/–; ##P < 0.05 versus Ad–/– DM. (B) Podocyte foot process fusion in Ad–/– mice was reduced with gAd treatment (compare with Figure 2D). (C) Semiquantitation of the degree of foot process effacement in WT mice, Ad–/– mice, and Ad–/– mice treated with gAd. Values represent percent foot process effacement of individual glomeruli. *P < 0.05 versus WT; **P < 0.05 versus Ad–/–. (D) AMPK activity was demonstrated in normal glomerular podocytes by double labeling with P-AMPK antibody and podocyte-specific synaptopodin (Synpo) antibody. (E) AMPK activity was reduced in glomeruli of Ad–/– mice and increased by adiponectin treatment. Mouse kidneys were immunostained by light microscopy with antibody specific for p-AMPKα as described Methods. Arrows denote p-AMPKα–positive podocytes. Insets show higher magnification of the same cells. Photomicrographs are representative of 50 glomeruli from each mouse kidney (n = 4 per group). (F) Quantitation of p-AMPKα–positive cells per glomerulus (n = 4 per group). *P < 0.05 versus WT; **P < 0.05 versus Ad–/–. Values are mean ± SEM. Original magnification, ×5,000 (B); ×40 (D and E); ×100 (E, insets).
A role for AMPK was also demonstrated, as AICAR administration restored albuminuria in Ad–/– mice (Figure 6A). Adiponectin administration significantly restored podocyte foot processes in Ad–/– mice (Figure 6, B and C). AMPK activity in glomeruli was measured with an antibody to p-AMPK and found to be present primarily in podocytes of glomeruli of WT mice (Figure 6D). AMPK activity was reduced in Ad–/– mouse glomerular cells and improved with adiponectin treatment (Figure 6, E and F).
Role of Nox4 in reduction of oxidant stress by adiponectin.
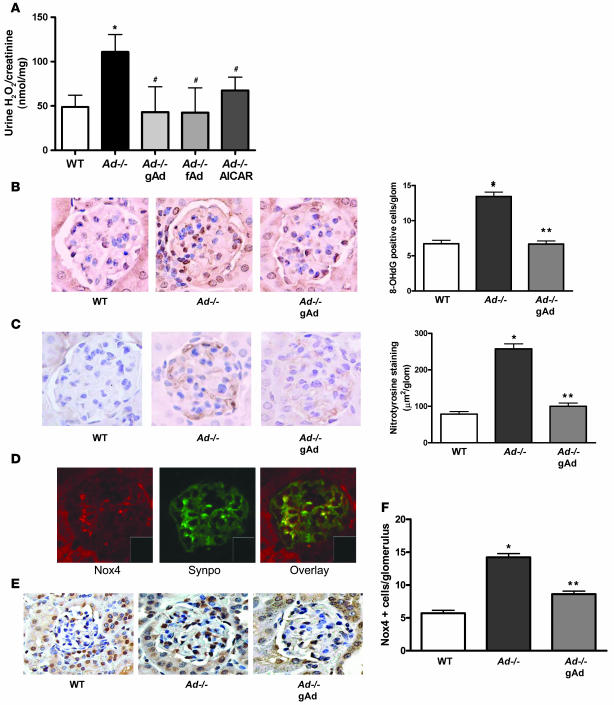
Because oxidant stress has been linked to podocyte dysfunction and albuminuria (38), we sought to determine whether exogenous adiponectin regulates oxidant stress in the Ad–/– mice and evaluated the source of oxidant production. Increased urinary levels of hydrogen peroxide in 4-month-old Ad–/– mice was reduced with treatment with gAd, fAd, or AICAR (Figure 7A). Furthermore, glomerular 8-hydroxydeoxyguanosine (8-OHdG) and nitrotyrosine were increased in Ad–/– mice and were restored with gAd treatment (Figure 7, B and C). Nox4, one of the several NADPH oxidases that have been cloned, is expressed in a variety of tissues, with the highest expression being in the kidney. As shown by real-time PCR, Nox4 was significantly increased in Ad–/– kidneys and was reduced to control levels with gAd treatment (Supplemental Figure 2). Nox1 and Nox2 were expressed at low levels in the kidney and did not increase in Ad–/– mice. As shown by immunofluorescence with double staining, glomerular Nox4 was clearly present in podocytes of WT kidneys (Figure 7D) as well as in other glomerular and tubular cells. Glomerular Nox4 was increased in Ad–/– kidneys and was reduced with gAd treatment (Figure 7, E and F).
Figure 7. Regulation of oxidant stress and Nox4 by adiponectin.
(A) Urinary levels of hydrogen peroxide were reduced by gAd, fAd, or AICAR treatment in Ad–/– mice (n = 7–10 per group). *P < 0.05 versus WT; #P < 0.05 versus Ad–/–. (B) Glomerular 8-OHdG was increased in Ad–/– kidneys and reduced with gAd, demonstrated by light microscopy immunostain and quantitation of 8-OHdG–positive cells per glomerulus (n = 4 per group). *P < 0.05 versus WT; **P < 0.05 versus Ad–/–. (C) Glomerular nitrotyrosine was increased in Ad–/– kidneys and reduced with gAd treatment, demonstrated by light microscopy immunostain and quantitation of nitrotyrosine staining per glomerulus (n = 4 per group). *P < 0.05 versus WT; **P < 0.05 versus Ad–/–. (D) Nox4 was present in podocytes, as well as other glomerular cells and tubular cells, as demonstrated by double labeling with synaptopodin in WT kidney. Insets show representative background staining without primary antibody. (E) Light microscopy immunostain demonstrated that Nox4 protein was increased in glomerular cells of Ad–/– kidneys and reduced with gAd treatment. Photomicrographs are representative of 50 glomeruli from each mouse kidney (n = 4 per group). (F) Quantitation of Nox4-positive cells per glomerulus (n = 4 per group). *P < 0.05 versus WT; **P < 0.05 versus Ad–/–. Values are mean ± SEM. Original magnification, ×40 (B–E); ×10 (D, insets).
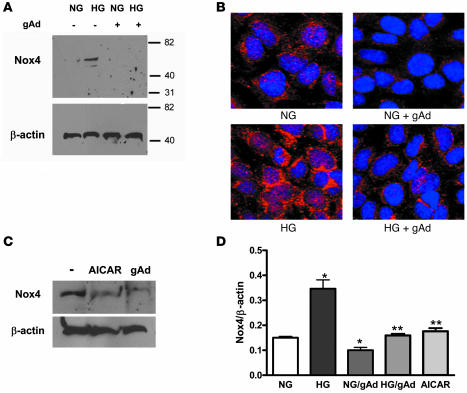
Nox4 protein was evident in podocyte cell culture grown in serum-free conditions and further increased with high glucose exposure (Figure 8, A, B, and D). As shown by confocal analysis, podocyte Nox4 was primarily perinuclear and at the cell periphery (Figure 8B). Addition of adiponectin was sufficient to suppress podocyte Nox4 (Figure 8, A, B, and D). Furthermore, the AMPK activator AICAR reduced Nox4 protein levels to a similar degree as did adiponectin (Figure 8, C and D).
Figure 8. Podocyte Nox4 is increased by high glucose exposure and reduced by adiponectin or AICAR.
(A) Podocytes grown in the presence of 3 μg/ml gAd for 24 h showed suppression of Nox4 with normal or high glucose exposure. Transferred proteins were immunoblotted with antibody to Nox4 and β-actin. (B) Similar studies were performed with podocytes grown on coverslips, demonstrating reduction of Nox4 protein. (C) AMPK activation with AICAR demonstrated Nox4 protein reduction to a degree similar to that shown by gAd in podocytes grown with high glucose exposure. Shown are representative immunoblots and confocal images from 5 separate experiments. (D) Quantitation of Nox4 relative to β-actin from the immunoblots (n = 5). Values are mean ± SEM. *P < 0.05 versus normal glucose; **P < 0.05 versus high glucose.
Discussion
In the present study, we report that plasma adiponectin level was inversely correlated with urinary albumin excretion in obese AAs. Ad–/– mice had increased levels of albuminuria and increased podocyte dysfunction, which indicates that adiponectin deficiency contributes to altered permeability, likely via podocyte dysfunction. AMPK regulation by adiponectin appears to contribute to the mechanism of podocyte dysfunction, because protective effects of adiponectin on podocyte permeability are blocked by AMPK inhibition and mimicked by an AMPK activator. Additionally, Nox4 is present in podocytes, is regulated by adiponectin, and may contribute to podocyte dysfunction. Administration of adiponectin to Ad–/– mice normalized albuminuria and oxidant stress, improved podocyte foot processes, increased glomerular AMPK activity, and reduced glomerular Nox4.
AAs have a disproportionate and excessive representation of end-stage renal disease (39). AAs also have high rates of obesity, which heightens risk for kidney disease and CVD. Low adiponectin levels have been identified in obese AAs and are also associated with susceptibility to diabetes (23, 24). A relationship between low adiponectin levels and albuminuria has been reported in males with essential hypertension (28) and in Japanese males and females with obesity (40). To our knowledge, our study is the first to link low adiponectin levels with albuminuria in the obese AA population. This correlation was noted in a population before the onset of diabetes and overt renal dysfunction. The degree of albuminuria in our cohort was within the so-called normal range and thus represents a very early manifestation of kidney disease in association with obesity and insulin resistance. The main conclusion supported by the clinical data is that low adiponectin levels are tightly correlated to this early rise in albuminuria.
Chronic kidney disease is a strong risk factor for CVD mortality (1, 3–5). To some degree, the increased risk of mortality and CVD in chronic kidney disease may be explained by levels of adipokines, including elevated levels of proinflammatory adipokines and reduced adiponectin (9, 11). Adiponectin reduction has been well documented in states of obesity and prediabetes (41), conditions often associated with microalbuminuria (10). It should be noted that subsequent to the development of overt proteinuria and renal insufficiency (27, 29) there is a reported increase in plasma adiponectin levels. However, several independent groups have reported that a region of chromosome 3q contains a susceptibility locus for diabetic nephropathy in patients with both type 1 (42, 43) and type 2 diabetes (44, 45), and one group evaluated 14 candidate genes on chromosome q and found the strongest linkage with a SNP for the promoter of adiponectin (20). Our present findings of a negative correlation between adiponectin and low levels of albuminuria in patients, a moderate increase in albuminuria in adiponectin-deficient mice, and a dramatic increase in the degree of albuminuria in diabetic mice as a result of adiponectin deficiency point to an important role for adiponectin in the initial development of increased albuminuria.
A vascular protective role of AMPK has been demonstrated in several cell types (17, 46, 47). Our present study demonstrated that AMPK plays a critical role in permeability of podocytes and that AMPK activity in podocytes is regulated by adiponectin. AMPK is a heterotrimeric signaling kinase and a critical energy-sensing pathway with important functions to stimulate glucose uptake. It has previously been demonstrated that the effect of adiponectin on various cell types involves AMPK as well as other pathways, such as the PPAR (48, 49) and cAMP-PKA pathways (17, 47). A recent study using rat glomerular epithelial cells demonstrated that AMPK contributes to high glucose exposure–induced cell hypertrophy (50). ZO-1 localization along the membrane is associated with tight junction adherence and normal function of the slit diaphragm. Adiponectin and AMPK activation promoted ZO-1 membrane localization, which suggests that ZO-1 may be a direct target protein for AMPK. Because the podocyte is the major cell type protecting the glomerulus from leaking albumin into the urinary space, additional podocyte proteins regulated by AMPK will be of major interest and are likely to be involved in linking obesity, hypoadiponectinemia, and albuminuria.
One potential pathway by which adiponectin and AMPK activation may provide protection against albuminuria and podocyte permeability is via reduction of oxidant stress (17, 18, 51). Nox4 is a recently described nonphagocytic NAPDH oxidase that is highly expressed in the kidney (30). Our present results demonstrated that podocytes expressed Nox4 and that adiponectin and AMPK regulated Nox4 protein in podocytes. Oxidant stress has been consistently linked with insulin resistance, obesity, and adiponectin deficiency. The kidney’s contribution to oxidant stress has been largely ignored in settings of insulin resistance. Our results demonstrated that systemic adiponectin deficiency caused upregulation of Nox4 in the kidney and podocytes, thus providing another critical link among obesity, insulin resistance, and oxidant stress. Of note, Ohashi et al. used a different adiponectin-deficient mouse and found that adiponectin deficiency accentuates albuminuria in the 5/6 nephrectomy model (52). Thus it is likely that adiponectin deficiency may be a critical risk factor in a variety of kidney diseases associated with albuminuria.
Our results suggest that several approaches may be successful in lowering the development of microalbuminuria and possibly CVD in at-risk populations. Identification of low adiponectin levels and increased albuminuria likely identifies a high risk profile with regard to kidney disease and CVD. In addition to obese AAs and obese Japanese (40), it is likely that similar findings will be made in other ethnic populations with obesity and/or insulin resistance (53) as well as type 1 diabetes (21, 22). Maneuvers to raise adiponectin levels, such as weight reduction, renin-angiotensin system blockade (54), and PPARγ agonists (48), may be beneficial for renal and potentially cardiovascular protection in at-risk populations. Treatment with metformin may be useful, because metformin raises AMPK activity independent of adiponectin (55). Inhibition of specific NADPH oxidase isoforms, such as Nox4, will likely reduce podocyte dysfunction in states of adiponectin deficiency and has previously been shown to benefit diabetic nephropathy in a rat model (56). Treatment with gAd or fAd is another potentially attractive option to treat podocyte dysfunction and albuminuria. It is possible that these approaches will only be successful in early-stage kidney disease, when podocyte function will be responsive to AMPK, and before there is widespread podocyte depletion, as noted in situations of severe proteinuria (57).
In summary, circulating adiponectin levels are inversely related to albuminuria in obese AAs without diabetes or overt kidney disease. Adiponectin plays a protective role to reduce albuminuria by directly affecting podocyte function via the AMPK pathway. Our results provide a strong pathobiologic rationale to intervene in the adiponectin-AMPK-Nox4 pathway to protect against albuminuria and potentially affect early renal disease as well as associated CVD.
Methods
Human subjects.
Subjects were recruited from an AA cohort that has been examined prospectively since late adolescence (58). Inclusion criteria were BMI greater than 30 and no history of known diabetes or kidney disease (serum creatinine, ≤1.4 mg/dl). Written informed consent was obtained from all subjects. Measurements included height; weight; calculated BMI; blood pressure; 3 separate urine ACRs assayed from timed overnight collections; and levels of adipocytokines, adiponectin, IL-6, and PAI-1. Insulin sensitivity was measured via an insulin clamp procedure as previously described (59). Urine albumin was measured by RIA (Diagnostic Products Corp.). Plasma adiponectin was measured with an RIA kit (Linco Inc.). Plasma IL-6 and PAI-1 were measured by ELISA (R&D Systems). The protocol was approved by the Thomas Jefferson University Institutional Review Board.
Animals.
Male Ad–/– mice on the C57BL/6 genetic background, as previously described (18, 31), were chosen for this study. The genotype of each mouse was determined by PCR analysis of mouse tail DNA as previously described (31). All animal procedures were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University. Mice were given standard rodent chow (Purina 5010) and water ad libitum. Urine was collected in Nalgene metabolic cages at various time points. A cohort of male WT and Ad–/– mice at 2 months of age were made diabetic with a multiple low-dose streptozotocin protocol as previously described (60). Blood glucose was measured with Accuchek (Roche). Urine in diabetic and nondiabetic mice was collected at baseline, 2 months after diabetes induction, and 4 months after diabetes induction (2, 4, and 6 months of age, respectively).
Interventional animal studies.
The mice were treated with recombinant human gAd (PepROTech) administrated 25 μg/mouse i.p. twice a day for 10 consecutive days, or fAd administrated using an AAV vector (AAV2/8 CMV fAd virus, 4 × 1011 GC/mouse i.p. for 10 days). A separate group of Ad–/– mice was treated with AICAR with a single i.p. dose of 300 mg/kg. The control animals were given PBS alone or control AAV. Twenty-four hour urines from each mouse were collected before treatment and on the last day of the treatment period. The urine albumin and creatinine were measured with a mouse Albuwell ELISA kit and a Creatinine Companion kit (Exocell Inc.; refs. 60, 61). As an index of oxidant stress, timed urine collections were also analyzed for hydrogen peroxide by Amplex red assay (Invitrogen) following the manufacturer’s protocol. Portions of liver, muscle, and kidney were snap-frozen in liquid nitrogen for RNA and protein isolation. An additional aliquot of normal kidney was frozen in OCT for immunofluorescent staining. Portions of kidney cortex were fixed in buffered formalin and embedded in paraffin, and a separate aliquot of kidney cortical tissue was cut into 1-mm3 pieces and fixed in 2.5% glutaraldehyde in Millonig solution and embedded in PolyBed 812 (Polysciences Inc.) for EM analysis. Individual capillary loops were evaluated and quantitated for the degree of foot process effacement by a single investigator following a previously published method (62).
Podocyte cell culture.
Conditionally immortalized mouse podocytes, kindly provided by P. Mundel (Mt. Sinai School of Medicine, New York, New York, USA), were cultured as previously described (63). Differentiated podocytes were cultured at 37°C for 8–10 days without IFN-γ in DMEM containing 5.5 mmol/l glucose and 5% FCS.
For the permeability assay, a modification of a previously published protocol was adopted (37). Differentiated podocytes (0.2 × 106 podocytes/well) plated on type I collagen–coated 24-well Transwell plates (Corning) were serum-starved overnight when confluent. Cells were then modulated with 3 μg/ml gAd (PepROTech Inc.), 3 μg/ml fAd (PepROTech Inc.), or 1 mM ARA (Sigma-Aldrich) for 24 h or with 1 mM AICAR for 1 h. Cells were then washed twice with PBS supplemented with 1 mM each MgCl2 and CaCl2. The upper compartment was refilled with 0.25 ml RPMI 1640 alone and the lower compartment with 0.5 ml RPMI 1640 supplemented with 40 mg/ml BSA and incubated for 2 hours at 37°C. Total protein concentration in the upper compartment was determined using a Bio-Rad protein assay (Bio-Rad Laboratories).
Immunoblotting.
Podocytes were cultured as described above, and, after differentiation in 6-well plates, the culture medium was replaced with serum-free DMEM with normal (5.5 mM d-glucose) or high glucose (25 mM d-glucose) for 24 h. In separate wells, podocytes were treated with 3 μg/ml gAd for 6 h or 1 mM AICAR for 1 h prior to modulation with glucose. Immunoblotting was performed as described previously (64). Primary antibodies included rabbit AdipoR2 polyclonal antibody (Alpha Diagnostics), p-AMPKα polyclonal antibody, rabbit anti-AMPKα monoclonal antibody (Cell Signaling Technology), and polyclonal rabbit antibody specific for Nox4, as previously described (65, 66). For verifying equal loading, antibody to β-actin was used.
Immunohistochemistry.
Immunocytochemistry was performed as described previously (67). Differentiated podocytes seeded on coverslips were serum-starved overnight, and gAd was added. After 24 h adiponectin introduction, cells were fixed in 3.7% paraformaldehyde. Primary antibodies included p-AMPKα (Thr172) rabbit monoclonal antibody (Cell Signaling Technology), Nox4 rabbit polyclonal antibody (Novus Biologicals), and ZO-1 (Invitrogen). Nuclear stain was performed with Hoechst 33342 (Invitrogen). Fluorescence images were obtained using a confocal laser fluorescence microscope (LSM-510; Carl Zeiss). ZO-1 linear staining was performed using computer-assisted image analysis. Immunostaining of paraffin-embedded mouse kidneys was performed as described previously (68). Primary antibodies included p-AMPKα (Thr172) rabbit monoclonal antibody, Nox4 rabbit polyclonal antibody (Novus Biologicals), anti–8-OHdG monoclonal antibody (Japan Institute for the Control of Aging), and nitrotyrosine monoclonal antibody (Cayman), used at 1:10 dilution per the manufacturers protocols. Quantitation of p-AMPKα, Nox4, and 8-OHdG–positive cells was performed on 20–50 glomeruli from 4 mice per group. Quantification of the nitrotyrosine-positive area in glomeruli was performed by color-subtractive, computer-assisted image analysis, as described previously (69). For localization studies in frozen mouse kidney tissue, immunofluorescence with overlay was performed with the primary antibodies, rabbit monoclonal antibody p-AMPKα (Thr172) and polyclonal rabbit anti-Nox4, and double staining was performed with the podocyte-specific mouse anti-synaptopodin antibody (Biodesign). Images were captured by confocal laser fluorescence microscope.
RNA isolation and quantitative real-time PCR.
Total RNA was isolated from liver, muscle, kidney, and differentiated podocytes using TRIzol reagent (Invitrogen) as previously described (70). Real-time PCR was performed as previously described (70). The primers for AdipoR1, AdipoR2, Nox1, Nox2, Nox4, and β-actin are listed in Supplemental Methods.
Statistics.
Data are summarized as arithmetic means ± SEM or medians. Data that were normally distributed were used in a Pearson correlation analysis. Data that did not meet the criteria of normally distributed data were used in a Spearman rank correlation. A P value less than 0.05 was considered significant. All reported P values are 2-sided. Analyses were carried out using Graph Pad Prism software version 4.03 and SPSS version 13.0 for the PC. Differences between data groups were evaluated for significance using independent t test of data or 1-way ANOVA and Newman-Keuls post-hoc tests.
Supplementary Material
Acknowledgments
These studies were performed with partial support from NIH grants DK 053867 and DK 076133 (to K. Sharma), DK 63018 and DK 71360 (to B. Goldstein), HL 051547 and DK 046107 (to B. Falkner), and HL 51586 (to L. Chan).
Footnotes
Nonstandard abbreviations used: AA, African American; ACR, albumin/creatinine ratio; AdipoR, adiponectin receptor; AICAR, 5-aminoimidazole-4-carboxamide-1-β-d-ribonucleoside; ARA, adenine 9-β-d-arabinofuranoside; CVD, cardiovascular disease; fAd, full-length adiponectin; gAd, globular adiponectin; Nox, NADPH oxidase; 8-OHdG, 8-hydroxydeoxyguanosine, PAI-1, plasminogen activator inhibitor–1; ZO-1, zonula occludens–1.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:1645–1656 (2008). doi:10.1172/JCI32691
Stephen R. Dunn’s present address is: Cancer Genomics Facility, Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, Pennsylvania, USA.
Portions of this work were presented in abstract form at the American Society of Nephrology meeting in Philadelphia, Pennsylvania, USA, on November 8–13, 2005.
See the related Commentary beginning on page 1619.
References
- 1.Amann K., Wanner C., Ritz E. Cross-talk between the kidney and the cardiovascular system. J. Am. Soc. Nephrol. 2006;17:2112–2119. doi: 10.1681/ASN.2006030204. [DOI] [PubMed] [Google Scholar]
- 2.de Zeeuw D., Parving H.-H., Henning R.H. Microalbuminuria as an early marker for cardiovascular disease. J. Am. Soc. Nephrol. 2006;17:2100–2105. doi: 10.1681/ASN.2006050517. [DOI] [PubMed] [Google Scholar]
- 3.Go A., Chertow G., Fan D., McCulloch C., Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Shlipak M.G., et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N. Engl. J. Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 5.Anavekar N.S., et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N. Engl. J. Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 6.Ruggenenti P., et al. Preventing microalbuminuria in type 2 diabetes. N. Engl. J. Med. 2004;351:1941–1951. doi: 10.1056/NEJMoa042167. [DOI] [PubMed] [Google Scholar]
- 7.Wachtell K., et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann. Int. Med. 2003;139:901–906. doi: 10.7326/0003-4819-139-11-200312020-00008. [DOI] [PubMed] [Google Scholar]
- 8.Davies M.R., Lund R.J., Mathew S., Hruska K.A. Low turnover osteodystrophy and vascular calcification are amenable to skeletal anabolism in an animal model of chronic kidney disease and the metabolic syndrome. J. Am. Soc. Nephrol. 2005;16:917–928. doi: 10.1681/ASN.2004100835. [DOI] [PubMed] [Google Scholar]
- 9.Becker B., et al. Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: The Mild and Moderate Kidney Disease Study. J. Am. Soc. Nephrol. 2005;16:1091–1098. doi: 10.1681/ASN.2004090742. [DOI] [PubMed] [Google Scholar]
- 10.Mykkanen L., et al. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes. 1998;47:793–800. doi: 10.2337/diabetes.47.5.793. [DOI] [PubMed] [Google Scholar]
- 11.Zoccali C., et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J. Am. Soc. Nephrol. 2002;13:134–141. doi: 10.1681/ASN.V131134. [DOI] [PubMed] [Google Scholar]
- 12.Scherer P. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. . 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 13.Fruebis J., et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl. Acad. Sci. U. S. A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waki H., et al. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology. 2005;146:790–796. doi: 10.1210/en.2004-1096. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein B., et al. Adiponectin: a novel adipokine linking adipocytes and vascular function. J. Clin. Endocrinol. Metab. 2004;89:2563–2568. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi T., et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007;13:332. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 17.Ouedraogo R., et al. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes. 2006;55:1840–1846. doi: 10.2337/db05-1174. [DOI] [PubMed] [Google Scholar]
- 18.Tao L., et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–1416. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 19.Matsuzawa Y. Therapy insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2006;3:35–42. doi: 10.1038/ncpcardio0380. [DOI] [PubMed] [Google Scholar]
- 20.Vionnet N., et al. Analysis of 14 Candidate genes for diabetic nephropathy on chromosome 3q in European populations: strongest evidence for association with a variant in the promoter region of the adiponectin gene. Diabetes. 2006;55:3166–3174. doi: 10.2337/db06-0271. [DOI] [PubMed] [Google Scholar]
- 21.Costacou T., et al. The prospective association between adiponectin and coronary artery disease among individuals with type 1 diabetes. The Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2005;48:41–48. doi: 10.1007/s00125-004-1597-y. [DOI] [PubMed] [Google Scholar]
- 22.Maahs D.M., et al. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111:747–753. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 23.Osei K., et al. Plasma adiponectin levels in high risk African-Americans with normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes. Obes. Res. . 2005;13:179–185. doi: 10.1038/oby.2005.23. [DOI] [PubMed] [Google Scholar]
- 24.Bush N., et al. Adiponectin is lower among African Americans and is independently related to insulin sensitivity in children and adolescents. Diabetes. 2005;54:2772–2778. doi: 10.2337/diabetes.54.9.2772. [DOI] [PubMed] [Google Scholar]
- 25.Ziyadeh F.N., Sharma K. Overview: combating diabetic nephropathy. J. Am. Soc. Nephrol. 2003;14:1355–1357. doi: 10.1097/01.ASN.0000065608.37756.58. [DOI] [PubMed] [Google Scholar]
- 26. United States Renal Data System. 2005. 2005 annual data report: atlas of end-stage renal disease in the United States. Division of Kidney, Urologic, and Hematologic Diseases, NIDDK, NIH. http://www.usrds.org/atlas_2005.htm . [Google Scholar]
- 27.Schalkwijk C.G., et al. Adiponectin is inversely associated with renal function in type 1 diabetic patients. J. Clin. Endocrinol. Metab. 2006;91:129–135. doi: 10.1210/jc.2005-1117. [DOI] [PubMed] [Google Scholar]
- 28.Tsioufis C., et al. Relation of microalbuminuria to adiponectin and augmented C-reactive protein levels in men with essential hypertension. Am. J. Cardiol. 2005;96:946. doi: 10.1016/j.amjcard.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 29.Looker H.C., et al. Adiponectin concentrations are influenced by renal function and diabetes duration in pima indians with Type 2 diabetes. J. Clin. Endocrinol. Metab. 2004;89:4010–4017. doi: 10.1210/jc.2003-031916. [DOI] [PubMed] [Google Scholar]
- 30.Geiszt M., Kopp J.B., Varnai P., Leto T.L. Identification of Renox, an NAD(P)H oxidase in kidney. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma K., et al. Increased beta-oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J. Biol. Chem. 2002;277:34658–34661. doi: 10.1074/jbc.C200362200. [DOI] [PubMed] [Google Scholar]
- 32.Djamali A., et al. BOLD-MRI assessment of intrarenal oxygenation and oxidative stress in patients with chronic kidney allograft dysfunction. Am. J. Physiol. Renal Physiol. 2007;292:F513–F522. doi: 10.1152/ajprenal.00222.2006. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L., Fujii S., Kosaka H. Effect of oestrogen on reactive oxygen species production in the aortas of ovariectomized Dahl salt-sensitive rats. J. Hypertens. 2007;25:407–414. doi: 10.1097/HJH.0b013e328010beee. [DOI] [PubMed] [Google Scholar]
- 34.Ouchi N., Shibata R., Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc. Med. 2006;16:141–146. doi: 10.1016/j.tcm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnabel E., Anderson J.M., Farquhar M.G. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J. Cell Biol. 1990;111:1255–1263. doi: 10.1083/jcb.111.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rincon-Choles H., et al. ZO-1 expression and phosphorylation in diabetic nephropathy. Diabetes. 2006;55:894–900. doi: 10.2337/diabetes.55.04.06.db05-0355. [DOI] [PubMed] [Google Scholar]
- 37.Rico M., et al. WT1-interacting protein and ZO-1 translocate into podocyte nuclei after puromycin aminonucleoside treatment. Am. J. Physiol. Renal Physiol. 2005;289:F431–F441. doi: 10.1152/ajprenal.00389.2004. [DOI] [PubMed] [Google Scholar]
- 38.Susztak K., Raff A.C., Schiffer M., Bottinger E.P. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. doi: 10.2337/diabetes.55.01.06.db05-0894. [DOI] [PubMed] [Google Scholar]
- 39.Freedman B.I., et al. A genome scan for all-cause end-stage renal disease in African Americans. Nephrol. Dial. Transplant. 2005;20:712–718. doi: 10.1093/ndt/gfh704. [DOI] [PubMed] [Google Scholar]
- 40.Yano Y., et al. Differential impacts of adiponectin on low-grade albuminuria between obese and nonobese persons without diabetes. J. Clin. Hypertens. (Greenwich) 2007;9:775–782. doi: 10.1111/j.1524-6175.2007.07321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kadowaki T., et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osterholm A.M., et al. Genome-wide scan for type 1 diabetic nephropathy in the Finnish population reveals suggestive linkage to a single locus on chromosome 3q. Kidney Int. 2006;71:140. doi: 10.1038/sj.ki.5001933. [DOI] [PubMed] [Google Scholar]
- 43.Moczulski D.K., Rogus J.J., Antonellis A., Warram J.H., Krolewski A.S. Major susceptibility locus for nephropathy in type 1 diabetes on chromosome 3q: results of novel discordant sib-pair analysis. Diabetes. 1998;47:1164–1169. doi: 10.2337/diabetes.47.7.1164. [DOI] [PubMed] [Google Scholar]
- 44.Imperatore G., et al. Sib-pair linkage analysis for susceptibility genes for microvascular complications among Pima Indians with type 2 diabetes. Pima Diabetes Genes Group. Diabetes. 1998;47:821–830. doi: 10.2337/diabetes.47.5.821. [DOI] [PubMed] [Google Scholar]
- 45.Bowden D.W., et al. A genome scan for diabetic nephropathy in African Americans. Kidney Int. 2004;66:1517–1526. doi: 10.1111/j.1523-1755.2004.00915.x. [DOI] [PubMed] [Google Scholar]
- 46.Ouchi N., et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J. Biol. Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu X., et al. Adiponectin suppresses I{kappa}B kinase activation induced by tumor necrosis factor-{alpha} or high glucose in endothelial cells: role of cAMP and AMP kinase signaling. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1836–E1844. doi: 10.1152/ajpendo.00115.2007. [DOI] [PubMed] [Google Scholar]
- 48.Yang B., et al. Serum adiponectin as a biomarker for in vivo PPARgamma activation and PPARgamma agonist-induced efficacy on insulin sensitization/lipid lowering in rats. BMC Pharmacol. 2004;4:23. doi: 10.1186/1471-2210-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamauchi T., et al. 2003Cloning of adiponectin receptors that mediate antidiabetic metabolic effects [erratum 2004, 431:1123]. Nature. 423762–769. 10.1038/nature01705 . [DOI] [PubMed] [Google Scholar]
- 50.Lee M.-J., et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am. J. Physiol. Renal Physiol. . 2006;292:F617–F627. doi: 10.1152/ajprenal.00278.2006. [DOI] [PubMed] [Google Scholar]
- 51.Alba G., et al. Stimulators of AMP-activated protein kinase inhibit the respiratory burst in human neutrophils. FEBS Lett. 2004;573:219. doi: 10.1016/j.febslet.2004.07.077. [DOI] [PubMed] [Google Scholar]
- 52.Ohashi K., et al. Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Arterioscler. Thromb. Vasc. Biol. 2007;27:1910–1917. doi: 10.1161/ATVBAHA.107.147645. [DOI] [PubMed] [Google Scholar]
- 53.Retnakaran R., et al. Does hypoadiponectinemia explain the increased risk of diabetes and cardiovascular disease in South Asians? Diabetes Care. 2006;29:1950–1954. doi: 10.2337/dc06-0867. [DOI] [PubMed] [Google Scholar]
- 54.Yenicesu M., et al. Blockade of the renin-angiotensin system increases plasma adiponectin levels in type-2 diabetic patients with proteinuria. Nephron. Clin. Pract. 2005;99:c115–c121. doi: 10.1159/000083929. [DOI] [PubMed] [Google Scholar]
- 55.Shaw R.J., et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gorin Y., et al. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J. Biol. Chem. 2005;280:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 57.Pagtalunan M.E., et al. Podocyte loss and progressive glomerular injury in type II diabetes. J. Clin. Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campbell K., Kushner H., Falkner B.2004Obesity and high blood pressure: a clinical phenotype for the insulin resistance syndrome in African Americans. J. Clin. Hypertens. 6364–370; quiz 371–372. . 10.1111/j.1524-6175.2004.03536.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stein E., Kushner H., Giddings S., Falkner B. Plasma lipid concentrations in nondiabetic African American adults: associations with insulin resistance and the metabolic syndrome. Metabolism. 2007;56:954–960. doi: 10.1016/j.metabol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Susztak K., et al. Molecular profiling of diabetic mouse kidney reveals novel genes linked to glomerular disease. Diabetes. 2004;53:784–794. doi: 10.2337/diabetes.53.3.784. [DOI] [PubMed] [Google Scholar]
- 61.Ziyadeh F., et al. Long-term prevention of renal insufficiency excess matrix gene expression and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-b antibody in db/db diabetic mice. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8015–8020. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jo Y.I., Cheng H., Wang S., Moeckel G.W., Harris R.C. Puromycin induces reversible proteinuric injury in transgenic mice expressing cyclooxygenase-2 in podocytes. Nephron Exp. Nephrol. 2007;107:e87–e94. doi: 10.1159/000108653. [DOI] [PubMed] [Google Scholar]
- 63.Mundel P., et al. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp. Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 64.Sharma K., et al. Involvement of transforming growth factor-{beta} in regulation of calcium transients in diabetic vascular smooth muscle cells. Am. J. Physiol. Renal Physiol. 2003;285:F1258–F1270. doi: 10.1152/ajprenal.00145.2003. [DOI] [PubMed] [Google Scholar]
- 65.Mahadev K., et al. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. . Mol. Cell. Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu T., et al. Reactive oxygen species production via NADPH oxidase mediates TGF-{beta}-induced cytoskeletal alterations in endothelial cells. Am. J. Physiol. Renal Physiol. 2005;289:F816–F825. doi: 10.1152/ajprenal.00024.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McGowan T., et al. TGF-beta-induced Ca(2+) influx involves the type III IP3 receptor and regulates actin cytoskeleton. Am. J. Physiol. Renal Physiol. 2002;282:F910–F920. doi: 10.1152/ajprenal.00252.2001. [DOI] [PubMed] [Google Scholar]
- 68.Fraser S., et al. Regulation of the energy sensor AMP-activated protein kinase in the kidney by dietary salt intake and osmolality. Am. J. Physiol. Renal Physiol. 2005;288:F578–F586. doi: 10.1152/ajprenal.00190.2004. [DOI] [PubMed] [Google Scholar]
- 69.Williams K.J., et al. Decorin deficiency enhances progressive nephropathy in diabetic mice. Am. J. Pathol. 2007;171:1441–1450. doi: 10.2353/ajpath.2007.070079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu Y., Casado M., Vaulont S., Sharma K. Role of upstream stimulatory factors in regulation of renal transforming growth factor-{beta}1. Diabetes. 2005;54:1976–1984. doi: 10.2337/diabetes.54.7.1976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.