Abstract
The ability to noninvasively assess physiological changes in solid tumors is desired for its diagnostic and therapeutic potential. In this issue of JCI, Matsumoto and colleagues reveal their development and use of a novel imaging approach, combining pulsed electron paramagnetic resonance imaging (EPRI) with conventional MRI to image squamous cell carcinoma tumor–bearing mice (See the related article beginning on page 1965). This method provides coregistered images of oxygenation and blood volume/flow with the underlying anatomy and concentrations of metabolites such as lactate and choline. This technique, combining functional and anatomic imaging, shows immediate preclinical applicability in monitoring factors that control tumor hypoxia and metabolism and may have future clinical potential for monitoring tumor response to treatment.
It has been known for well over two decades that tumor angiogenesis results in blood vessels that are structurally and functionally abnormal, contributing to inefficient tumor perfusion and hypoxia (1, 2). Hypoxia has an impact on patient survival for a wide range of solid tumors and is associated with poor prognosis following radiation, chemotherapy, and surgery (3–5). Interest in the hypoxic status of tumors has further increased with the discovery that hypoxia regulates dozens of genes that alter cellular behavior and result in a more malignant tumor phenotype (6). However, in spite of the known importance of hypoxia in tumor pathophysiology, there has yet to be identified a method that can noninvasively and repeatedly measure partial pressure of oxygen (oxygenation; pO2) of tumors with precision. Additionally, the multifaceted causality of tumor hypoxia has not been addressed; both metabolic rate and perfusion can influence hypoxia, but to date there has not been an integrated method that can assess these multiple physiologic parameters.
An important milestone in multimodal imaging is achieved
The study by Matsumoto and colleagues in this issue of the JCI represents the pièce de résistance of multiparametric tumor imaging (7). They have demonstrated the ability to obtain noninvasive, accurate measurements of tissue pO2 in a live animal with an integrated imaging platform that provides overlay of acquired oxygen maps with further anatomic, physiologic, and metabolic information. This work is truly groundbreaking, and if implemented on a wider basis, it could revolutionize the way in which we study and understand the principles controlling tumor hypoxia at the preclinical level. Although implementing this technique in the clinical environment will be challenging, successful translation of this research method into clinical use would create a true paradigm shift in how technology is used in diagnostic and therapeutic oncology.
The current study (7) describes a novel platform that uses pulsed electron paramagnetic resonance imaging (EPRI), a method fundamentally similar to MRI that utilizes an injected oxygen-sensitive chemical probe to obtain 3D maps of tissue pO2. The authors combine EPRI with MRI by exploiting the common radio frequency of 300 MHz between EPRI and MRI signals at their magnetic field strengths of 10 mT and 7 T, respectively. This elegant design allows for serial monitoring and multiparametric analysis of the tumor environment, merging the refined oxygen-imaging ability of EPRI with the multitude of powerful parameters available through MRI and magnetic resonance spectroscopy (MRS).
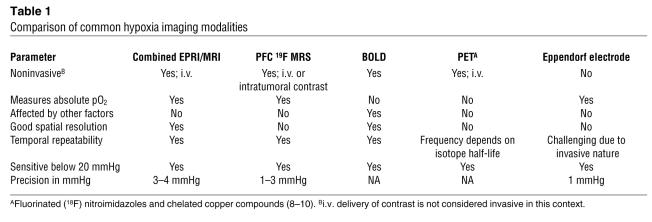
The method outlined by Matsumoto et al. (7) offers a unique capacity for hypoxia imaging by providing 3D images of absolute tissue pO2 with a resolution of 3–4 mmHg. No other hypoxia imaging modality can currently match this ability (Table 1). While polarographic electrodes and optical methods are also capable of assessing absolute oxygen content in mmHg, they are severely limited in their imaging capability (8); polarography, the current gold standard for measuring tissue pO2, is invasive and provides only point or line measurements, and optical methods have limited penetration depths in the order of millimeters to centimeters. PET imaging of oxygen-dependent hypoxia marker compounds is the most commonly used clinical method for determining tumor hypoxia, with a variety of available fluorinated (18F) nitroimidazoles and chelated copper compounds. These radioactive tracers are bioreduced and consequently trapped inside cells with low oxygen tension (8–10). However, PET imaging provides only a relative measure of hypoxia and is influenced by the cellular uptake and retention of the radiotracers. This is in contrast to the pulsed EPRI system, which provides quantitative information on hypoxic intensity, enabling severely hypoxic (<1 mmHg) tumor regions to be discriminated from mildly hypoxic (approximately 10 mmHg) tumor regions, at a spatial resolution far superior to that of PET.
Table 1 .
Comparison of common hypoxia imaging modalities
Competing MRI methods of imaging hypoxia such as blood oxygen level–dependent (BOLD) and dynamic contrast–enhanced (DCE) MRI also have limitations, particularly when compared with this novel method. BOLD MRI provides a measure of relative hemoglobin oxygen saturation, but confounding influences of blood flow, arterial saturation, microvessel density, hematocrit, and oxygen consumption complicate interpretation of the BOLD signal and limit the ability to infer tissue pO2 (8, 9, 11). DCE MRI has also been used to infer differences in tumor pO2, but the parameters of perfusion, permeability, and extracellular volume fraction obtained from this method are only tangentially related to hypoxia; studies have reported weak correlates between these endpoints and the hypoxic status of tumors (12, 13). Perfluorocarbon (PFC) 19F MRS provides the most comparable imaging capabilities, as PFCs acquire absolute pO2 with a resolution of 1–3 mmHg (14). However, the main disadvantage of PFC MRI/MRS is its spatial resolution, which is inferior to that of pulsed EPRI.
While EPRI shows great promise for hypoxia imaging, until now it has been limited by its lack of ability to provide anatomical information. In this issue, Matsumoto et al. (7) demonstrate that they have overcome this limitation and provide a further adjunct to the anatomical information by incorporating multiple magnetic resonance parameters. The authors obtained magnetic resonance angiograms, apparent diffusion coefficient (ADC) maps, and arterial spin-labeling, corresponding to parameters of blood volume, cellularity/necrosis, and blood flow, respectively. This approach is particularly significant as the multiparametric possibilities are not limited to the analysis conducted in this experiment; BOLD or DCE MRI could be performed as easily as the ADC or arterial spin-labeling methods.
A comprehensive view of the tumor microenvironment requires metabolic information
From the overlaid functional and anatomical images obtained, Matsumoto et al. (7) selected regions of interest within the tumors and performed proton MRS to measure levels of metabolites such as lactate and choline. This is the first report of an imaging modality providing metabolic information simultaneously with pO2 values, furthering the elegance of this study. Yet, from this laudatory introduction arises a note of caution: imaging technology is only as valuable as its validation and interpretation. Matsumoto et al. conclude that their findings, showing high levels of lactate at normal pO2 levels, support the predominance of aerobic glycolysis in normoxic regions of tumor, also known as the Warburg effect (15). However, the role of lactate in tumor metabolism has much greater complexity than acknowledged with these conclusions. Without additional information to aid and support interpretation of the imaging results, it is difficult to claim that the images provide evidence for the Warburg effect.
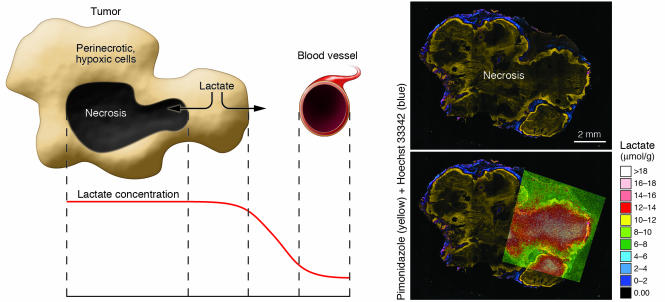
Tumor metabolism is a dynamic process of nutrient delivery and breakdown, which influences the tissue concentration of its substrates and products in a complicated manner. It has been well described that cancer cells, as most other cells, compensate for hypoxia by increasing their rate of glycolysis, a phenomenon known as the Pasteur effect (16). While the Pasteur effect in cancer cells was first described in the early 1930s, in part by Otto Warburg himself, it has never been found that lactate production decreases with hypoxia (16–18). Moreover, regions of chronic severe hypoxia in experimental tumors usually surround a necrotic core, which functions as a sink to accumulate lactate (Figure 1). Considering these factors, a situation in which lactate levels are lower in hypoxic as compared with normoxic tumor areas is highly unlikely, even if individual cancer types exhibit a strong Warburg effect. A possible explanation is that short-term changes in hypoxia, also known as fluctuating hypoxia, lead to metabolite concentrations that do not represent the current pO2 state of the tissue. In this scenario, the EPRI takes a “snapshot” of the oxygen status of the tumor, which may not be representative of the tumor over time. The authors themselves illustrate this influence by the fluctuations of EPRI pO2 values with carbogen breathing. As fluctuating hypoxia tends to be perfusion dominated, this would coincide with an area that has higher blood volume. Another explanation is that local tissue hypoglycemia could be restricting lactate production in tumors. However, such a lack of substrate will most likely lead to cell death shortly thereafter. In short, the authors’ finding of lactate being higher in normoxic than hypoxic tissue is unexpected and necessitates further explanation. This is particularly important when a novel method is used to measure these parameters. We would therefore like to underscore that imaging modalities, while powerful methods to provide insight into the tumor environment, are only as valuable as their validation and interpretation.
Figure 1. Mechanisms underlying increased lactate concentrations in hypoxic and necrotic versus normoxic, well-perfused tumor areas.
Necrosis occurs within the hypoxic core of the (experimental) tumor. Lactate produced by perinecrotic, hypoxic cells is cleared through the microvasculature. Lacking a route of drainage, lactate accumulates in the necrotic cavity. In addition, hypoxia leads to an increase in baseline lactate production rate via the Pasteur effect (16). Consequently, the concentration of lactate in hypoxic tumor areas is determined by oncogenic/hypoxic lactate production, lactate backflow from necrosis, and other factors, such as vascular efficiency and substrate availability. The right panel illustrates these principles in a human cervical carcinoma (SiHa) xenografted in a mouse. The administered hypoxia marker pimonidazole (yellow) labels hypoxic cells, whereas external Hoechst 33342 (blue) marks better-perfused and oxygenated parts of the tumor. Lactate was determined from cryoslices using quantitative bioluminescence microscopy. The images were color encoded and coregistered with the pimonidazole/Hoechst image.
Potential for clinical translation
On a final note, Matsumoto et al. (7) highlight the clinical implications of this combined imaging modality, particularly with respect to utilizing this method for dose contouring with intensity-modulated radiation therapy (IMRT). IMRT, also referred to as dose painting, is a technique used in radiation oncology in which the dose can be contoured to subvolumes within the tumor (19). For instance, a subvolume of a hypoxic area within the tumor could be targeted with higher radiation dose through IMRT. From a clinical perspective, the results of Matsumoto and colleagues are quite profound. They imply that a single diagnostic test could provide insight into tumor pO2 status, necrosis, metabolism, and perfusion, all with anatomic overlay. However, the clinical benefit and ease of implementation remains unknown. While there are studies illustrating the feasibility of performing hypoxia-guided IMRT (20, 21), of including hypoxia information in dose optimization algorithms (22), and of modeling tumor control probability (23, 24), there are currently no clinical data to support that a patient benefit exists. The majority of the current literature has failed to account for changes in hypoxic regions over the course of treatment that may render an original treatment plan ineffective or even more toxic. Sovik et al. have incorporated fluctuating hypoxic regions into their planning and have shown that a maximum tumor control probability would require IMRT replanning twice weekly (25). The clinical utility of the combined EPRI/MRI modality in IMRT dose painting will be uncertain until clinical trials assessing patient outcome and cost-benefit analyses are performed.
Currently, this combined modality requires a 7-T MRI machine. There are only about twenty 7-T or higher field strength MRI machines in use around the world, and those in clinical use are for research purposes (26). Theysohn et al. recently reported that 7-T magnets require scan times approximately twice as long as 1.5-T machines and that 7-T machines also elicit a broader range of complaints from patients. However, they also concluded that, in general, the complaints were not serious and that 7-T imaging was well tolerated by the majority of patients. Further data collection will be necessary to better determine the effects and acceptance of 7-T clinical MRI machines. As a possible alternative, future designs may attempt to find a common resonance at a more clinically useful field strength.
Despite some clinical challenges, this combined pulsed EPRI/MRI technique is relatively limitless in its preclinical applications, and the ability to noninvasively and repeatedly measure multiple tumor parameters will be an exceedingly powerful tool for assessing and refining our knowledge of the tumor environment. For example, the ability to assess multiple tumor parameters concomitantly will heighten the elegance of studies in transgenic mice or in spontaneous tumor models (27). The repeatability and noninvasive nature of this technique will be important for following tumor development and assessing therapeutic response. Successful translation into the clinic will provide clinicians with a greater understanding of how patients respond to therapy, and the metabolic and hypoxic information obtained could be used to decide treatment options in a patient- and tumor physiology–specific manner. In clinical trials, this method would aid in determining treatment schedules based on physiologic response.
In summary, the elegant method of combining pulsed EPRI with MRI/MRS provides a powerful tool to perform multiparametric analysis of tumor physiology, metabolism, and anatomy. It is clear that this method will be exceedingly valuable preclinically to better understand important interrelationships between perfusion, pO2, and metabolism. Eventual implementation of this technique into the clinic would translate this potential into patient benefit. Furthermore, even though this method has been initially highlighted in oncology, the imaging technique could be applied to many other diseases that involve hypoxia, such as ischemic heart disease, stroke, and inflammatory diseases.
Acknowledgments
Our thanks to Benjamin L. Viglianti, Andrew N. Fontanella, and Kelly Kennedy for their thoughtful discussions regarding this commentary. We also thank Chamali Wickramasekara for technical help and the creativity of Isabel Cardenas-Navia. This work was supported by NIH/NCI grants CA40355 and CA42745.
Footnotes
Nonstandard abbreviations used: BOLD, blood oxygen level dependent; DCE, dynamic contrast enhanced; EPRI, electron paramagnetic resonance imaging; IMRT, intensity-modulated radiation therapy; MRS, magnetic resonance spectroscopy; PFC, perfluorocarbon; pO2, partial pressure of oxygen (oxygenation).
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:1616–1619 (2008). doi:10.1172/JCI35543.
See the related article beginning on page 1965.
References
- 1.Folkman J. Tumor angiogenesis. Adv. Cancer Res. 1974;19:331–358. doi: 10.1016/s0065-230x(08)60058-5. [DOI] [PubMed] [Google Scholar]
- 2.Secomb T.W., Hsu R., Dewhirst M.W., Klitzman B., Gross J.F. Analysis of oxygen transport to tumor tissue by microvascular networks. Int. J. Radiat. Oncol. Biol. Phys. 1993;25:481–489. doi: 10.1016/0360-3016(93)90070-c. [DOI] [PubMed] [Google Scholar]
- 3.Nordsmark M., et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother. Oncol. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 4.Hockel M., et al. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 5.Hockel M., et al. Tumor hypoxia in pelvic recurrences of cervical cancer. Int. J. Cancer. 1998;79:365–369. doi: 10.1002/(SICI)1097-0215(19980821)79:4<365::AID-IJC10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Vaupel P., Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist. 2004;9(Suppl. 5):4–9. doi: 10.1634/theoncologist.9-90005-4. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto S., et al. Low-field paramagnetic resonance imaging of tumor oxygenation and glycolytic activity in mice. J. Clin. Invest. 2008;118:1965–1973. doi: 10.1172/JCI34928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manzoor A., Yuan H., Palmer G., Viglianti B., Dewhirst M.2008Imaging hypoxia. In Molecular imaging: principles and practice. R. Weissleder, et al., editors. B.C. Decker. Hamilton, Ontario, Canada. In press [Google Scholar]
- 9.Padhani A.R., Krohn K.A., Lewis J.S., Alber M. Imaging oxygenation of human tumours. Eur. Radiol. 2007;17:861–872. doi: 10.1007/s00330-006-0431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis J.S., Welch M.J. PET imaging of hypoxia. Q J Nucl. Med. 2001;45:183–188. [PubMed] [Google Scholar]
- 11.Neeman M., Dafni H., Bukhari O., Braun R.D., Dewhirst M.W. In vivo BOLD contrast MRI mapping of subcutaneous vascular function and maturation: validation by intravital microscopy. . Magn. Reson. Med. 2001;45:887–898. doi: 10.1002/mrm.1118. [DOI] [PubMed] [Google Scholar]
- 12.Cooper R.A., et al. Tumour oxygenation levels correlate with dynamic contrast-enhanced magnetic resonance imaging parameters in carcinoma of the cervix. Radiother. Oncol. 2000;57:53–59. doi: 10.1016/S0167-8140(00)00259-0. [DOI] [PubMed] [Google Scholar]
- 13.Lyng H., et al. Assessment of tumor oxygenation in human cervical carcinoma by use of dynamic Gd-DTPA-enhanced MR imaging. J. Magn. Reson. Imaging. 2001;14:750–756. doi: 10.1002/jmri.10016. [DOI] [PubMed] [Google Scholar]
- 14.Zhao D., Jiang L., Mason R.P. Measuring changes in tumor oxygenation. Methods Enzymol. 2004;386:378–418. doi: 10.1016/S0076-6879(04)86018-X. [DOI] [PubMed] [Google Scholar]
- 15.Gatenby R.A., Gillies R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder T., et al. Spatial heterogeneity and oxygen dependence of glucose consumption in R3230Ac and fibrosarcomas of the Fischer 344 rat. . Cancer Res. 2005;65:5163–5171. doi: 10.1158/0008-5472.CAN-04-3900. [DOI] [PubMed] [Google Scholar]
- 17.Zu X.L., Guppy M. Cancer metabolism: facts, fantasy, and fiction. Biochem. Biophys. Res. Commun. 2004;313:459–465. doi: 10.1016/j.bbrc.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 18.Robey I.F., Lien A.D., Welsh S.J., Baggett B.K., Gillies R.J. Hypoxia-inducible factor-1alpha and the glycolytic phenotype in tumors. Neoplasia. 2005;7:324–330. doi: 10.1593/neo.04430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling C.C., et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int. J. Radiat. Oncol. Biol. Phys. 2000;47:551–560. doi: 10.1016/S0360-3016(00)00467-3. [DOI] [PubMed] [Google Scholar]
- 20.Chao K.S., et al. A novel approach to overcome hypoxic tumor resistance: Cu-ATSM-guided intensity-modulated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2001;49:1171–1182. doi: 10.1016/s0360-3016(00)01433-4. [DOI] [PubMed] [Google Scholar]
- 21.Lee N.Y., et al. Fluorine-18-labeled fluoromisonidazole positron emission and computed tomography-guided intensity-modulated radiotherapy for head and neck cancer: a feasibility study. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:2–13. doi: 10.1016/j.ijrobp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alber M., Paulsen F., Eschmann S.M., Machulla H.J. On biologically conformal boost dose optimization. Phys. Med. Biol. 2003;48:N31–N35. doi: 10.1088/0031-9155/48/2/404. [DOI] [PubMed] [Google Scholar]
- 23.Malinen E., Sovik A., Hristov D., Bruland O.S., Olsen D.R. Adapting radiotherapy to hypoxic tumours. Phys. Med. Biol. 2006;51:4903–4921. doi: 10.1088/0031-9155/51/19/012. [DOI] [PubMed] [Google Scholar]
- 24.Thorwarth D., Eschmann S.M., Paulsen F., Alber M. Hypoxia dose painting by numbers: a planning study. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:291–300. doi: 10.1016/j.ijrobp.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 25.Sovik A., et al. Radiotherapy adapted to spatial and temporal variability in tumor hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:1496–1504. doi: 10.1016/j.ijrobp.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 26.Theysohn J.M., et al. Subjective acceptance of 7 Tesla MRI for human imaging. MAGMA. 2007 doi: 10.1007/s10334-007-0095-x. doi: [DOI] [PubMed] [Google Scholar]
- 27.Kirsch D.G., et al. A spatially and temporally restricted mouse model of soft tissue sarcoma. Nat. Med. 2007;13:992–997. doi: 10.1038/nm1602. [DOI] [PubMed] [Google Scholar]