Abstract
Obesity predisposes toward renal disease independently of diabetes and hypertension. In this issue of the JCI, Sharma and colleagues assessed the role of adiponectin, an adipose-derived hormone, in the pathogenesis of albuminuria (see the related article beginning on page 1645). Obese African Americans had reduced adiponectin levels associated with albuminuria. Adiponectin deficiency in mice induced oxidative stress, fusion of podocyte foot processes in the kidney glomerulus, and urinary albumin excretion. Adiponectin treatment reversed these abnormalities, likely through activation of AMPK. The benefits of adiponectin were observed in diabetic and nondiabetic mice. These findings suggest that adiponectin is a biomarker for kidney disease and may be targeted for prevention and treatment.
Obesity and kidney disease
The obesity epidemic has been linked to rising incidences of type 2 diabetes, cardiovascular disease, nonalcoholic fatty liver disease, sleep apnea, and cancer (1). Studies also indicate that obesity increases the risk of kidney disease independently of diabetes and hypertension (2, 3). Renal blood flow and glomerular filtration rate are elevated in obesity and are related to increased levels of the protein albumin in the urine (albuminuria) (4). Epidemiological studies suggest that microalbuminuria, defined as a urine albumin/creatinine ratio of 30–300 μg/mg, increases cardiovascular morbidity (5). Furthermore, an albumin/creatinine ratio considered to be within the normal range (10–30 μg/mg) is associated with higher cardiovascular risk (6). Insulin resistance, oxidative stress, and inflammation have all been implicated in albuminuria and declining kidney function, but the underlying mechanisms are unclear (4).
Hypoadiponectinemia is related to albuminuria
In the current issue of the JCI, Sharma et al. describe a role of adiponectin in the pathogenesis of albuminuria (7). Adiponectin is secreted exclusively by adipocytes (8). The monomer is a 30-kDa protein with 3 distinct domains: N-terminal hypervariable region, collagenous stalk, and C-terminal globular domain (8, 9). Adiponectin circulates in plasma as various complexes: high–molecular weight (HMW; 12- to 36-mer), low–molecular weight (hexamer), and trimeric forms. Total and HMW adiponectin levels are more abundant in females and decline in obesity (8, 9). Low adiponectin levels are related to higher prevalence of type 2 diabetes, inflammation, and atherosclerosis, and these abnormalities are reversed by adiponectin treatment (8, 10). The insulin-sensitizing effect of thiazolidinediones is related to increased HMW adiponectin levels (9, 10).
Previously, adiponectin receptors AdipoR1 and AdipoR2 — containing 7-transmembrane domains that are structurally and functionally distinct from G protein–coupled receptors — have been described (8). AdipoR1 is more widely expressed and enriched in muscle, while AdipoR2 is abundant in liver. Binding of adiponectin to AdipoR1 and AdipoR2 increases AMPK activation and PPARα signaling, resulting in suppression of gluconeogenesis, stimulation of fatty acid oxidation, and amelioration of diabetes (8, 11). AdipoR1- and AdipoR2-mediated signal transduction has been implicated in steatosis, inflammation, and oxidative stress, all key abnormalities associated with obesity and the metabolic syndrome (8).
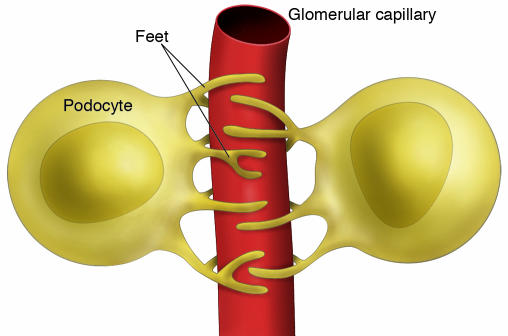
Sharma et al. found that plasma adiponectin concentration was inversely related to urinary albumin excretion in obese African Americans, a group prone to obesity and chronic kidney disease (7). Conversely, BMI, blood pressure, lipid levels, and plasma levels of IL-6 and plasminogen activator inhibitor–1 (PAI-1) were not associated with albuminuria in this group. To establish a role of adiponectin in the pathogenesis of albuminuria, Sharma et al. compared wild-type mice with adiponectin-deficient mice. Blood pressure, glucose levels, and lipid levels were not affected in Ad–/– mice fed a regular rodent diet; however, albuminuria was significantly higher, worsened with age, and was exacerbated by diabetes. Hydrogen peroxide levels increased in the urine of Ad–/– mice, consistent with oxidative stress. Electron microscopic examination revealed segmental fusion of the feet processes of podocytes (interdigitated cells that closely invest the glomerular capillary network in the kidney, as shown in Figures 1 and 2, and act in part as a filter for large macromolecules) in Ad–/– mice, although the thickness of the glomerular basement membrane and the structures of endothelial and mesangial cells were not altered by adiponectin deficiency. Albumin permeability was increased in a monolayer culture of podocytes from Ad–/– mice, consistent with albuminuria in this model.
Figure 1. Schematic demonstrating the interdigitation of podocyte foot processes that surround glomerular capillaries.
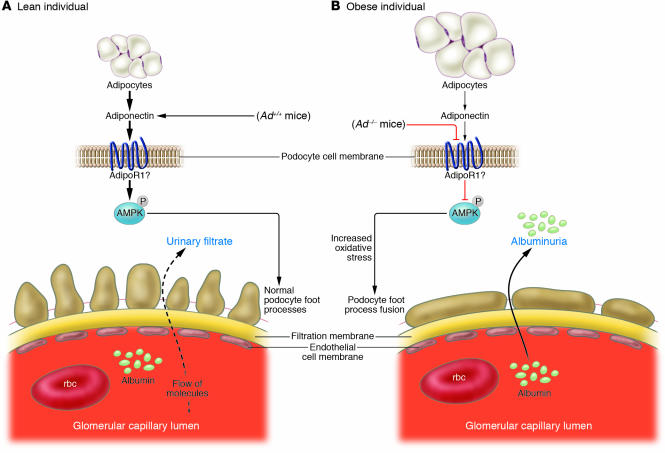
Figure 2. Schematic illustration of the effects of adiponectin on podocytes.
(A) In lean individuals, high levels of adiponectin phosphorylate and activate AMPK, presumably via AdipoR1, which prevents oxidative stress and the fusion of podocyte foot processes as well as limits albumin excretion. (B) As Sharma et al. demonstrate in this issue of the JCI (7), total adiponectin deficiency in Ad–/– mice or partial deficiency in obesity diminishes AMPK activity, increases oxidative stress, promotes the fusion of podocyte foot processes, and leads to higher urinary albumin excretion.
The authors showed that AdipoR1 was highly expressed in podocytes (7). AMPK phosphorylation was increased in podocytes by adiponectin or the AMPK activator 5-aminoimidazole-4-carboxamide-1-β-d-ribonucleoside (AICAR; Figure 2A). The distribution of the tight junction protein zonula occludens–1 (ZO-1) was disrupted in podocytes from Ad–/– mice and restored by adiponectin or AICAR treatment. Although the involvement of AdipoR1 in albuminuria and how this is coupled to AMPK signaling requires further study, these results demonstrate major effects of adiponectin on the kidney.
Adiponectin decreases oxidative stress and albuminuria
To determine whether adiponectin is causally related to kidney disease, the authors assessed effects of adiponectin treatment on urine albumin and hydrogen peroxide excretion (7). These parameters were related to podocyte structure, activity of AMPK, and expression of oxidative enzymes. Ad–/– mice were treated with the full-length or globular forms of adiponectin. Restoration of plasma adiponectin to levels measured in wild-type mice blunted albuminuria in nondiabetic as well as diabetic Ad–/– mice. Remarkably, adiponectin reversed the fusion of podocyte foot processes in Ad–/– mice. AMPK activity was attenuated in podocytes and glomeruli, and the expression of the NADPH oxidase Nox4, but not Nox1 or Nox2, was enhanced in the absence of adiponectin in Ad–/– mice. As predicted, adiponectin treatment restored AMPK activity and decreased Nox4 expression in parallel with improvements in urinary albuminuria and hydrogen peroxide levels.
Adiponectin as a biomarker of kidney disease: ready for prime time?
These carefully conducted studies demonstrate an important link between adiponectin and albuminuria (Figure 2) (7). The experiments in rodents and cell culture suggest that adiponectin signaling via AMPK regulates oxidative stress, segmental fusion of podocyte foot processes, and albuminuria. The capacity of adiponectin to reverse these abnormalities in Ad–/– mice is independent of glucose. These results are in agreement with exacerbation of albuminuria following surgical resection of kidneys in Ad–/– mice (12), which resulted in glomerular hypertrophy, podocyte injury, oxidative stress, inflammation, and fibrosis. Adiponectin decreased albuminuria, glomerular hypertrophy, and tubulointerstitial fibrosis, and these changes were associated with restoration of VCAM-1, monocyte chemoattractant protein–1, TNF-α, TGF-β1, collagen type I/III, and Nox to the levels found in wild-type mice. An antiinflammatory role for adiponectin in the kidney is in agreement with its similar actions in the vasculature, liver, and colon (13–15).
Low plasma adiponectin concentration could potentially serve as a biomarker for early detection of kidney disease. However, the inverse relationship between adiponectin and proteinuria among obese African Africans found in the current study (7) has not been consistently observed in others (16, 17). The plasma adiponectin concentration may reflect the extent of kidney damage, metabolic state, and ethnic background of the population under study (7, 15, 16). As mentioned earlier, adiponectin exists as HMW, hexamer, and trimer complexes, yet these forms of the protein were not measured in patients or mice in the current study (7).
The authors’ findings in Ad–/– mice raise an intriguing possibility that increasing adiponectin levels or stimulating AMPK activity may blunt albuminuria and prevent the progression of kidney disease. However, there are pitfalls in the translation of these results. Recombinant adiponectin was administered in mice with total adiponectin deficiency. As is often the case in hormone replacement, Ad–/– mice are expected to be hyperresponsive to adiponectin treatment. Because there are no patients with total adiponectin deficiency, the question remains as to whether patients with partial adiponectin deficiency will benefit from treatments that increase adiponectin levels. Previous studies in diabetic patients suggest this may be the case: rosiglitazone increased adiponectin levels, enhanced insulin sensitivity, and suppressed albuminuria in patients with type 2 diabetes (18, 19). AMPK activation also has the potential to ameliorate kidney disease, as evidenced by the effects of metformin and AICAR to increase AMPK phosphorylation and inhibit renal hypertrophy in diabetic rats (20).
Conclusions
The article by Sharma et al. (7) advances our understanding of renal pathophysiology. Until the discovery of leptin, the prevailing view of adipose tissue was that of a passive storage site for triglycerides. It is now well established that adipocytes secrete proteins that actively control energy homeostasis, glucose and lipid metabolism, neuroendocrine and cardiovascular function, and various physiological systems. Adiponectin is a logical candidate for exploring the link between adipose tissue and kidney function. The tools for assessing the relationships among adiposity, adiponectin, metabolism, and renal pathophysiology in rodents and humans are readily available, making the clinical translation of key elements of this paper feasible. The demonstration of the validity of adiponectin as a biomarker of albuminuria in humans is a crucial step in this process and may ultimately facilitate the prevention and treatment of kidney disease.
Footnotes
Nonstandard abbreviations used: AdipoR, adiponectin receptor; AICAR, 5-aminoimidazole-4-carboxamide-1-β-d-ribonucleoside; HMW, high molecular weight; Nox, NADPH oxidase; ZO-1, zonula occludens–1.
Conflict of interest: R.S. Ahima has received research support from Biomeasure/Ipsen Inc. and served on scientific advisory boards of Biomeasure/Ipsen Inc. and Ethicon Endo-Surgery (Johnson & Johnson).
Citation for this article: J. Clin. Invest. 118:1619–1622 (2008). doi:10.1172/JCI35655.
See the related article beginning on page 1645.
References
- 1.Ogden C.L., Yanovski S.Z., Carroll M.D., Flegal K.M. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 2.Coresh J., et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 3.Kramer H., et al. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am. J. Kidney Dis. . 2005;46:587–594. doi: 10.1053/j.ajkd.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Locatelli F., Pozzoni P., Del Vecchio L. Renal manifestations in the metabolic syndrome. . J. Am. Soc. Nephrol. . 2006;17(4 Suppl. 2):S81–S85. doi: 10.1681/ASN.2005121332. [DOI] [PubMed] [Google Scholar]
- 5.de Zeeuw D., Parving H.H., Henning R.H. Microalbuminuria as an early marker for cardiovascular disease. J. Am. Soc. Nephrol. 2006;17:2100–2105. doi: 10.1681/ASN.2006050517. [DOI] [PubMed] [Google Scholar]
- 6.Wachtell K., et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann. Intern. Med. 2003;139:901–906. doi: 10.7326/0003-4819-139-11-200312020-00008. [DOI] [PubMed] [Google Scholar]
- 7.Sharma K., et al. Adiponectin regulates albuminuria and podocyte function in mice. J. Clin. Invest. . 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadowaki T. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pajvani U.B., et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J. Biol. Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 10.Nawrocki A.R., et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J. Biol. Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi T., et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 12.Ohashi K., et al. Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Arterioscler. Thromb. Vasc. Biol. 2007;27:1910–1917. doi: 10.1161/ATVBAHA.107.147645. [DOI] [PubMed] [Google Scholar]
- 13.Ouedraogo R., et al. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J. Clin. Invest. 2007;117:1718–1726. doi: 10.1172/JCI29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu A., et al. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J. Clin. Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishihara T., et al. Effect of adiponectin on murine colitis induced by dextran sulfate sodium. Gastroenterology. 2006;131:853–861. doi: 10.1053/j.gastro.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Yano Y., et al. Differential impacts of adiponectin on low-grade albuminuria between obese and nonobese persons without diabetes. J. . Clin. Hypertens. (Greenwich) . 2007;9:775–782. doi: 10.1111/j.1524-6175.2007.07321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Looker H.C., et al. Adiponectin concentrations are influenced by renal function and diabetes duration in Pima Indians with type 2 diabetes. . J. Clin. Endocrinol. Metab. 2004;89:4010–4017. doi: 10.1210/jc.2003-031916. [DOI] [PubMed] [Google Scholar]
- 18.Pistrosch F., et al. Rosiglitazone improves glomerular hyperfiltration, renal endothelial dysfunction, and microalbuminuria of incipient diabetic nephropathy in patients. Diabetes. 2005;54:2206–2211. doi: 10.2337/diabetes.54.7.2206. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki Y., Cersosimo E., Triplitt C., DeFronzo R.A. Rosiglitazone decreases albuminuria in type 2 diabetic patients. Kidney Int. 2007;72:1367–1373. doi: 10.1038/sj.ki.5002516. [DOI] [PubMed] [Google Scholar]
- 20.Lee M.J., et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am. J. Physiol. Renal Physiol. 2007;292:F617–F627. doi: 10.1152/ajprenal.00278.2006. [DOI] [PubMed] [Google Scholar]