Abstract
Activated microglia can release a variety of proinflammatory cytokines that play a crucial role in the pathogenesis of multiple sclerosis (MS). IL-23, a novel proinflammatory cytokine, is required for the induction of experimental autoimmune encephalomyelitis. Previously we demonstrated that IL-23 is expressed in MS lesions and that microglia are one cellular source of IL-23 in MS patients. In the present study we investigated the inducible expression and regulation of p19, a key subunit of IL-23, in human microglia. We demonstrated the inducible expression of IL-23p19 by lipopolysaccharide-stimulated microglial cells. Using signaling pathway-specific inhibitors, we showed that blocking p38 MAP kinase or NF-κB signaling pathway significantly reduced p19 expression in microglia. The regulatory role of p38 MAP kinase in p19 expression was further confirmed by decreased expression in microglia transduced with dominant negative p38. We concluded that the p38 MAP kinase and NF-κB signaling pathways play an important role in regulation of IL-23p19 expression on human microglia, and are thus potential therapeutic targets in the treatment of MS.
Keywords: IL-23, microglia, multiple sclerosis, experimental autoimmune encephalomyelitis
Introduction
IL-23, a heterodimer cytokine in the IL-12 family, is composed of IL-12p40 and p19 subunits (Oppmann et al., 2000). Cytokines IL-12 and IL-23 are both produced by monocytes, but they promote two distinct T-cell subsets. In contrast to the role of IL-12 in inducing IFN-γ-producing Th1 cells, IL-23 drives the expansion of a novel T-cell population, Th17, characterized by the secretion of IL-17A, IL-17F, IL-6 and tumor necrosis factor (TNF) (Park et al., 2005; Veldhoen et al., 2006; Mangan et al., 2006; Zhou et al., 2007). Importantly the IL-23-driven Th17 cells are linked to autoimmune inflammation and mediated tissue destruction in chronic inflammatory disease such as multiple sclerosis (MS). In experimental autoimmune encephalomyelitis (EAE), which serves as the animal model of MS, IL-23-driven TIL17 cells can transfer demyelinating disease into the brain, indicating that IL-23 is a highly potent inducer of CNS immune pathology (Langrish et al., 2005). In addition, therapeutic treatment with anti-IL-23p19 antibodies in the relapsing and remitting EAE model reduced the serum level of proinflammatory cytokines, and prevented disease relapse by inhibiting epitope spreading initiated inside the brain (Chen et al., 2006). Thus, IL-23 plays a critical role in the development of neuroinflammatory disease.
Microglia are cells of the monocyte/macrophage lineage that reside in brain parenchyma in two major states: nonactivated (ramified, or resting) and activated (ameboid). Microglia have been proposed to play a role in host defense and tissue repair in the CNS. However, activation of microglia and consequent release of proinflammatory and /or cytotoxic factors, such as IL-1β, TNF-α, nitric oxide and reactive oxygen species, are believed to contribute to the pathophysiology of several neurodegenerative diseases, including MS and Alzheimer’s disease. In our previous studies, we identified human microglia, represented by CD11b+CD68+ as one of the major cellular sources of IL-23 in MS brain sections, and IL-23p19 production inside the brain correlated with MS disease severity (Li et al., 2007). Thus, therapeutic targets for precise and specific fine tuning of IL-23 responses including IL-23 production and its signaling will inhibit the inflammatory immune response and consequently benefit MS patients.
Aimed at therapeutic reduction of IL-23, we further investigated the intrinsic signaling pathways that mediate IL-23 production in microglia. Here we report the inducible expression of IL-23p19 in human microglia stimulated by LPS, and the involvement of p38 mitogen activated protein (MAP) kinase and NF-κB signal pathways in the regulation of IL-23p19 expression using pharmacological inhibitors and genetic manipulation.
Materials and Methods
Microglia cell cultures and activation
Because of the limited availability of naïve microglia from human brain, microglial cell lines are a necessary alternative to human microglia in in vitro studies and have provided significant information for the biology of human microglia in vivo (Nagai et al., 2005). Thus, in the present study we used commercially available human microglial cell lines to elucidate the regulation and inhibition of IL-23p19 production. The studies on human microglia were performed in accordance with the guidelines of the Thomas Jefferson University Ethics Review Board. Microglial cells were purchased from Clonexpress Company (Gaitherburg, MD) (lot numbers of microglial cells denote their origin from different donors), and cultured according to the manufacturer’s instructions. Briefly, microglial cells are isolated initially as a free floating population of cells from fetal brain tissue samples digested with collagenase and plated in a proprietary medium for 1–2 weeks. Microglial cells are further manipulated and grown in a proprietary medium specially developed for these cells. At this stage the cells grow as a mixed population containing both attached cells as well as a population of free-floating cells. The cells express CD45, CD14, CD68, and chemokine receptors. The cells are grown in 50:50 DMEM: F-12 supplemented with 5% FBS and 10ng/ml of M-CSF. Microglia-enriched populations were prepared from primary cultures by collecting cells that freely floated in the medium and were continuously cultured for 7 days. At this time the less adherent astrocytes were then floated off. Microglia were cultured for another 7 days and detached using trypsin (0.25%) and DNase (50 µg/ml). Microglia were replated at 5.5 × 105 cells/ml in their conditioned media and were stimulated with 0.1 µg/ml purified lipopolysaccharide (LPS) (Sigma Aldrich, St. Louis, MO).
Morphology conversion and confocal imaging of IL-23p19 expression in cultured human microglia
To visualize the morphological changes during cell activation, microglia were stained with FITC-conjugated RCA-I (1:50 RCA-I; Vector Lab, Burlingame, CA) for 1h at room temperature and treated with 0.1 µg/ml LPS. The morphology conversion of activated microglia was viewed with a Nikon Eclipse 600 fluorescent microscope. RCA-1 is a specific histochemical marker for microglia in the normal human brain, but does not react with astrocytes, oligodendrocytes, or neurons (Mannoji, Yeger, and Becker, 1986). The viability of cells was >97% as determined by trypan blue.
To determine whether cultured microglia in vitro produce IL-23p19, human microglia were activated by the above stimuli and IL-23p19 secretion was analyzed by double-immunofluorence staining as previously described (Li et al., 2007). Briefly, microglial cells were fixed by acetone and immunostained with purified anti-p19 antibody (Rat IgG2a; clone I178 at 1:200, Axxora, CA) and mAb against CD11b+ (clone 2LPM19c, Dako, CA), a general marker for microglia. After washing, secondary antibodies labeled with Alexa Fluor 486 or Alexa Fluor 546 were incubated for 1 hour at room temperature. Microglial cells incubated with purified isotype antibody (Rat IgG2a; Axxora) as the first antibody served as negative control for p19. Fluorescent images were acquired using a Zeiss LSM 510 Confocal microscope.
Signal-specific inhibition on p19 mRNA expression and IL-23 protein
To study signal pathways involved in IL-23p19 regulation, activated human microglia were cultured in the presence of signal-specific inhibitors, including genistein, SP600125, PD098590 (Sigma-Aldrich, St. Louis, MO), SB203580 and IKK-2 inhibitor IV (Calbiochem, La Jolla, CA). These inhibitors were dissolved in DMSO. Less than 0.1% DMSO was added to the cells. Real-time PCR was performed to quantify mRNA levels in microglial cells. The TaqMan probe for IL-12p40 and IL-23p19 and two endogenous controls, 18s RNA and β-actin, were purchased from Applied Biosystems (Forster City, CA). Total RNA extraction and reverse transcript into cDNA were carried out following the instructions of the cDNA synthesis kit (Invitrogen, CA). PCR was performed in 96-well microtiter plates under the following conditions: 2 min at 50°C, 10 min at 95°C and 40 cycles of 15 sec at 95°C and 1 min at 60°C. Quantification of gene expression was performed using standard curves and comparative Ct methods. In the standard curve method, the plasmids containing targeted sequences were used as a PCR template to set up a standard curve. A standard graph of the cycle threshold (Ct) values obtained from serial dilutions (10 to 106 copies/well) of the plasmid was constructed for both p19 and human β-actin. The Ct values from unknown samples were plotted on the respective standard curves, and the copy number was calculated with Sequence Detection System software (version 1.1; Applied Biosystems; Forster City, CA). By a comparable method, 18S rRNA was used as endogenous control. The Ct of 18S rRNA was subtracted from the Ct of the target gene to yield the ΔCT. Change in expression of the normalized target gene as a result of an experimental manipulation was expressed as 2−ΔΔCT where −ΔΔCT=ΔCT samples—CT controls. Each sample was analyzed in triplicate. To control for cross-contamination, a sample consisting of distilled water was also submitted.
The concentration of IL-23 in the supernatants of activated microglia was measured with ELISA kits (Bender MedSystems, Burlingame, CA) in which the capture Ab specifically recognizes IL-23p19 subunit and the detection Ab is anti-IL-12p40 mAb.
Western blot
Cell lysates of microglia were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. The primary antibodies were anti-total and three subtypes of anti-active MAP kinase, including ERK, JNK and p38 MAP kinase (Cell Signaling Technology, MA). Immunoreactive bands were visualized using an enhanced chemiluminescence detection kit according to the manufacturer’s instructions (Amersham, NJ).
Adenovirus infection and detection of β-Galactosidase-positive cells
Full-length p38 was obtained by PCR and cloned into pcDNA3. The mutation of dual-activating phosphorylation of p38, T180A and Y182F was constructed by QuikChange system (Stratagene), which cannot be activated by phosphorylation. Mutations were confirmed by DNA sequencing. Adenovirus harboring dominant negative p38 (Ad-dnp38) and β-galactosidase (AdLacZ) were constructed using the AdenoX adenovirus construction kit (BD-Clontech). Viral titer was determined by plaque assay in 293T cells. For adenoviral transductions, microglial cells were plated at 5×105 per well in 24-well tissue culture plates and fed with culture medium. The medium was aspirated 24 h later and replaced with culture medium containing adenovirus. Infection proceeded for 48 h, after which the medium was replaced with fresh complete medium containing LPS at 0.1 µg/ml. To determine the transfection efficiency, staining for expression of the β-galactosidase marker gene was performed. Transfected cells were fixed and incubated for 18 h in X-gal stain (2 mM MgCl2, 1 mg/ml X-gal, 5mM K3Fe(CN)6, and 5 mM K4Fe(CN)6 in PBS). To estimate the percentage of cells that were infected, the total cell number and lacZ-positive cells were counted in five random fields.
Statistical analysis
The expression levels of p19 in different types of tissue, or with different treatment of human microglia, were compared using the Mann-Whitney U test. P-values < 0.05 were considered significant.
Results
Inducible production of IL-23 in activated microglia
We have previously demonstrated IL-23p19 expression colocalized in CD11b+CD68+ cells in MS patients’ brain tissue sections and verified that macrophages/microglia are one of the cellular sources of IL-23 in the pathogenesis of MS (Li et al., 2007). In order to study the regulation of IL-23p19 in microglia, highly enriched microglial cells were generated after several weeks’ culture in vitro. Stained with FITC-conjugated RCA-1, the purity of microglial cells was validated by flow cytometry (data not shown) and microglia cell morphology conversion (Fig. 1A, B). Resting microglia appeared ramified with the characteristics of a long branch and small cell body (Fig. 1A), while activated microglia gradually lost the ramified morphology, and became typically amoeboid with a reduction in the branching pattern and the appearance of round macrophages (Fig. 1B) (Hailer et al., 1997).
Fig. 1. Inducible production of IL-23 in activated mciroglia.
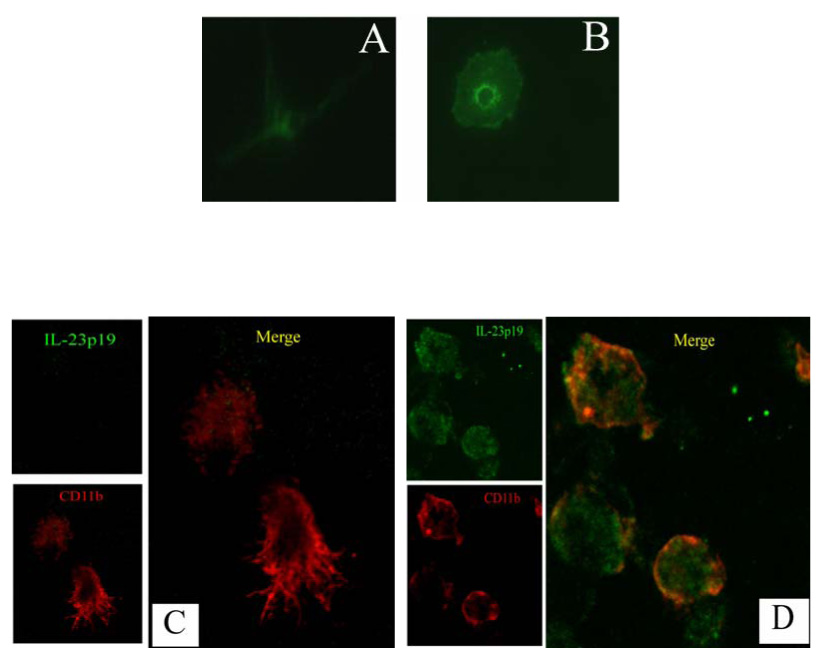
Isolated microglia from fetal brain tissues were continue to culture in vitro for several weeks to obtain highly purified microglial cells. Microglia were treated with LPS (0.1 µg/ml) to activate. (A, B): the shape conversion of high purified microglia in response to LPS stimulation. Microglia were stained with FITC-conjugated RCA-I, a specific marker for human microglia. Fluorescence microscopy shows cultured microglia undergoing morphological changes in response to stimuli. Resting microglia (A) appear ramified with long branches; while cells became amoeboid with round shapes following stimulation for 48 h (B). Magnification × 40. (C, D) Double-fluorescence immunostaining analysis of IL-23p19 expression (green) in resting (C) and activated (D) microglia represented by CD11b (red). No green staining was obtained in microglia that were incubated with isotype rat IgG2a as the first antibody (data not shown). Magnification × 40.
We next used immunofluorescence staining with anti-IL-23p19 and CD11b to analyze IL-23p19 at the protein level in human microglia by confocal microscopy. As seen in Fig. 1C, no immunostaining signals of IL-23p19 were detected inside the resting microglial cells. By contrast, obvious green staining was observed, representing IL-23p19 production in activated microglia (Fig 1D). In addition, with red staining of CD11b, morphological conversion was observed from the resting status of long branches into the activated status of round, macrophage-like shapes. Taken together, our data not only confirm the production of IL-23p19 in cultured microglia, but also demonstrate its inducible expression in response to stimuli.
Kinetics of IL-23p19 mRNA expression in activated microglia
To investigate the kinetics of IL-23p19 expression in activated microglial cells, we cultured these cells with different doses of LPS and, at different time points, we examined its expression using real-time PCR. Microglial cells were treated with varying concentrations of LPS for 24 hours. Without stimulation, the mRNA expression of IL-23p19 was scarcely detectable. LPS at more than 0.01 µg/ml induced a dramatic increase in IL-23p19 mRNA above the value in medium controls. Thus, IL-23 p19 mRNA was strongly upregulated in a dose-dependent manner in response to LPS (Fig. 2A).
Fig. 2. Kinetics of IL-23p19 mRNA expression in microglia.

Microglia were cultured with different doses of LPS and, at different time points, IL-23p19 mRNA expression was examined using real-time PCR. (A) Microglia were cultured with various concentrations of LPS (0, 0.01, 0.1, 0.5, and 1 µg/ml). After 24 h, p19 mRNA expression was determined by real-time PCR. Columns refer to mean values and bars to SEM of 3 independent experiments. (B) Microglia were cultured with 0.1 µg/ml LPS and harvested at different time points after culture (0, 4, 12, 24, 36, 48 hours). p19 mRNA expression was determined by real-time PCR. Symbols refer to mean values and bars to SEM of 3 independent experiments. (C) Microglia from 4 individual donors were cultured with 0.1 µg/ml LPS for 24 h. IL-23p19 mRNA expression was determined by real-time PCR. Un-stimulated microglia served as control. Columns refer to mean values and bars to SEM of 4 individual donors.
At different time points we observed the expression patterns of IL-12p40 and IL-23p19 in LPS-stimulated microglia. IL-23p19 mRNA expression was upregulated in the first 4 hours, reached its peak at 24 hours, and dramatically decreased afterwards. LPS at 0.1 µg/ml induced a gradual increase in IL-12p40 mRNA expression, reached its maximal expression around 24 hours, and maintained a higher expression than that of IL-23p19 afterwards (Fig. 2B).
We also assessed IL-23p19 mRNA expression in human microglia from different donors. These microglial cells were cultured in 12-well plates and treated with LPS at a concentration of 0.1 µg/ml for 24 hours. Activated microglia from all four donors expressed significantly increased levels of IL-23p19 mRNA (Fig. 2C) compared to resting microglia.
Different signal pathways regulate LPS-induced p19 expression
The inducible expression of IL-23p19 led us to delineate the signal pathways involved in its regulation in microglia. Here microglia were incubated with LPS, a strong stimulus for microglia activation, following the addition of several pharmaceutical inhibitors in the culture. These inhibitors have been widely used to specifically block corresponding signal pathways (Hebert and O'Callaghan, 2000; Wilms et al., 2003; Suzuki et al., 2004), and their optimal concentrations were determined based on the manufacturer’s recommendations and our preliminary results (data not shown). The potential contributions of intrinsic signaling pathway to IL-23p19 regulation were reflected by varied IL-23p19 expression in the presence of pharmaceutical inhibitors.
Microglia were pre-treated with pharmaceutical inhibitors 1 hour before LPS-stimulation, and continued to culture for 24 h. IL-23 expression was assayed by real-time PCR and ELISA. Compared to the copy number in microglia treated with LPS (Fig. 3A), approximately 45 ± 10% (p<0.01) of p19 expression was observed in microglia treated with SB203580, an inhibitor of p38 MAP kinase, which means more than half of IL-23p19 expression was inhibited. A less reduction (15 ± 5 %; p<0.05) of p19 was found in microglia treated with SP600125, an inhibitor of JNK kinase; while there was no significant change of p19 copy number in the presence of PD098059, a specific inhibitor of ERK1/2 kinase. In addition, approximately 63 ± 10 % (p< 0.01) reduction was observed in the presence of genistein (Akiyama et al., 1987; Gould, Rembold, and Murphy, 1995), implying that tyrosine phosphorylation is involved in the regulation of p19 expression. The NF-κB specific inhibitor, IKK-2 inhibitor IV (Karin, Yamamoto, and Wang, 2004; Podolin et al., 2005), reduced p19 mRNA by 71 ± 7% (p<0.01), indicating that NF-kB signaling is important in the regulation of p19 expression. The decreased expression of p19 was not due to the potential toxicity of DMSO, as cells with 0.1% DMSO had p19 expression similar to those with medium (data not shown). The IL-23 protein level was assayed by ELISA in the supernatants collected from the above microglia. The level of production of IL-23 was similar to its mRNA expression, except in SP600125-treated microglia where there was no significant change in IL-23 production compared with LPS-stimulated microglia (Fig. 3B).
Fig. 3. Signal pathway specific inhibitors regulate LPS-induced p19 expression.

Microglia were pretreated for 1h with signal pathway specific inhibitors including SB203580 (SB), PD98059 (PD), SP600125 (SP) at a concentration of 20 µM, genistein (GE) at 50 µM and IKK-2 inhibitor IV at 2.5 µM, followed by adding 0.1 µg/ml LPS. These concentrations are optimal for inhibition as shown by us (data not shown) and others. After 24 h, p19 mRNA was assayed by real-time PCR (A) and IL-23 protein in supernatant was assayed by ELISA (B). The viability of cells was >97% as determined by trypan blue. Values in brackets indicate the reduced percentage of p19 expression compared with that in LPS-stimulated microglia. Data are shown as the mean values and SEM of 3 independent experiments. p values represent comparison between LPS-stimulated microglia without inhibitor and with various signal pathway specific inhibitors. * p< 0.05 and ** p<0.01.
Activation of p38 MAPK is required for p19 expression
MAPK cascades, in particular p38 MAPK, are thought to play a key role in LPS- and cytokine-induced cytokine expression in microglia (Koistinaho and Koistinaho, 2002). We evaluated whether the activation of MAPK signaling pathways is involved in LPS-induced IL-23p19 expression. The status of activation of MAPK was determined by detecting their dually phosphorylated (Thr/Tyr) forms by western blotting against specific anti-phosphokinase Abs. We showed that LPS in human microglia can elicit rapid phosphorylation of three MAPKs of ERK, JNK (data not shown) and p38 (Fig. 4A). We also determined the dose-dependent impaired activation of three MAPKs by their corresponding inhibitors, including PD098059 vs. ERK, SP600125 vs. JNK (data not shown), and SB203580 vs. p38 (Fig. 4B). IL-23 production was assayed in the supernatants from the treated microglia, and displayed a significant reduction in the presence of SB203580 (> 20 µM) (Fig. 4C), but not in the presence of PD098059 and SP600125. These data indicate that p19 expression is regulated by signaling mediated by activated p38 MAPK.
Fig. 4. The requirement of activated p38 MAPK for the regulation of IL-23 production.

Microglia were cultured with indicated concentrations of LPS for 20 min and lysed for western blot. Activation of p38 MAPK was determined by anti-phospho-p38 mAb. Serial diluted SB203580 was pre-incubated 1 h after LPS addition. IL-23 concentration was assayed by ELISA. (A) LPS elicits rapid activation of p38 demonstrated by its phosphorylation in western blot. (B) Presence of SB203580 inhibits activation of p38. (C) The reduction of IL-23 in the microglia incubated with varied concentrations of SB203580. The viability of cells was 95–97% as determined by trypan blue. *, p<0.05, compared with the group of LPS+SB203580− microglia. One representative of two is shown.
Dominant-negative p38 blocked LPS-induced p19 expression in microglia
SB203580 has been shown to have no effect on ERK or JNK activity in vitro (Cuenda et al., 1995). However, it has also been reported that SB203580 can block not only p38 MAPK, but also JNK at high concentrations (Clerk and Sugden, 1998). To confirm the specificity of SB203580, which blocks only p38 MAPK but not JNK, we constructed adenovirus vectors encoding the dominant negative p38 that causes constitutive inactivation of p38 MAPK in transfected cells. As a control, adenovirus-encoding LacZ (Ad-LacZ) was transduced in parallel into microglia, and illustrated high transduction efficiency in microglia by Lac Z-staining (Fig. 5A). The concentration of IL-23 in the supernatants of transduced microglia assayed by ELISA displays a similar reduction of IL-23 in the dominant-negative p38 transduced microglia, as seen in the presence of pharmaceutical inhibitor SB203580 (Fig. 5B). Our data further confirmed the importance of the p38 MAPK signal pathway in the regulation of p19 expression in human microglial cells. There is no significant difference in IL-23 production between Ad-LacZ and wild-type microglia.
Fig. 5. Dominant-negative p38 blocked LPS-induced p19 expression in microglia.

Microglia were transduced with adenovirus-encoding dominant negative p38 (Ad-dnp38). Adenovirus-encoding lac Z (Ad-lacZ) was used as control. (A) x-gal staining indicated nearly 100% transduction efficiency 36 h after transduction. (B) Transduced microglia were stimulated with LPS for 18 h. The viability of cells was >97% as determined by trypan blue. Supernatant was assayed for IL-23 production by ELISA. **p < 0.01; *, p < 0.05. One of three experiments is represented.
Discussion
In the present study, we demonstrate that IL-23 is induced in cultured human microglia in vitro, and that the intrinsic signaling of p38 MAPK and NF-κB is involved in the regulation of IL-23 expression. We further confirm that phosphorylated Threonine 180 and Tyrosine 182 of p38, which cause activation of the p38 signaling pathway, is necessary for IL-23p19 production in human microglia.
In the pathogenesis of MS and EAE, it is believed that auto-aggressive myelin-reactive T lymphocytes migrate into the CNS, where they recognize their cognate target antigen and initiate an inflammatory cascade leading to tissue damage (Zamvil and Steinman, 2003). Microglia in the brain can activate naïve T cells or reactivate myelin-specific T cells, thereby initiating neuroinflammation that causes damage to the myelin sheath and injury to the nervous system. Proinflammatory cytokine IL-23, which supports the development of a novel subset of CD4+ inflammatory T cells known as Th17 cells, is considered to play a critical role in MS pathogenesis (Langrish et al., 2005; Park et al., 2005). With immunohistological staining, our previous studies in brain tissue of MS patients indicated IL-23p19 expression on CD11b+ cells including activated macrophages, microglia and dendritic cells (DCs). Here, the inducible expression of IL-23 detected in protein levels was further confirmed in activated human microglial cells. These findings imply the formation of the IL-23 / IL-17 axis in the local milieu, which potentially causes tissue destruction in the pathogenesis of MS. Thus, therapeutically targeting IL-23 is predicted to limit the inflammatory cascade and reduce tissue damage.
Developing therapies that interfere with signaling routes and gene expression, aimed at attenuating the proinflammatory response, could prove to be a promising avenue for the treatment of autoimmune disease. A danger signal, such as LPS, can trigger several intracellular signaling pathways, including three MAPKs in the early stage of gene expression, and the IkappaB kinase (IKK)-NF-κB pathway in human monocytes (Miller, Ernst, and Bader, 2005). These signaling pathways in turn activate a variety of transcription factors including NF-κB and AP-1, which coordinate the induction of many genes that encode inflammatory mediators. Activation of mitogen-activated protein (MAP) kinase has also been detected in MS brain (Natarajan et al., 2004) and reported to regulate expression of some proinflammatory cytokines, including TNF-α and IL-12 (Bhat et al., 1998; Feng et al., 1999). Recent studies have suggested that the MAP kinase pathway, especially p38 MAPK, is involved in the regulation of NF-κB activity (Jijon et al., 2004; Olson et al., 2007), and collaborates synergistically to induce proinflammatory cytokine gene expression and release (Craig et al., 2000). Besides NF-κB signal pathway, p38 MAP kinase is also essential in Jak-STAT signaling (Kovarik et al., 1999; Kovarik et al., 2001). Our study reports that LPS can quickly trigger the activation of MAP kinase in human microglia, suggesting the early signaling stage of the MAP kinase signaling pathway. Used as readout to reflect the activity of intrinsic signaling, IL-23 expression was dramatically reduced in response to impaired activation of NF-κB and p38 MAP kinase signaling by pharmacological inhibitors and genetic manipulation. Specifically, targeting Threonine180 and Tyrosine182 of p38 impaired activation of p38 signaling, and significantly reduced IL-23 production, suggesting possible therapeutic target sites for the regulation of IL-23 production. Although it has also been reported that inhibition of p38 MAPK and JNK activation enhanced the expression of IL-23p19 in PMA-treated human monocytic THP-1 cells (Utsugi et al., 2006), several studies in different model systems have shown results consistent with our observations. For example, it has been found that inhibition of p38 MAP kinase abrogates IL-23p19 expression in Mycobacterium tuberculosis-induced human macrophages (Yang et al., 2006); IL-23p19 expression induced by IL-17 was completely blocked by inhibitors of phosphatidylionositol kinase/Akt, NF-kB and p38 MAP kinase in synovial fibroblasts of rheumatoid arthritis (Kim et al., 2007); and c-Rel (one component of NF-kB signaling) played an essential role in TLR-induced IL-23p19 expression in murine DCs (Carmody et al., 2007). Although SP600125, a specific inhibitor of the JNK signal pathway, slightly decreased mRNA expression of IL-23p19 (15%), there was no significant difference in protein level. Together, our results indicate the involvement of p38 MAP kinase and NF-κB signal pathway in the regulation of IL-23 expression. In human inflammatory bowel disease, administration of the p38 inhibitor resulted in a significant decrease in disease activity index, as well as improved mucosal healing (Hommes et al., 2002). Our studies suggest that p38 MAP kinase, positioned in the early stage of signal transduction, could be a potential therapeutic target for precise and specific fine tuning of IL-23 production in human microglia in the treatment of human MS.
In conclusion, we in the present study detected IL-23p19 expression on human microglia in vitro by northern blot and double-fluorescence immunostaining. We further demonstrated that both the p38 MAP kinase and the NF-κB signaling pathways play important roles in the regulation of IL-23p19 production in human microglia. Delineation of the differential regulatory pathways involved will lead to novel therapeutic targets for precise and specific fine-tuning of cytokine responses, and thus immunomodulation, in autoimmune diseases.
Acknowledgements
This study was supported by the NIH, the National Multiple Sclerosis Society, and the Groff Foundation. We thank Katherine Regan for editorial assistance.
Abbreviations
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- APC
antigen presenting cell
- MS
multiple sclerosis
- MAP kinase
Mitogen Activated Protein kinase
- p19
interleukin IL-23 subunit p19
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J. Neurosci. 1998;18:1633–1641. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody RJ, Ruan Q, Liou HC, Chen YH. Essential roles of c-Rel in TLR-induced IL-23 p19 gene expression in dendritic cells. J. Immunol. 2007;178:186–191. doi: 10.4049/jimmunol.178.1.186. [DOI] [PubMed] [Google Scholar]
- Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin. Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerk A, Sugden PH. The p38-MAPK inhibitor, SB203580, inhibits cardiac stress-activated protein kinases/c-Jun N-terminal kinases (SAPKs/JNKs) FEBS Lett. 1998;426:93–96. doi: 10.1016/s0014-5793(98)00324-x. [DOI] [PubMed] [Google Scholar]
- Craig R, Larkin A, Mingo AM, Thuerauf DJ, Andrews C, McDonough PM, Glembotski CC. p38 MAPK and NF-kappa B collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J. Biol. Chem. 2000;275:23814–23824. doi: 10.1074/jbc.M909695199. [DOI] [PubMed] [Google Scholar]
- Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- Dean JL, Sully G, Clark AR, Saklatvala J. The involvement of AU-rich element-binding proteins in p38 mitogen-activated protein kinase pathway-mediated mRNA stabilisation. Cell Signal. 2004;16:1113–1121. doi: 10.1016/j.cellsig.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Feng GJ, Goodridge HS, Harnett MM, Wei XQ, Nikolaev AV, Higson AP, Liew FY. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J. Immunol. 1999;163:6403–6412. [PubMed] [Google Scholar]
- Gould EM, Rembold CM, Murphy RA. Genistein, a tyrosine kinase inhibitor, reduces Ca2+ mobilization in swine carotid media. Am. J. Physiol. 1995;268:C1425–C1429. doi: 10.1152/ajpcell.1995.268.6.C1425. [DOI] [PubMed] [Google Scholar]
- Hailer NP, Heppner FL, Haas D, Nitsch R. Fluorescent dye prelabelled microglial cells migrate into organotypic hippocampal slice cultures and ramify. Eur. J. Neurosci. 1997;9:863–866. doi: 10.1111/j.1460-9568.1997.tb01436.x. [DOI] [PubMed] [Google Scholar]
- Hebert MA, O'Callaghan JP. Protein phosphorylation cascades associated with methamphetamine-induced glial activation. Ann. N. Y. Acad. Sci. 2000;914:238–262. doi: 10.1111/j.1749-6632.2000.tb05200.x. [DOI] [PubMed] [Google Scholar]
- Hommes D, van den BB, Plasse T, Bartelsman J, Xu C, Macpherson B, Tytgat G, Peppelenbosch M, Van DS. Inhibition of stress-activated MAP kinases induces clinical improvement in moderate to severe Crohn's disease. Gastroenterology. 2002;122:7–14. doi: 10.1053/gast.2002.30770. [DOI] [PubMed] [Google Scholar]
- Jijon H, Allard B, Jobin C. NF-kappaB inducing kinase activates NF-kappaB transcriptional activity independently of IkappaB kinase gamma through a p38 MAPK-dependent RelA phosphorylation pathway. Cell Signal. 2004;16:1023–1032. doi: 10.1016/j.cellsig.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat. Rev. Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- Kim HR, Cho ML, Kim KW, Juhn JY, Hwang SY, Yoon CH, Park SH, Lee SH, Kim HY. Up-regulation of IL-23p19 expression in rheumatoid arthritis synovial fibroblasts by IL-17 through PI3-kinase-, NF-kappaB- and p38 MAPK-dependent signalling pathways. Rheumatology. (Oxford) 2007;46:57–64. doi: 10.1093/rheumatology/kel159. [DOI] [PubMed] [Google Scholar]
- Koistinaho M, Koistinaho J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia. 2002;40:175–183. doi: 10.1002/glia.10151. [DOI] [PubMed] [Google Scholar]
- Kovarik P, Mangold M, Ramsauer K, Heidari H, Steinborn R, Zotter A, Levy DE, Muller M, Decker T. Specificity of signaling by STAT1 depends on SH2 and C-terminal domains that regulate Ser727 phosphorylation, differentially affecting specific target gene expression. EMBO J. 2001;20:91–100. doi: 10.1093/emboj/20.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovarik P, Stoiber D, Eyers PA, Menghini R, Neininger A, Gaestel M, Cohen P, Decker T. Stress-induced phosphorylation of STAT1 at Ser727 requires p38 mitogen-activated protein kinase whereas IFN-gamma uses a different signaling pathway. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13956–13961. doi: 10.1073/pnas.96.24.13956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chu N, Hu A, Gran B, Rostami A, Zhang GX. Increased IL-23p19 expression in multiple sclerosis lesions and its induction in microglia. Brain. 2007;130:490–501. doi: 10.1093/brain/awl273. [DOI] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Mannoji H, Yeger H, Becker LE. A specific histochemical marker (lectin Ricinus communis agglutinin-1) for normal human microglia, and application to routine histopathology. Acta Neuropathol. (Berl) 1986;71:341–343. doi: 10.1007/BF00688060. [DOI] [PubMed] [Google Scholar]
- Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- Nagai A, Mishima S, Ishida Y, Ishikura H, Harada T, Kobayashi S, Kim SU. Immortalized human microglial cell line: phenotypic expression. J Neurosci Res. 2005;81:342–348. doi: 10.1002/jnr.20478. [DOI] [PubMed] [Google Scholar]
- Natarajan C, Sriram S, Muthian G, Bright JJ. Signaling through JAK2-STAT5 pathway is essential for IL-3-induced activation of microglia. Glia. 2004;45:188–196. doi: 10.1002/glia.10316. [DOI] [PubMed] [Google Scholar]
- Olson CM, Hedrick MN, Izadi H, Bates TC, Olivera ER, Anguita J. p38 mitogen-activated protein kinase controls NF-kappaB transcriptional activation and tumor necrosis factor alpha production through RelA phosphorylation mediated by mitogen- and stress-activated protein kinase 1 in response to Borrelia burgdorferi antigens. Infect. Immun. 2007;75:270–277. doi: 10.1128/IAI.01412-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolin PL, Callahan JF, Bolognese BJ, Li YH, Carlson K, Davis TG, Mellor GW, Evans C, Roshak AK. Attenuation of murine collagen-induced arthritis by a novel, potent, selective small molecule inhibitor of IkappaB Kinase 2, TPCA-1 (2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3- thiophenecarboxamide), occurs via reduction of proinflammatory cytokines and antigen-induced T cell Proliferation. J. Pharmacol. Exp. Ther. 2005;312:373–381. doi: 10.1124/jpet.104.074484. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J. Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsugi M, Dobashi K, Ishizuka T, Kawata T, Hisada T, Shimizu Y, Ono A, Mori M. Rac1 negatively regulates lipopolysaccharide-induced IL-23 p19 expression in human macrophages and dendritic cells and NF-kappaB p65 trans activation plays a novel role. J. Immunol. 2006;177:4550–4557. doi: 10.4049/jimmunol.177.7.4550. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Wilms H, Rosenstiel P, Sievers J, Deuschl G, Zecca L, Lucius R. Activation of microglia by human neuromelanin is NF-kappaB dependent and involves p38 mitogen-activated protein kinase: implications for Parkinson's disease. FASEB J. 2003;17:500–502. doi: 10.1096/fj.02-0314fje. [DOI] [PubMed] [Google Scholar]
- Yang CS, Song CH, Lee JS, Jung SB, Oh JH, Park J, Kim HJ, Park JK, Paik TH, Jo EK. Intracellular network of phosphatidylinositol 3-kinase, mammalian target of the rapamycin/70 kDa ribosomal S6 kinase 1, and mitogen-activated protein kinases pathways for regulating mycobacteria-induced IL-23 expression in human macrophages. Cell Microbiol. 2006;8:1158–1171. doi: 10.1111/j.1462-5822.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- Zamvil SS, Steinman L. Diverse targets for intervention during inflammatory and neurodegenerative phases of multiple sclerosis. Neuron. 2003;38:685–688. doi: 10.1016/s0896-6273(03)00326-x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]


