Abstract
Mucopolysaccharidosis type I (MPS I) is one of the most common lysosomal storage diseases with progressive neurological dysfunction. To characterize the chronological behavioral profiles and identify the onset of functional deficits in a MPS I mouse model (IDUA−/−), we evaluated anxiety, locomotor behavior, startle, spatial learning and memory with mice at 2-, 4-, 6- and 8- months of age. In automated open-field test, IDUA−/− mice showed hypoactivity as early as 2- months of age and altered anxiety starting from 6-months of age during the initial exploratory phase, even through normal habituation was observed at all ages. In the marble-burying task, the anxiety-like compulsive behavior was normal in IDUA−/− mice at almost all tested ages, but significantly reduced in 8-month old male IDUA−/− mice which coincided with the rapid death of IDUA−/− males starting from 7-months of age. In the Morris water maze, IDUA−/− mice exhibited impaired proficient learning only at 4-months of age during the acquisition phase. Spatial memory deficits were observed in IDUA−/− mice during both 1- and 7-days probe trials at 4- and 8-months of age. The IDUA−/− mice performed normally in a novel object recognition task at younger ages until 8 month old when reduced visual cognitive memory retention was noted in the IDUA−/− mice. In addition, 8-month old IDUA−/− mice failed to habituate to repeated open-field exposure, suggesting deficits in nonaversive and non-associative memory. In acoustic startle assessment, significantly more non-responders were found in IDUA−/−, but normal performance was seen in those that did show a response. These results presented a temporal evaluation of phenotypic behavioral dysfunctions in IDUA−/− mice from adolescent to maturity, indicating the impairments, with different age of onset, in locomotor and anxiety-like compulsive behaviors, spatial learning and memory, visual recognition, and short-term non-associative memory retention. This study would also provide guidelines for the experimental designs of behavioral evaluation on innovative therapies for the treatment of MPS type I.
Keywords: animal model, lysosomal storage disease, Hurler syndrome, behavior profiles, spatial learning, open-field, locomotion
1. Introduction
Mucopolysaccharidosis type I (MPS I) is one of the most common lysosomal storage diseases (LSD). As a group, LSDs have a collective incidence of ~1 in 7,000 live births and 65% of these affect the central nervous system (CNS) (Neufeld, 1991). MPS I is caused by the deficiency of alpha-L-iduronidase (IDUA) and the subsequent systemic accumulation of glycosaminoglycans (GAGs). The clinical features in patients with MPS I are associated with progressive systemic tissue pathology and multi-organ dysfunction. In severe forms of MPS I (i.e., Hurler syndrome), the CNS complications are devastating and characterized by hydrocephalus, learning delays and mental retardation culminating in dementia. If untreated, children with MPS I usually die before the age of 10. One of the current treatments for MPS I, allogeneic bone marrow transplantation (BMT), is effective in prolonging a child’s life and ameliorating many of the clinical manifestations of the disease (Church et al., 2007; Krivit et al., 1999). However, clinical benefits of BMT for CNS abnormalities vary dramatically and seem to be associated with the age of treatment. For example, when children with MPS I are treated with allogeneic BMT early in life (less than 2-years old) there is an attenuation of CNS deterioration or in some cases preservation of normal intellectual function (Peters et al., 1996; Shapiro et al., 1995). In contrast to early BMT therapy, little benefit was shown if CNS deterioration was apparent prior to treatment (Souillet et al., 2003; Staba et al., 2004). Thus, optimal prevention of CNS deterioration in MPS is possible only when intervention (treatment) occurs before the onset of neurological dysfunction. Therefore, early diagnosis and determination of the optimal timing for intervention or a window for treatment could be crucial to successful therapy. However, in the human literature, there are only a few detailed case history studies on clinical outcomes or disease progression that address CNS aspects of the neuronopathic forms of LSD and in particular MPS I. In addition, because of the limitation of BMT with the risk of significant mortality and the high rate of engraftment failure (Schiffmann and Brady, 2002), innovative therapies with consistent long-term clinical benefits are still needed.
A transgenic IDUA knockout murine model (IDUA−/−, MPS I), generated by disruption of the open reading frame with an insertion in exon 6 (Clarke et al., 1997), has made it possible for the systematic evaluation of the neurological deficits associated with MPS I. In this regard, some behavioral impairments have been reported by us (Hartung et al., 2004; Pan et al., 2003) and others (Reolon et al., 2006) using this mouse model. We found that animals subjected to 3 consecutive short-term open field tests, 5 min each with an inter-trial interval of 30 (Pan et al., 2003) or 90 (Hartung et al., 2004) min, failed to habituate to the testing apparatus (i.e., showed sustained activity) compared to wildtype animals on the third trial. In the study by Reolon et al.(Reolon et al., 2006), IDUA−/− mice performed similarly to controls during a 5 min open-field test including the number of line crossings and latency to begin moving after being placed in the apparatus, except that IDUA−/− mice showed a reduced number of rears compared to controls. Furthermore, no differences were observed in a novel object recognition task or in an inhibitory step-down avoidance task with a 90 min retention interval. However, when animals were examined 24 h after inhibitory avoidance training, the IDUA−/− mice showed deficits in memory. Taken together, these data suggest that the IDUA−/− mice have an altered locomotor response to an open-field upon multiple presentations and have some degree of memory inhibition, suggesting this mouse is representative of some defects seen in human patients with MPS I (i.e., Hurler syndrome).
While the previous data identified some specific phenotypes of IDUA−/− mice compared to controls, the progressive deterioration of CNS function with age has not been addressed. In fact, the previous two studies examined animals only at a single time point (4-months old by us or 5–7 months mixed age by Reolon), and also did not consider potential sex differences. These considerations are especially important because the progressive nature of the disease, gender differences, complications arising from other symptoms (e.g., musculoskeletal dysfunctions) on behavioral appearance, or the combination of these may influence behavioral outcomes. Therefore, a detailed evaluation is needed to determine when the IDUA−/− mice begin to demonstrate specific behavioral phenotypes (e.g., locomotor changes), if sexual dimorphism exists in the progression of the disease, if other cognitive abilities, such as spatial learning and memory, are affected, and if anxiety-related behaviors might also be changed (since altered anxiety can influence learning and memory).
In the present report, we examined IDUA−/− mice repeatedly, every other month from 2–8 months of age, in behavioral assessments measuring anxiety, locomotor behavior, and spatial learning and memory. These tasks were selected because of previous data and because of the brain pathology associated with IDUA−/− mice. In particular, abnormal lysosomal storage, i.e., cytoplasmic vacuolation, has been found in the neurons of cerebral cortex and Purkinje cells of the cerebellum as early as 2-month of age (Clarke et al., 1997). Abnormal GAG accumulation and/or GM2 ganglioside staining has been demonstrated in the hippocampus, neostriatum, cerebellum and cortical areas as well as other regions in adult MPS I mice (older than 3-months) (Chung et al., 2007; Hartung et al., 2004). Both the Morris water maze and novel object recognition task are known to be dependent upon the hippocampus, and other regions for proficient learning of these tasks (Clark et al., 2000; Squire and Zola, 1996; Zola et al., 2000). In order to determine if sensorimotor deficits existed we tested animals for their ability to navigate to a cued platform in the Morris water maze at 2 months of age. We also examined animals at 8 months to determine if they were responsive to acoustic startle stimuli since hearing can be affected in IDUA−/− mice (Schachern et al., 2007) and the cerebellum is also involved in acoustic startle. Animals were examined in the repeated open-field habituation test at 8 months of age to confirm our previous results and as a comparison to the automated locomotor tests that we did at all ages. Finally, anxiety-related behavior was examined because the progression of systemic symptoms with age can affect stress levels in MPS I, and changes in anxiety can affect learning and memory tasks. The overall aims of these assessments were to characterize the chronological behavioral profiles in MPS I mice and identify the onset of functional deficits.
2. Results
2.1. Body weights, metabolic accumulation and survival curve
Body weights were recorded weekly to monitor the overall health of the mice and weights from every third week beginning on week 8 were subjected to analysis (Fig.1A). Regardless of genotype, all animals gained weight continually over the 8 month testing period [Age, F(8, 408) =310.83, p <0.0001]. The IDUA−/− mice weighed more than their normal littermates [Genotype, F(1, 55.1) = 13.82, p <0.0005] in an age-dependent manner [Genotype × Age, F(8, 408) = 4.93, p <0.0001]. Body weight was similar between both groups at 8 weeks of age. However the IDUA−/− mice weighed significantly more than controls beginning at 11 weeks of age for female and 14 weeks for male, and remained heavier until the end of the 34-week testing period, with the same trend observed in both genders. Interestingly, the majority of the body weight changes occurred during 8- to 14-weeks of development, coinciding with appearance of liver pathology, which was indistinguishable at 8-weeks, but emerged to the characteristic lysosomal storage accumulation at 14-week of age (data not shown). As expected, the males weighed more than the females (Sex, p < 0.0001) and this disparity grew larger across weeks (Sex × Week, p <0.0001).
Fig. 1.
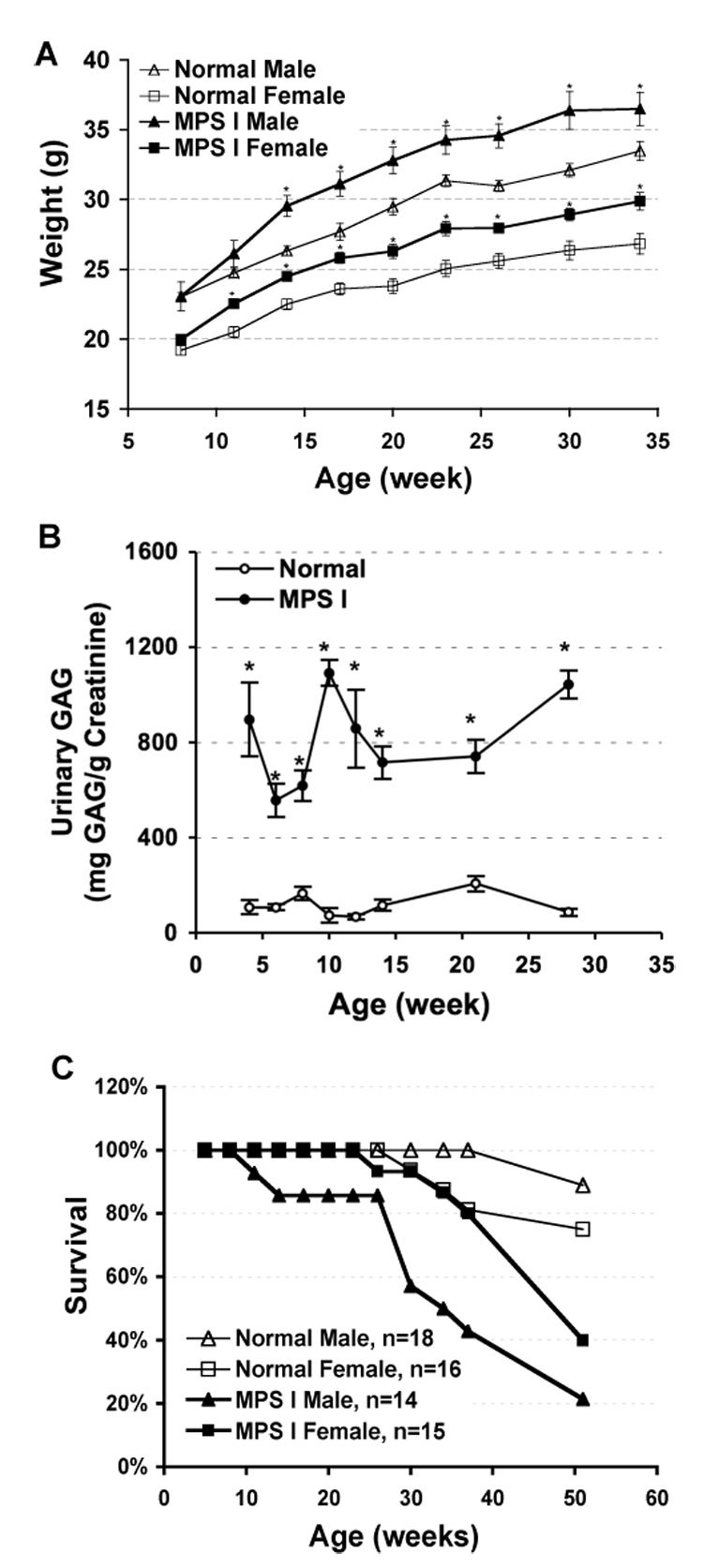
(A) Mean body weights ± SEM for male and female IDUA−/− mice and their control heterozygote littermates. N = 13–18 for normal male, 14–16 for normal female, 7–14 for IDUA−/− male and 13–15 for IDUA−/− female. (B) Urinary GAG accumulation in normal (n=4–7) and MPS I mice (n=4–6). (C) Survival curve for normal and IDUA−/− mice from weaning up to 1-yr of age. * P < 0.001.
To monitor the systemic metabolic status of MPS I mice during development, GAG accumulation was evaluated in urine of MPS I mice and normal heterozygous controls from 4-weeks to 7-months of age (Fig. 1B). Significant difference (p<0.001) in urinary GAG accumulation was demonstrated between MPS I mice and normal carrier as early as 4-weeks of age and throughout the 7-month observation periods. Similar GAG accretion in urine was detected in MPS I mice at all age tested points.
The overall survival of affected MPS I mice and normal heterozygous littermates were determined up to 1-year of age (Fig. 1C). There were only 21% (3/14) male and 40% (6/15) female MPS I mice alive by 12-months, distinctly lower than heterozygous controls (89% or 16/18 of males and 75% or 12/16 of females) that were randomly caged together by same gender. The median survival age in male IDUA−/− mice (32-weeks) was much shorter than their female counterparts (48-weeks). Interestingly, the rapid loss of MPS I mice started at about 7-months of age in males; while the survival rate in MPS I females remained indistinguishable from the control group until 9-month of age.
2.2. Elevated Zero Maze
At 2-months of age, animals were tested for anxiety-related behavior in the elevated zero maze. No significant differences were noted between genotypes or sex for anxiety measurements such as open quadrant entries, time in the open, or number of head dips. Regardless of gender, the time spent in open was 105.9 ± 6.7 s for control mice and 103.6 ± 5.3 s for IDUA−/− mice. A trend was seen for the number of head dips (Gene, F3.21, p= 0.08) (data not shown).
2.3. Locomotor Behavior
Spontaneous locomotor behavior was examined at 2-, 4-, 6- and 8-months of age in order to determine if animals failed to habituate to the task as previously shown in a repeated open-field test (Hartung et al., 2004; Pan et al., 2003) and to determine if IDUA−/− mice had motoric decrements associated with age. At all ages of testing, all animals, regardless of sex and genotype, showed habituation to the test arenas as evidenced by a decrease in horizontal activity during the 90 min testing period, Interval, p < 0.0001 (Fig. 2A). In addition, the IDUA−/− mice were hypoactive compared to unaffected littermates at all ages [Genotype, F(1,57.5) = 4.31, p < 0.05 (2 months), F(1,56.2) = 5.84, p < 0.02 (4 months), F(1,48.6) = 5.71, p < 0.03 (6 months)], except that at 8-months the effect fell short of statistical significance [F(1,46) = 3.88, p =0.08]. Interestingly, this hypoactivity in the IDUA−/− mice was dependent upon the interval examined [Genotype × Interval, F(8,351) = 2.05, p = 0.04 (2 months), F(8,350) = 1.94, p = 0.05 (4 months), F(8,300) = 2.46, p < 0.02 (6 months), and F(8,368) = 2.11, p < 0.04 (8 months)]. At 2 months of age, significant hypoactivity in the IDUA−/− mice was observed during the first 30 min of testing (i.e., intervals 1–3), and at 4 months reductions were observed during the first 30 min as well as the last 10 min (interval 9). At 6 and 8 months of age, the IDUA−/− mice had decreased locomotion during the first 20 min, and at 6 months of age a reduction was also observed at intervals 5 and 6. No other significant main effects or interactions were observed at 2, 4, or 6 months of age. At 8 months the females, regardless of genotype, had greater horizontal activity compared to the males (p < 0.004).
Fig. 2.
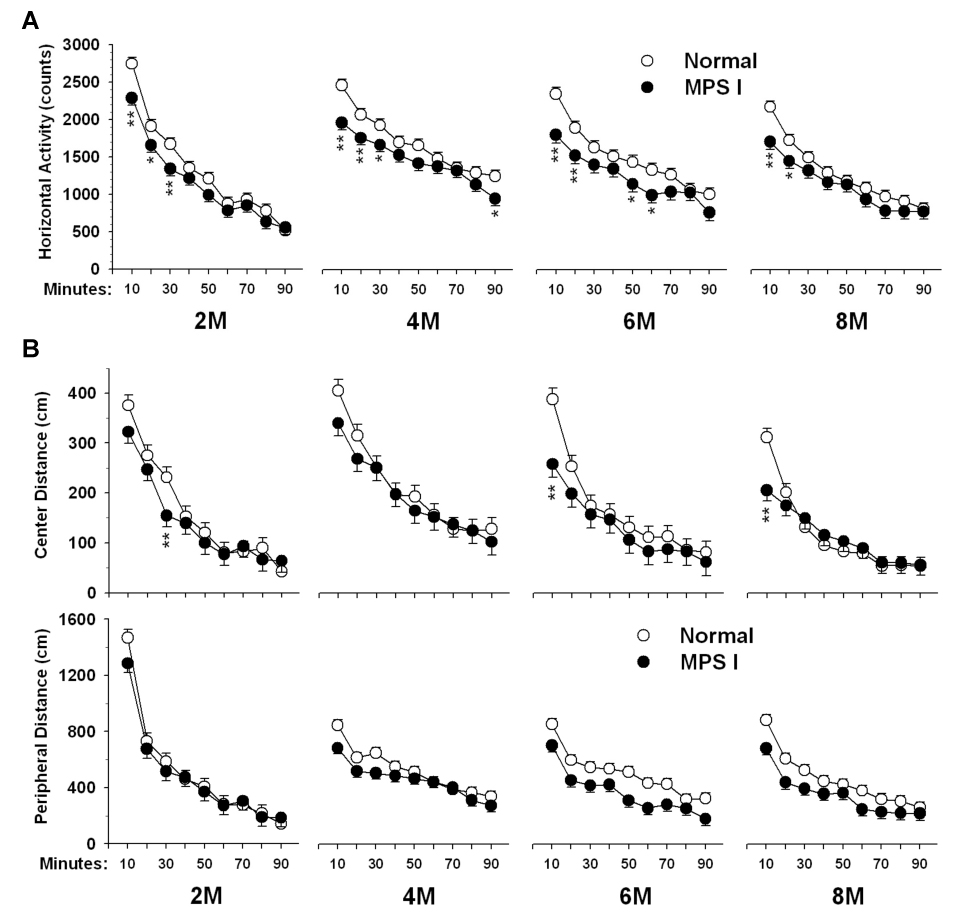
Spontaneous locomotor behavior in IDUA−/− and control mice from 2-months (2M) to 8-months (8M) of age. Animals were placed in the automated Digiscan apparatus for 90 min of free exploration. Data are mean ± SEM for (A) horizontal activity (total number of beam breaks) and (B) distance traveled in the center. N of controls and IDUA−/− = 33 and 27 for 2M, 33 and 26 for 4M, 31 and 21 for 6M, 30 and 20 for 8M, respectively. *p < 0.05.
The distance traveled in the center of the arenas was also measured since this can be an indication of altered anxiety especially if animals remain thigmotaxic throughout the entire test period (Fig 2B). Regardless of the age of testing or the genotype of the animals, the distance traveled in the center decreased over the test period, Interval, p < 0.0001. At 2 months, consistent with the reduction in horizontal activity, the IDUA−/− mice had a decrease in the distance traveled in the center during the third interval, Genotype×Interval, F(8,360) = 2.17, p < 0.03. No gene differences in center distance were observed at 4 months. At 6 months, the IDUA−/− mice showed reduced distance traveled in the center during the first interval, Genotype × Interval, F(8,293) = 2.02, p < 0.05, and this was the result of the female IDUA−/− mice, Genotype×Sex×Interval, F(8,293) = 1.95, p = 0.05. At 8 months, the IDUA−/− mice again traveled in the center less than controls during the first interval, Genotype×Interval, F(8,292) = 3.45, p < 0.0008. No sex differences or main effects were observed for center distance, nor were there interactions between genotype and any other factors. These data indicate that most of the effect in the IDUA−/− mice occurs during the initial exploratory phase of testing.
2.4. Marble Burying
Immediately following the locomotor activity test at each age, animals were brought to an adjacent suite, and tested for defensive marble burying behavior. At 2, 4, and 6 months of age, no differences were observed between genotypes or sexes for the latency to begin burying the marbles or the number of visible marbles at the end of the 30 min test period. However, at the oldest age (8 months), the IDUA−/− male mice buried fewer marbles than control males as reflected by a significant Genotype × Sex interaction (F(1,46) = 6.23, p < 0.02) (Fig 3). The females at 8 months, regardless of genotype, began to bury the marbles sooner than males (Sex, p < 0.03).
Fig. 3.
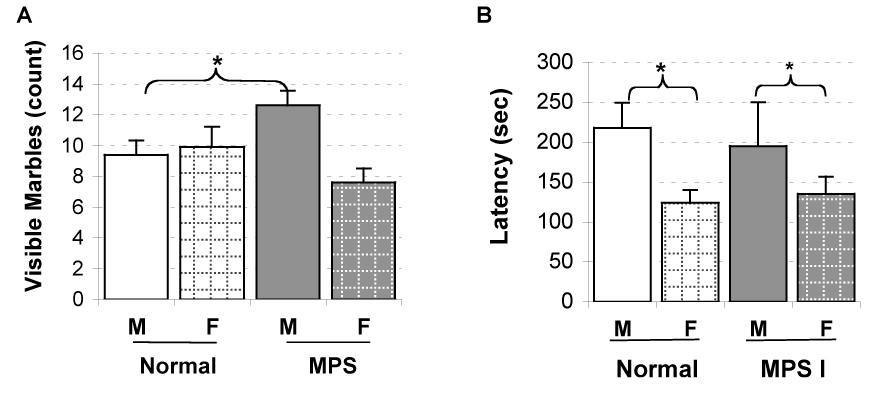
Anxiety-like marble burying behavior in mice at 8-months of age. Animals were exposed to an arena with blue marbles for 30 min immediately following the locomotor task. Data are mean ± SEM for (A) the number of visible marbles and (B) the latency to start burying. N = 18 male (M) and 12 female (F) of controls, and 8 male and 13 female of IDUA−/− mice. *P < 0.05 between male and female mice in each genetic group.
2.5. Morris water maze learning and memory
Prior to hidden platform learning in the Morris water maze (MWM), the animals were first tested in a cued platform version of the task at 2 months of age. This experience reduces the number of mice that float or jump off the platform after locating it, and teaches them the basic requirements of the task (Vorhees and Williams, 2006). All animals, regardless of genotype or sex, found the platform faster over the 6-day test period. No other differences were found between the genotypes or genders, and no interactions were observed (data not shown).
Animals were subjected to the MWM hidden platform version of the task at each age point and the dependent measures were the average time and cumulative distance to target. During this phase of testing, the platform was in a constant position at each age, but the position of the platform was changed between ages so that new learning was required. As shown in Fig. 4, the overall time taken to find the platform in the acquisition phase decreased significantly after 6 days of repeated trials for both groups at all test ages, indicating that learning for the platform position at each age occurred (Day, p < 0.0001). However, at 4 months of age, the IDUA−/− mice took longer to reach the platform than controls [Genotype, F(1,57.9) = 10.38, p < 0.0001]. This longer latency was evident beginning on the third day of testing and persisted thereafter [Genotype × Day, F(5,215) = 2.54, p <0.03]. These results were further confirmed by analysis of cumulative distance from the platform measured every 0.1 s (not shown). No significant genotype differences were observed at later test ages.
Fig. 4.
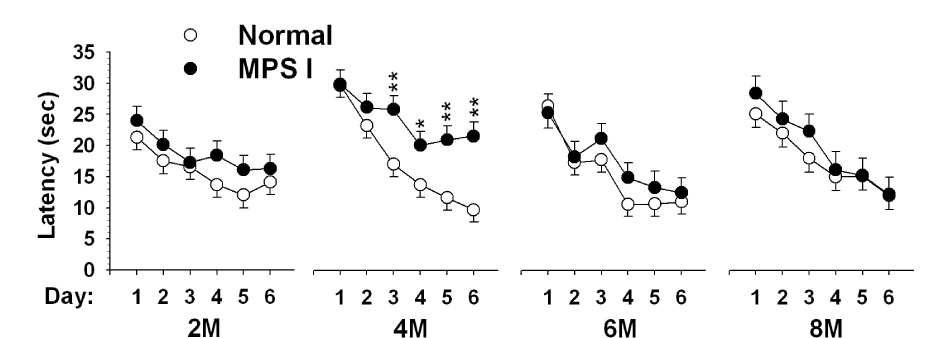
Learning capability for hidden platform in Morris water maze over a 6-day acquisition phase for IDUA−/− (I/I) and control mice (+/I) from 2-months (2M) to 8-months (8M) of age. The mean latencies to find the submerged platform were shown in each group with both male and female, as no gender differences were observed at all test ages. N of controls and IDUA−/− mice = 32–33 and 26 for 2M, 33 and 26 for 4M, 29–31 and 21 for 6M, 29 and 19 for 8M, respectively.
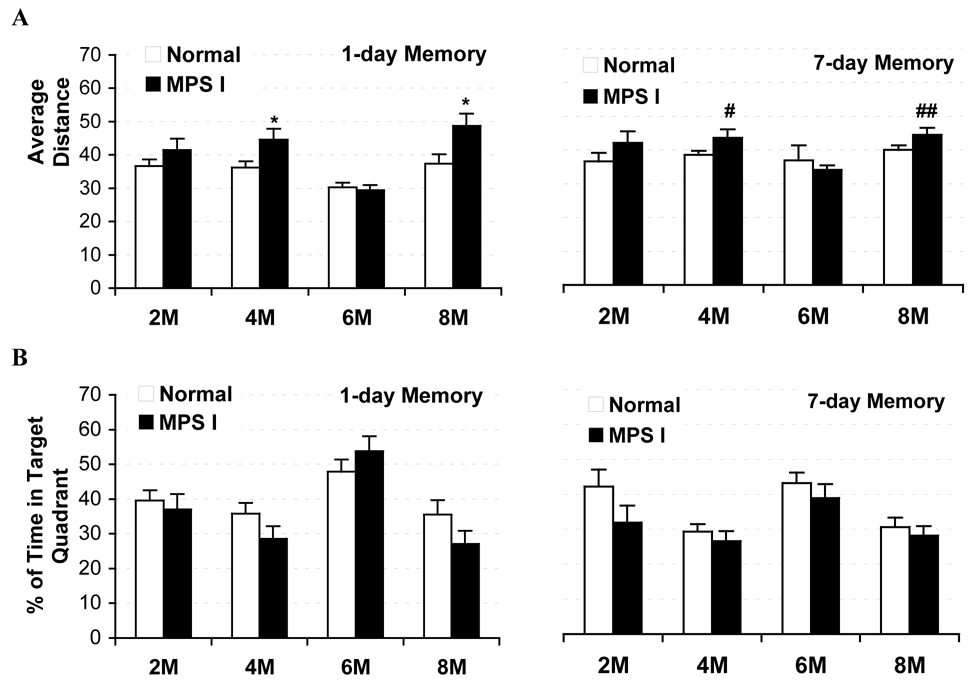
Two separate memory trials (30 s each) were performed at each test age: one conducted 24 h and one conducted 7 days after the last learning trial. The average distance to the target position (where the platform had been located), percentage time spent in target quadrant and number of platform site crossings were calculated for each trial. As shown in Figure 5A, there was a tendency for the IDUA−/− mice to swim further away from the platform than control animals during the 1 day memory trial starting at 2-months of age (p=0.16), reaching significance at 4- and 8-months, Genotype, F(1,55) = 5.64, p<0.03 and F(1,19) = 4.56, p < 0.05, respectively. Consistent with this observation, MPS I mice spent less time in the target quadrant than the controls at 4- (29% vs. 36%, p = 0.13) and 8-month (27% vs. 36%, p=0.16), although these differences were not reaching significance (Figure 5B). No differences were observed at 6 months when both control and MPS I groups had unexpectedly reduced distance to target and more time in target quadrant (48% and 54%) compared to data obtained from the same group of mice at the other testing ages. In the 7-day delay memory trial, similar trends were also seen for average distance to target at 4 and 8-months (p=0.06 and p=0.08, respectively), with only moderately less time spent in target quadrant observed in MPS I at the early age (32% vs. 42%, p=0.12, at 2-month). There were no significant effects on the number of platform site crossings (not shown).
Fig. 5.
Memory trials in Morris water maze, over probe phases where the platform was removed from the pool, for IDUA−/− and control mice from 2-months (2M) to 8-months (8M) of age. The average distance to the platform site (A) and the percentage of time spent in the target quadrant (B) were evaluated during 1-day later and 7-day later probe trials. For 1-day later probe trials, N of control and IDUA−/− mice = 25 and 22 for 2M, 32 and 25 for 4M, 20 and 6 for 6M, 13 and 10 for 8M and for 7-day later probe trials, N of control and IDUA−/− mice = 10 and 15 for 2M, 26 and 22 for 4M, 30 and 22 for 6M, 29 and 19 for 8M, respectively. *P < 0.05; # p=0.06; ## p=0.08.
2.6. Novel object recognition
To assess recognition memory, MPS I mice and littermate controls were tested for novel object recognition following the MWM 1 day memory trial at 4, 6, and 8 months of age (Fig 6). Because cued platform testing occurred at 2 months and we wanted to keep the amount of testing fairly consistent between months, animals were not tested at 2 months. Both genotypes showed similar percent of novel object exploration at 4 or 6 months of age with no gender difference within each group (Fig. 6A). However, at 8 months of age, the IDUA−/− mice had a reduction in the percentage of time spent on the novel object with total exploration time similar to controls, Genotype, F(1,46) = 7.34, p < 0.01. The control animals spent 66 ± 2.5% of the time exploring the novel object during the test phase, whereas the IDUA−/− mice only spent 54 ± 3.7% of the time exploring the novel object. This effect was slightly more pronounced among males (70% vs. 53%) although there was no genotype by sex interaction (Fig. 6B).
Fig. 6.
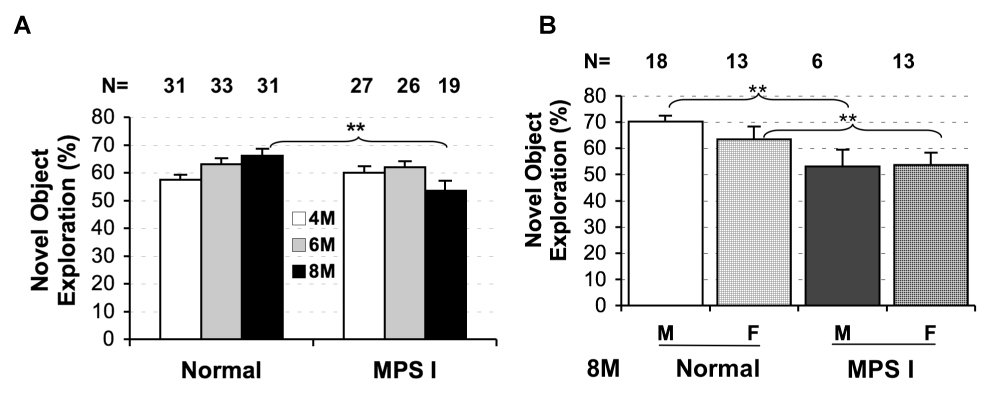
Novel object recognition memory tested at 4-, 6- and 8-months of age. Animals were tested for novel object recognition two-days after the 1-day probe trial in MWM. Data are mean ± SEM for percentage of time exploring the novel object in IDUA−/− and control groups at each test age (A) or with (B) male and females separated at 8-months of age. **P < 0.01.
2.7. Repeated open-field habituation
A repeated open-field habituation test was done at 8 months in order to demonstrate that the IDUA−/− mice in this study responded similarly to the task as we have found previously at a younger age (4-month). Mice were exposed to the same open-field for 3 repeated trials (5-min per trial) with a 30 min inter-trial interval (Fig 7). The difference between the first and third exposure was determined for grid crossings, rear frequency and time spent grooming. The normal mice showed a 55% reduction in locomotor activity, whereas the IDUA−/− mice only showed a 31% reduction in activity (p < 0.001). The reduction in locomotor activity was associated with an increase in time spent grooming in control animals (i.e., 40% more in trial 3 relative to trial 1). In contrast, a reduction in grooming was seen in the IDUA−/− mice with 20% less (p < 0.03). The IDUA−/− mice also had a greater reduction in rearing on the last trial compared to controls (p < 0.001).
Fig. 7.
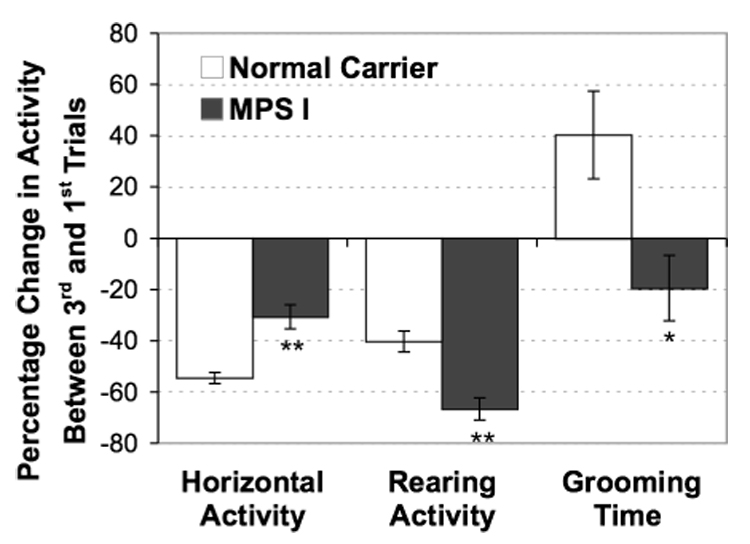
Repeat-trial open-field behavior in mice at 8-months of age. Each mouse was allowed to freely explore the arena for three trials with 5 min each and 30 min inter-trial intervals. N = 29 animals in control group and 19 in IDUA−/− group. * p < 0.05, ** p < 0.001.
2.8. Acoustic Startle
Because it has been suggested that the IDUA−/− mice may have hearing problems with advancing age, we examined the acoustic startle response of the animals at 8 months of age. Examination of the data showed that 61% of the IDUA−/− mice did not respond to any of the startle stimuli presented compared to 9.7% of the controls that did not respond, χ² = 14.76, df(1), p < 0.0001 (not shown). In the animals that did show a startle response, there were no differences between genotypes or sexes.
3. Discussion
The major goal of this study was to evaluate the progression of CNS functional deficits during development using seven behavioral assessments with consideration of potential sexual dimorphism in IDUA−/− mice at the age of 2-months to 8-months when increased mortality of IDUA−/− mice started. We found consistently that the IDUA−/− mice were less spontaneously active than controls during the initial exploratory phase of testing in an automated open-field test as early as 2-months and thereafter, with normal habituation observed at all ages. At approximately 3-months of age, IDUA−/− mice started to weigh more than controls with differences seen first in females. An impairment in spatial-learning and long-term memory occurred at 4-months of age in MPS I mice during Morris water maze testing. At 8-months of age, IDUA−/− mice showed deficits in novel object recognition memory-. Meanwhile, abnormal anxiety on marble burying behavior was observed in male MPS I mice coinciding with their elevated death rate starting at 7-months of age. The deficit in repeat-trial open-field habituation observed in MPS I mice confirmed previous findings and indicate that although the mice have lower exploratory activity during the automated locomotor test, they have reduced habituation after repeated short-term testing, perhaps because they fail to recall the test environment as well as controls, a finding consistent with the novel object deficit seen at this age.
MPS I is a progressive disease affecting many organs, including skeleton, joints, eyes, heart, liver, spleen as well as the CNS. Severe mental retardation and death in childhood are observed in patients with Hurler syndrome, the most severe form of MPS I. The cellular mechanism(s) by which lysosome storage accumulation can lead to neurodegeneration are not understood, and the onset of neurological deficits are not well-defined. The murine IDUA knock-out model was reported to represent the severe form of MPS I. Brain pathology studies have been reported previously by us and others, indicating abnormal lysosomal storage in the neurons of cerebral cortex as early as 2-month of age (Clarke et al., 1997), the Purkinje cells of cerebellum and perivascular cells in the hippocampus as early as 3-months of age (Pan et al. unpublished observation, (Chung et al., 2007; Hartung et al., 2004). An inflammatory component of CNS disease was also indicated by abnormal activation of microglial cells in the cortex of MPS I mice occurring at 6-months of age (Ohmi et al., 2003). In this study, we performed a temporal evaluation of behavioral changes in male and female MPS I mice and employed multiple assessments with overlapping functions to identify the potential complications arising from other abnormalities associated with this disease. It appears that the onsets of most behavioral deficits occurred around 3–4 months of age or older except the hypoactivity detected at 2-months in initial exploratory phase of locomotor task, even though abnormal systemic metabolism was indicated in MPS I mice as early as 4-weeks of age by significantly higher urinary GAG accretion. The age of onset of particular behavioral deficits varies among different LSD mouse models, and can be affected by strain background. Similar onset of impaired learning and memory in Morris water maze was observed in MPS IIIB (Crawley et al., 2006), however, earlier manifestation was detected in male affected MPS VII mice at 6-weeks of age (O'Connor et al., 1998).
Potential sex differences on body weight, survival and different behavioral tests were investigated in this study. The abnormally heavier body weights in IDUA−/− mice appeared first in female mice at 11-weeks of age, followed by male mice 3-weeks later. This bodyweight change may be attributed, at least partially, to the systemic metabolic defects, such as hepatosplenomegaly, as liver pathology emerges as early as 3-months of age. Heavier liver weights and spleen weights have been reported in MPS IIIA mice with significant age by disease group interaction (Crawley et al., 2006; Hemsley and Hopwood, 2005). In addition, the reduced locomotor activity levels, observed in IDUA−/− mice as early as 2-months of age in an automated open-field test, may also play a role, although no differences of genotype by sex were shown in horizontal activity. Interestingly, the median survival age in male IDUA−/− mice (32-weeks) was much shorter than their female counterparts (48-weeks), indicating potentially more rapid progression of detrimental symptoms in male affected mice.
Sex differences on behavioral performance have been reported in some LSD disease models such as MPS type IIIA mice (Crawley et al., 2006; Hemsley and Hopwood, 2005). Studies on hormone therapeutic effects have shown that higher estrogen levels could result in significantly improved neurological symptoms and greater longevity in Saposin A−/−-deficient mice, which is a model for late-onset globoid cell leukodystrophy (Matsuda et al., 2001). The administration of estradiol was reported to inhibit brain macrophages (Vegeto et al., 2003), while macrophage activation was implicated in the CNS pathology in MPS I mice (Ohmi et al., 2003), as well as several other LSD models including MPS IIIB, metachromatic leukodystrophy (Hess et al., 1996) and Niemann-Pick disease type C (German et al., 2002). However, in this current study, no significant gene by sex differences were observed in most of the behavioral assessments tested at all ages, except in the marble burying test at 8-months of age. The marble burying test has been a model of non-associative, impulsive or anxiety-like behavior often used to examine the efficacy of anxiolytic drugs (Saadat et al., 2006). Decreased marble burying activities were observed in male IDUA−/− mice with no changes found in the latency to bury compared to the control males, while there were no gene by sex differences detected in overall locomotor activity, and no reduction in horizontal activities compared to an earlier age (6-months). It has been reported that disparate effects between male and female animals were observed in response to chronic stress (Bowman et al., 2003; Galea et al., 1997). The abnormal performance detected in male but not female IDUA−/− mice is in agreement with other studies that female animals were insensitive in response to stress paradigms known to affect males (Bowman et al., 2003; Luine, 2002; Mitra et al., 2005).
The current study clearly demonstrated that MPS I mice are less active in the open field than their normal heterozygous littermates as early as 2-months of age, especially during the initial exploratory period (first 30 min). The basal activity levels (activities after 60 min of exposure) were nevertheless similar between groups. In the elevated zero-maze test, however, normal exploratory performance was observed in 2-months old IDUA−/− mice. The musculoskeletal defects in IDUA−/− mice may have played a role in the reduced exploratory activity, as features of dysostosis multiplex were visible by radiography (e.g., widening and thickeness to the ribs and zygomatic arches) at 4-weeks of age and became more prominent with age (Clarke et al., 1997). On the other hand, the horizontal activities in IDUA−/− mice decreased gradually with age and repeated exposures in parallel with the control group, indicating no further progression of the hypoactivity (unlike the rapid progression of the bone disease). Nevertheless, hypoactivity in the automated open field test can be used as a model for early onset of behavioral abnormalities in MPS I.
Our previous studies have demonstrated impaired habituation of IDUA−/− mice at 3-months (Pan et al., 2003) and 4-months (Hartung et al., 2004) of age after three repeated trials to an open field with a 30 min intertrial interval, a type of nonaversive short-term memory. Consistent with those findings, 8-month old IDUA−/− mice continued to show impaired memory for the test arena, indicated by less reduction in exploration in the 3rd versus the 1st trial, and no increase in grooming, a self-directed behavior evoked by familiarity (rather than fear in this test). In contrast to grooming, rearing is a voluntary and risk-assessing behavior and is correlated with hippocampal theta rhythms (van Lier et al., 2003). Inconsistent with observations at earlier ages, 8-month old IDUA−/− mice exhibited more reduction in the 3rd trial in rearing with similar numbers of rearing found in the 1st trial in comparison to the controls. This age difference could be due to the complication by the progression of dysostosis multiplex as more pressure on knees and joints may contribute to rearing changes. Thus, the repeat-trial open field test can be a model for mid-age onset of non-associative memory impairment in IDUA−/− mice with locomotion and grooming as reliable parameters.
Spatial-learning and memory deficits evaluated by Morris water maze have been associated with damage to the hippocampus in rats (Aggleton et al., 1986; Morris et al., 1982) and mice (Logue et al., 1997; Paylor et al., 1992). In this study, impaired spatial learning and memory was evident in IDUA−/− mice at 4-months of age. Significant separation in search times to find the hidden platform appeared by day 3 of learning (17 and 26 s, unaffected and IDUA−/− mice, respectively) and throughout the rest of learning (9 and 22 s by day 6, unaffected and IDUA−/− mice, respectively). This observation was further confirmed by analysis of cumulative distance from the platform which reduces concern over potential differences in swimming speed. It is not clear why the learning deficit seen at 4-months of age were not consistently seen at later ages when mice entered the same environment and learned new platform locations, but practice effects may be a factor. By 8 months of age, the mice had been tested in the maze on three previous occasions and they may have learned a general strategy that at each retest the platform could be found in the same relative location but in a different quadrant. It has been shown that repeated testing in MWM is less sensitive to group differences than when animals are naïve to the test (Vorhees and Williams, 2006). However based on the current experimental design, we cannot rule out the possibility that 4 month Morris water maze finding is not reliable (false positive). In addition, selection bias can be another factor, where the sickest animals died and only the healthier ones were available for re-test at the older age. Testing naive groups at each age will be needed to resolve this point. The IDUA−/− mice also showed deficits in reference memory during the 1-day and 7-day probe trials at 4-months and 8-months of ages. Abnormal and inconsistent behavior was observed in both IDUA−/− and control groups at 6-months of age when significantly less average distance to target and more percent time in target quadrant were found compared with data obtained from earlier and later ages (4-months and 8-months). This suggests that extraneous factors may have masked genotype differences during the 6-months of age probe trials. Testing errors also reduced the number of valid data files at this test age. Nevertheless, the data suggest that the Morris water maze can be used to assess mid-age onset of impaired spatial learning and memory in IDUA−/− mice.
Memory for familiar objects was assessed using the novel object recognition test, a nonspatial and non-aversively motivated memory test associated with hippocampal (Brooks et al., 2005) and perirhinal cortex function (Wan et al., 1999; Xiang and Brown, 1999). In our study, a significant reduction of novel object preference in IDUA−/− mice was present at 8-months of age. This deficit in visual recognition learning could not be attributed to potential corneal clouding often found in patients with Hurler’s syndrome, because competent vision was suggested for 8-month old IDUA−/− mice by their normal spatial learning performance in the acquisition phase of the Morris water maze. Thus, the novel object recognition test can be considered as a model for late onset visual cognitive dysfunctions in IDUA−/− mice.
In summary, our results demonstrated multiple behavioral deficits with different ages of onset in a murine model of Hurler syndrome, including impairment in locomotor and anxiety-like behavior, spatial learning and memory, visual recognition memory, and short-term non-associative memory retention. These findings provide a framework for the design of future evaluations of innovative therapies for the treatment of MPS type I.
4. Materials & Methods
4.1 Experimental subjects
Mice of the B6.129-iduatm1Clk strain (Clarke et al., 1997) with a knock-out of the idua gene in C57BL/6 congenic line (IDUA−/− ; more than 11 successive generations of backcrossing) were obtained from Jackson Laboratory (Bar Harbor, ME), and backcrossed in-house for two generations upon arrival. The experimental groups were generated using heterozygous females and homozygous males as breeding pairs in a pathogen-free facility (with micro-isolator) at Cincinnati Children’s Research Foundation (CCRF) in the vivarium fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). IDUA−/− mice and their normal littermate controls (IDUA−/+) from 10 individual litters were the subjects for these experiments. At 4 weeks of age, offspring were separated from the dam, genotyped and housed (3–4 per cage) by sex. At 6 weeks of age, animals were transferred to conventional housing under a 14 h light/10 h dark cycle (lights on at 700 h) with temperature (19 ± 1°C) and humidity (50 ± 10%) controlled. Mice were given 8–10 days to acclimate to these conditions prior to entering the initial behavioral test. Body weights were recorded at multiple time points throughout the study to monitor the changes in body mass and identify animals with abnormal weight loss. All experimental procedures were performed according to the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at CCRF.
4.2. Quantitation of urinary GAG
Based on methods previously described (Hartung et al., 2004; Pan et al., 2003), we have developed an assay to quantitate GAG excretion in as little as 40 ul of murine urine. Briefly, urine aliquots were diluted 1:2 and 1:10 with sodium formate buffer (pH3.0), and mixed in duplicate with freshly prepared 1,9-dimethylmethylene blue (DMB) solution (0.35uM in sodium formate buffer, pH3.0). Absorbance of the color reaction was measured at 535 nm within 30 min on DU640 spectrophotometer (Beckman Coulter Co.) and compared with standard curve generated with heparan sulfate solutions (Sigma). Further dilution was made if reaction turned pinkish. Urinary creatinine was quantitated by incubating diluted samples with freshly made picric acid/sodium hydroxide solution (10% saturated picric acid and 0.09M NaOH) for 20 min, followed by measuring absorbance at 535nm and calculated with standard curve established using creatinine reference solutions (Sigma).
4.3. Behavioral Assessment
To determine if IDUA−/− mice displayed a progressive decline in locomotor behavior, increased anxiety, or decrements in cognition, we examined mice in several tests every other month for 4 test periods beginning at 2 months of age. These tests were as follows: locomotor ability in automated test chambers, defensive marble burying, Morris water maze (MWM), and a test of novel object recognition (excluding 2 months of age because of testing animals in the MWM cued-platform at this age). Animals also were assessed one time only at 2 months in the elevated zero maze and the cued platform phase of the MWM and at 8 months in the repeated open-field habituation test and acoustic startle. Both males and females were assessed. All behavioral tests were performed between 1200 h and 1700 h during the light portion of the light/dark cycle.
4.3.1 Elevated Zero Maze
The elevated zero maze is conceptually similar to the elevated plus maze, with the exception that there is no center area that can complicate interpretation of the plus maze (Shepherd et al., 1994). We examined animals at 2 months of age as described previously (Williams et al., 2003). The apparatus consists of a circular runway divided in 4 equal quadrants. Two quadrants have high black walls (30 cm) on both sides of the runway and the opposite two quadrants are open except for a short (1 cm), clear acrylic curb to demarcate the edge. Animals were placed in the apparatus in one of the walled quadrants and the test continued for 5 min. Time in the open, number of open entries (2x open entries = number of transitions since there is no unscored zone at the intersection as in the elevated plus maze), and number of head dips over the side of open quadrants were scored for each animal from video recordings made of each session.
4.3.2. Spontaneous Locomotion
At least 1 h after the elevated zero maze at 2 months, animals were placed in Digiscan rxyz-16 activity monitors (41 cm L × 41 cm W × 30 cm H; Accuscan Electronics, Columbus, OH) and spontaneous locomotor activity was measured in 10 min intervals for 90 min. To begin the test, an animal was placed in the apparatus and following 90 min of exploration the task was ended and animals were returned to the homecage. At 4, 6, and 8 months of age animals began the sequence of behavioral assessments in the automated open-field. The dependent variables were horizontal activity, which is the measure of all beam breaks, and center distance (cm). Arenas were cleaned with 70% ethanol between animals. Locomotor activity data from the 90 minute test were analyzed in 10 min intervals.
4.3.3. Marble Burying
At all ages tested, animals were brought to an adjacent suite immediately following the spontaneous locomotor task, and examined for marble burying, a test of anxiety (Njung'e and Handley, 1991) with modification. Specifically, 15 blue marbles, 1.4 cm in diameter (blue produced greater burying compared to clear marbles in a preliminary study), were evenly placed in 5 rows of 3 in a cage measuring 28 × 17 × 12 cm. A cardboard template was used to ensure equal and consistent positioning of marbles (4.5 cm apart, 4.5 cm from the long edge, and 3.5 cm from the short end). Fresh wood chip bedding (3 cm deep) was placed in each cage and a filter top was used to cover the cage. Animals were given 30 min of exposure to the marbles, and the dependent measures were latency to begin burying and number of marbles visible at the end of the test.
4.3.4. Morris Water Maze
Spatial learning and memory were tested in the MWM. The apparatus was a 122 cm circular stainless steel tank painted white on the interior. Animals began a series of tests in the MWM using procedures described elsewhere (Vorhees and Williams, 2006). The first phase consisted of cued trials (only examined at 2 months of age), where animals were required to find a submerged square platform (10 cm²) that had an orange spherical “cue” (40 mm diameter) mounted 7 cm above the platform on a steel rod. Curtains were drawn around the maze to reduce visibility of extramaze cues, and the animals were given 2 trials per day for 5 days. Both the platform position and start position were changed at random on each trial to inhibit reliance on spatial cues. Latency to reach the platform was recorded on each trial. The cued procedure introduces the animals to the main task requirements (e.g., swimming, the fact that the platform is not near the perimeter, and climbing on the platform to escape).
Subsequent to the cued version at 2 months or the Monday after marble burying at 4, 6, and 8 months, animals were tested in the spatial (hidden platform) learning phase (Vorhees and Williams, 2006). The platform initially was located in the southwest quadrant at 2 months, then was shifted to the northeast, northwest, and southeast quadrants at the 4, 6, and 8 month test periods, respectively. During these hidden-platform phases the curtains were open, and the platform remained stationary throughout each phase. Animals were given 4 daily trials for 6 days with a 15 s inter-trial interval. Each trial lasted up to 1 min and latency to reach the platform was recorded and animals were tracked using Smart tracker software (San Diego Instruments, San Diego, CA) that digitizes images received from a camera located over the maze. Latency to reach the platform on learning trials was used as the principal measure because we have previously shown that it correlates >0.95 with path length and cumulative distance from the platform; other indices were analyzed to corroborate the latency findings. When an animal was unsuccessful at locating the platform within the 1-min limit, it was removed from the water and placed on the platform for 15 s.
One day after the end of each of the 6-day acquisition phases, a single 30 s probe trial was given with a new starting position and the platform was removed to determine if the animals had learned the location sufficiently to be able to swim back to the location where the platform had been. In addition, a delayed probe trial was employed 6 days later at each of the 4 assessment ages to evaluate long-term reference memory of the mice. No platform was used in the 30 s trial with the start position identical to the Day 7 probe trial.
4.3.5. Novel Object Recognition
The novel object recognition test was assessed at 4, 6, and 8 months of age two days after the hidden platform phase of the MWM. In order to keep the testing period similar at all months of testing, a 2 month test was not included because cued-platform phase of the MWM was included at this age only. The test was conducted over a two day period and the arenas were opaque (31 W × 33 L × 12 H cm) and males and females were tested in separate chambers. Animals were habituated to the test arena for 10-min on the first day. The second day was divided into two phases: familiarization and novel object recognition. During the initial familiarization stage, two identical objects were placed in the center of the arena equidistant from the walls and each other. The mice were placed in the center of the arena between the two objects for a maximum of 10 min or until it had completed 30 s of cumulative object exploration. Object recognition was scored when the animal was within 0.5 cm of an object with its nose toward the object. Exploration was not scored if a mouse reared above the object with its nose in the air or climbed on an object. Mice were returned to the homecage after familiarization and retested 1 h later, except now the arenas had a novel object (never seen before at any test age) and a third replicate of the original object used at that age. Scoring of object recognition was performed in the same manner as during the familiarization phase. The objects for mice to discriminate consisted of gray metal half-spheres, clear glass jars, green rectangular plastic Lego stacks, and brown ceramic miniature basketballs. A halogen light set at medium intensity was used during all stages of the experiment. Arenas were cleaned with 70% ethanol after each trial.
4.3.6. Repeated open-field test
At 8-months of age, the repeated open-field test was performed three days after the delayed probe trial of MWM to confirm our previous findings in this task. The open-field apparatus (60 × 60 cm) consisted of a white Plexiglas box with 25 squares (12 × 12 cm) painted on the floor (16 outer and 9 inner). Briefly, the mouse was placed in one of the four corners of the apparatus and allowed to explore the whole field for 5 min. Activity was monitored and quantified for ambulation (number of inner and outer squares crossed), rearing and time spent grooming by an observer who did not know the genotype of the animal during testing. Each mouse was tested for three repeated trials with 30-minute inter-trial intervals.
4.3.7. Acoustic Startle/Pre-pulse Inhibition
The final test at 8 months of age was acoustic startle reactivity and it was performed after MWM testing during the same time period each day. Testing was conducted in an SR apparatus (San Diego Instruments, San Diego, CA). Animals were placed in an acrylic cylindrical test chamber mounted on a platform fitted with a piezoelectric force transducer attached to the underside of the platform. The platform was positioned inside a sound-attenuated test chamber. A 5 min acclimation period preceded test trials. Animals were tested for a total of 12 min using a 4 × 4 Latin square design balanced for 4 trial types: no stimulus, startle stimulus with no pre-pulse, 74 dB pre-pulse or 76 dB pre-pulse. Each set of 16 trials was repeated 3 times for a total of 48 trials. Trials of the same type were averaged together. The intertrial interval was 8 s and on prepulse trials the interstimulus interval was 70 ms (signal onset to onset). The startle stimulus was a 20 ms 110 dB SPL mixed frequency signal and the recording window was 100 ms after startle signal onset. The apparatus was cleaned with 70% ethanol between animals.
4.4. Data analysis
Any data collected within 3-days of the death of a particular animal were eliminated from the analysis. Data were analyzed using a mixed general linear model analysis of variance (ANOVA; SAS Proc Mixed, Cary, NC). Genotype (normal heterozygous IDUA+/− or affected IDUA−/−) and sex (female or male) were between subject factors, whereas measures taken repetitively on the same animal, such as weights, time interval for locomotor testing, or day for maze testing, were treated as repeated measure (within) factors. Separate analyses were run for each behavior tested at each month. Variance-covariance models were selected based on best-fit statistics for each dependent variable. The models were either compound symmetry or first-order autoregressive. Significant interactions were further analyzed using the slice ANOVA option within Proc Mixed. Proc Mixed provides adjusted degrees of freedom and they do not always match those used in standard ANOVAs and can be fractional. For clarity of presentation, only significant Genotype main effects or interactions are provided with F values. Data are presented as mean ± SEM and statistical significance was accepted at p ≤ 0.05.
Acknowledgments
This research was supported in part by Translational Research Initiative grant from Cincinnati Children’s Research Foundation (DP), and the National Institutes of Health (AI061703 to DP, DA006733 to CVV, and DA014269 to MTW). The authors gratefully acknowledge Mary Moran for data analysis and Nicole Worsham for animal maintenance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Hunt PR, Rawlins JN. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behav Brain Res. 1986;19:133–146. doi: 10.1016/0166-4328(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Brooks SP, Pask T, Jones L, Dunnett SB. Behavioural profiles of inbred mouse strains used as transgenic backgrounds. II: cognitive tests. Genes Brain Behav. 2005;4:307–317. doi: 10.1111/j.1601-183X.2004.00109.x. [DOI] [PubMed] [Google Scholar]
- Chung S, Ma X, Liu Y, Lee D, Tittiger M, Ponder KP. Effect of neonatal administration of a retroviral vector expressing alpha-l-iduronidase upon lysosomal storage in brain and other organs in mucopolysaccharidosis I mice. Mol Genet Metab. 2007;90:181–192. doi: 10.1016/j.ymgme.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Church H, Tylee K, Cooper A, Thornley M, Mercer J, Wraith E, Carr T, O'Meara A, Wynn RF. Biochemical monitoring after haemopoietic stem cell transplant for Hurler syndrome (MPSIH): implications for functional outcome after transplant in metabolic disease. Bone Marrow Transplant. 2007;39:207–210. doi: 10.1038/sj.bmt.1705569. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LA, Russell CS, Pownall S, Warrington CL, Borowski A, Dimmick JE, Toone J, Jirik FR. Murine mucopolysaccharidosis type I: targeted disruption of the murine alpha-L-iduronidase gene. Hum Mol Genet. 1997;6:503–511. doi: 10.1093/hmg/6.4.503. [DOI] [PubMed] [Google Scholar]
- Crawley AC, Gliddon BL, Auclair D, Brodie SL, Hirte C, King BM, Fuller M, Hemsley KM, Hopwood JJ. Characterization of a C57BL/6 congenic mouse strain of mucopolysaccharidosis type IIIA. Brain Res. 2006;1104:1–17. doi: 10.1016/j.brainres.2006.05.079. [DOI] [PubMed] [Google Scholar]
- Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- German DC, Liang CL, Song T, Yazdani U, Xie C, Dietschy JM. Neurodegeneration in the Niemann-Pick C mouse: glial involvement. Neuroscience. 2002;109:437–450. doi: 10.1016/s0306-4522(01)00517-6. [DOI] [PubMed] [Google Scholar]
- Hartung SD, Frandsen JL, Pan D, Koniar BL, Graupman P, Gunther R, Low WC, Whitley CB, McIvor RS. Correction of metabolic, craniofacial, and neurologic abnormalities in MPS I mice treated at birth with adeno-associated virus vector transducing the human alpha-l-iduronidase gene. Mol Ther. 2004;9:866–875. doi: 10.1016/j.ymthe.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Hemsley KM, Hopwood JJ. Development of motor deficits in a murine model of mucopolysaccharidosis type IIIA (MPS-IIIA) Behav Brain Res. 2005;158:191–199. doi: 10.1016/j.bbr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Hess B, Saftig P, Hartmann D, Coenen R, Lullmann-Rauch R, Goebel HH, Evers M, von Figura K, D'Hooge R, Nagels G, De Deyn P, Peters C, Gieselmann V. Phenotype of arylsulfatase A-deficient mice: relationship to human metachromatic leukodystrophy. Proc Natl Acad Sci U S A. 1996;93:14821–14826. doi: 10.1073/pnas.93.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivit W, Peters C, Shapiro EG. Bone marrow transplantation as effective treatment of central nervous system disease in globoid cell leukodystrophy, metachromatic leukodystrophy, adrenoleukodystrophy, mannosidosis, fucosidosis, aspartylglucosaminuria, Hurler, Maroteaux-Lamy, and Sly syndromes, and Gaucher disease type III. Curr Opin Neurol. 1999;12:167–176. doi: 10.1097/00019052-199904000-00007. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Luine V. Sex differences in chronic stress effects on memory in rats. Stress. 2002;5:205–216. doi: 10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- Matsuda J, Vanier MT, Saito Y, Tohyama J, Suzuki K. A mutation in the saposin A domain of the sphingolipid activator protein (prosaposin) gene results in a late-onset, chronic form of globoid cell leukodystrophy in the mouse. Hum Mol Genet. 2001;10:1191–1199. doi: 10.1093/hmg/10.11.1191. [DOI] [PubMed] [Google Scholar]
- Mitra R, Vyas A, Chatterjee G, Chattarji S. Chronic-stress induced modulation of different states of anxiety-like behavior in female rats. Neurosci Lett. 2005;383:278–283. doi: 10.1016/j.neulet.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Neufeld EF. Lysosomal storage diseases. Annu. Rev. Biochem. 1991;60:257–280. doi: 10.1146/annurev.bi.60.070191.001353. [DOI] [PubMed] [Google Scholar]
- Njung'e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav. 1991;38:63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- O'Connor LH, Erway LC, Vogler CA, Sly WS, Nicholes A, Grubb J, Holmberg SW, Levy B, Sands MS. Enzyme replacement therapy for murine mucopolysaccharidosis type VII leads to improvements in behavior and auditory function. J Clin Invest. 1998;101:1394–1400. doi: 10.1172/JCI1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH, Neufeld EF. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc Natl Acad Sci U S A. 2003;100:1902–1907. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D, Gunther R, Low WC, Larson B, Walkley SU, Frandsen J, Kafri T, McIvor RS, Whitley CB. Metabolic and neuropathologic correction of Hurler syndrome by a single intravenous injection of lentiviral vector to newborn mice. Molecular Therapy. 2003;7:s76. [Google Scholar]
- Paylor R, Morrison SK, Rudy JW, Waltrip LT, Wehner JM. Brief exposure to an enriched environment improves performance on the Morris water task and increases hippocampal cytosolic protein kinase C activity in young rats. Behav Brain Res. 1992;52:49–59. doi: 10.1016/s0166-4328(05)80324-9. [DOI] [PubMed] [Google Scholar]
- Peters C, Balthazor M, Shapiro EG, King RJ, Kollman C, Hegland JD, Henslee-Downey J, Trigg ME, Cowan MJ, Sanders J, Bunin N, Weinstein H, Lenarsky C, Falk P, Harris R, Bowen T, Williams TE, Grayson GH, Warkentin P, Sender L, Cool VA, Crittenden M, Packman S, Kaplan P, Lockman LA, Anderson J, Krivit W, Dusenbery K, Wagner J. Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood. 1996;87:4894–4902. [PubMed] [Google Scholar]
- Reolon GK, Braga LM, Camassola M, Luft T, Henriques JA, Nardi NB, Roesler R. Long-term memory for aversive training is impaired in Idua(−/−) mice, a genetic model of mucopolysaccharidosis type I. Brain Res. 2006;1076:225–230. doi: 10.1016/j.brainres.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Saadat KS, Elliott JM, Green AR, Moran PM. High-dose MDMA does not result in long-term changes in impulsivity in the rat. Psychopharmacology (Berl) 2006;188:75–83. doi: 10.1007/s00213-006-0470-8. [DOI] [PubMed] [Google Scholar]
- Schachern PA, Cureoglu S, Tsuprun V, Paparella MM, Whitley CB. Age-related functional and histopathological changes of the ear in the MPS I mouse. Int J Pediatr Otorhinolaryngol. 2007;71:197–203. doi: 10.1016/j.ijporl.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann R, Brady RO. New prospects for the treatment of lysosomal storage diseases. Drugs. 2002;62:733–742. doi: 10.2165/00003495-200262050-00002. [DOI] [PubMed] [Google Scholar]
- Shapiro EG, Lockman LA, Balthazor M, Krivit W. Neuropsychological outcomes of several storage diseases with and without bone marrow transplantation. Journal of Inherited Metabolic Disease. 1995;18:413–429. doi: 10.1007/BF00710053. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated "zero-maze" as an animal model of anxiety. Psychopharmacology (Berl) 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Souillet G, Guffon N, Maire I, Pujol M, Taylor P, Sevin F, Bleyzac N, Mulier C, Durin A, Kebaili K, Galambrun C, Bertrand Y, Froissart R, Dorche C, Gebuhrer L, Garin C, Berard J, Guibaud P. Outcome of 27 patients with Hurler's syndrome transplanted from either related or unrelated haematopoietic stem cell sources. Bone Marrow Transplant. 2003;31:1105–1117. doi: 10.1038/sj.bmt.1704105. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Ischemic brain damage and memory impairment: a commentary. Hippocampus. 1996;6:546–552. doi: 10.1002/(SICI)1098-1063(1996)6:5<546::AID-HIPO7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Staba SL, Escolar ML, Poe M, Kim Y, Martin PL, Szabolcs P, Allison-Thacker J, Wood S, Wenger DA, Rubinstein P, Hopwood JJ, Krivit W, Kurtzberg J. Cord-blood transplants from unrelated donors in patients with Hurler's syndrome. N Engl J Med. 2004;350:1960–1969. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- van Lier H, Coenen AM, Drinkenburg WH. Behavioral transitions modulate hippocampal electroencephalogram correlates of open field behavior in the rat: support for a sensorimotor function of hippocampal rhythmical synchronous activity. J Neurosci. 2003;23:2459–2465. doi: 10.1523/JNEUROSCI.23-06-02459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci U S A. 2003;100:9614–9619. doi: 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Aggleton JP, Brown MW. Different contributions of the hippocampus and perirhinal cortex to recognition memory. J Neurosci. 1999;19:1142–1148. doi: 10.1523/JNEUROSCI.19-03-01142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Moran MS, Vorhees CV. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology (Berl) 2003;168:329–338. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]
- Xiang JZ, Brown MW. Differential neuronal responsiveness in primate perirhinal cortex and hippocampal formation during performance of a conditional visual discrimination task. Eur J Neurosci. 1999;11:3715–3724. doi: 10.1046/j.1460-9568.1999.00790.x. [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]