Abstract
Although it has been shown that pro-inflammatory cytokines such as interleukin-1β (IL-1β) facilitate perception of noxious inputs at the spinal level, the mechanisms have not been understood. This study determined the cell type that produces IL-1β, the co-localization of IL-1 receptor type I (IL-1RI) and Fos and NR1 in the spinal cord, and the effects of IL-1 receptor antagonist (IL-1ra) on NR1 phosphorylation and hyperalgesia in a rat model of inflammatory pain. Phosphorylation of NR1, an essential subunit of the NMDA receptor (NMDAR), is known to modulate NMDAR activity and facilitate pain. Hyperalgesia was induced by injecting complete Freund’s adjuvant (CFA, 0.08 ml, 40 μg Mycobacterium tuberculosis) into one hind paw of each rat. Paw withdrawal latency (PWL) was tested before CFA (−48 h) for baseline and 2 and 24 h after CFA to assess hyperalgesia. IL-1ra was given (i.t.) 24 h before CFA to block the action of basal IL-1β and 2 h prior to each of two PWL tests to block CFA-induced IL-1β. Spinal cords were removed for double immunostaining of IL-1β/neuronal markers and IL-1β/glial cell markers, IL-1RI/Fos and IL-1RI/NR1, and for western blot to measure NR1 phosphorylation. The data showed that 1) astrocytes produce IL-1β, 2) IL-1RI is localized in Fos- and NR1-immunoreactive neurons within the spinal dorsal horn, and 3) IL-1ra at 0.01 mg/ rat significantly increased PWL (P<0.05) and inhibited NR1 phosphorylation compared to saline control. The results suggest that spinal IL-1β is produced by astrocytes and enhances NR1 phosphorylation to facilitate inflammatory pain.
Keywords: IL-1β, NMDA receptor, Hyperalgesia, Phosphorylation, Spinal cord, Rat
1. Introduction
Recent studies suggest that pro-inflammatory cytokines such as IL-1β facilitate transmission and processing of noxious inputs at the spinal level (Watkins et al. 2003; Raghavendra et al. 2004). IL-1β is reported to be up-regulated in the spinal cord during inflammatory (Samad et al. 2001; Raghavendra et al. 2004) and neuropathic pain (Winkelstein et al. 2001; Raghavendra et al. 2003). Intrathecal (i.t.) IL-1β administration induces mechanical (Reeve et al. 2000) and thermal hyperalgesia (Sung et al. 2004). IL-1 receptor antagonist (IL-1ra) produces anti-allodynic effects in rat models of neuropathic pain (Milligan et al. 2001; Sweitzer et al. 2001). These data support a role for spinal cord IL-1β in pain.
However, the mechanisms of IL-1β pain facilitation are not understood. It has been demonstrated that IL-1β enhances responses to C-fiber stimulation, wind-up phenomena, and post-discharge of wide-dynamic range neurons in the spinal dorsal horn of anesthetized rats (Reeve et al. 2000). Thus, neurons that are activated during pain may have IL-1 receptors, which allows IL-1β to modulate their activities. Further, neurons contain an NMDA receptor (NMDAR), a glutamate-gated ionotropic channel that plays an important role in the spinal transmission and modulation of nociceptive inputs (Petrenko et al. 2003). Phosphorylation of NR1, an essential subunit of NMDAR, is known to modulate NMDAR activity and facilitate transmission of nociceptive inputs in inflammatory and neuropathic pain models (Zou et al. 2000; Gao et al. 2005; Zhang et al. 2005). Interestingly, it has also been shown that IL-1β enhances NMDAR-mediated inward current and increase of intracellular Ca2+ (Viviani et al. 2003; Yang et al. 2005). These studies lead us to hypothesize that 1) the IL-1 receptor is localized in NMDAR-containing spinal neurons; 2) IL-1β increases NR1 phosphorylation in spinal cord neurons to facilitate pain and IL-1ra blocks inflammation-induced NR1 phosphorylation to suppress pain. In the present study, we determined that IL-1 receptor type I (IL-1RI) is co-localized with Fos, which is expressed in activated neurons during pain, and with NR1 in the spinal cord. We also evaluated the effects of IL-1ra on NR1 phosphorylation and pain in a rat model of inflammatory pain.
2. Methods
2.1 Experimental design
Two sets of experiments were conducted, the first using immunostaining to localize cells and the second on IL-1ra. The first experiment comprised three sub-experiments. In sub-experiment 1, complete Freund’s adjuvant (CFA)-inflamed rats were randomly divided into 2 h post-CFA injection and 24 h post-CFA injection groups (n=3 per group). Another 3 rats, 24 h post-saline injection, were used as control. Previous studies(Milligan et al. 2001; Samad et al. 2001) have used Enzyme-Linked ImmunoSorbent Assay (ELISA) to show up-regulation of IL-1β in the spinal cord during inflammatory and neuropathic pain but have not defined what kind of cell produces IL-1β. To determine the cell type that produces IL-1β during inflammatory pain, the spinal cord was used for double immunofluorescence staining of IL-1β with glial fibrillary acidic protein (GFAP), OX-42 and NeuN, the respective markers of astrocytes, microglia and neurons.
In sub-experiment 2, spinal cord sections were double stained for IL-1RI and Fos to determine whether neurons containing IL-1RI are activated during peripheral inflammation. Since fos is an immediately early gene and is expressed in minutes and peaks in hours following stimulation (Presley et al. 1990), we only used spinal cords from the 2 h group.
In sub-experiment 3, spinal cord sections from the 24 h group were double stained for IL-1RI and NR1 to determine whether IL-1RI is localized in NMDAR-containing neurons.
In experiment 2, IL-1ra was administered to the inflammatory pain rat model to determine whether IL-1β modulates NR1 phosphorylation and pain. Rats were given CFA to induce inflammation in a single hind paw and randomly divided (n=7 per group) into a control group and two IL-1ra (Amgen) groups, 0.001 and 0.01 mg/rat (2 μl). I.t. IL-1ra was given three times, i.e. 24 h before CFA to block the action of basal IL-1β and 2 h prior to each of two hyperalgesia tests to block the CFA-induced IL-1β. The control group received saline (2 μl, i.t.) on the same schedule. Paw withdrawal latency (PWL) tests were conducted before CFA (−48 h) for baseline and 2 and 24 h after CFA to measure thermal hyperalgesia. After the 24 h post-CFA behavioral test, the spinal cords were removed for western blot testing to measure NR1 phosphorylation. A group of rats (n=5) with saline-injected hind paws was used as control for the CFA-induced NR1 phosphorylation.
2.2 Intrathecal Cannulation
Male Sprague-Dawley rats weighing 200–220 g (Harlan) were kept under controlled conditions (22°C±0.5°C, relative humidity 40–60%, 7 am to 7 pm alternate light-dark cycles, food and water ad libitum). The animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Maryland School of Medicine.
Rats were prepared for i.t. injection under pentobarbital sodium anesthesia (50 mg/kg i.p.). The atlanto-occipital membrane was exposed, and a 7.0-cm length of PE-10 tubing was inserted into the subarachnoid space through a slit made in the membrane. The catheter was advanced to the level of the lumbar spinal cord and filled with saline (approximately 7–10 μl), and the outer end was plugged. The animals were allowed to recover for seven days after the operation prior to the experimentation; those with gross signs of motor impairment were excluded from the study. At the end of the experiments, the location of the distal end of the catheter was verified when the spinal cord was removed.
2.3 Induction of Hyperalgesia
Inflammatory hyperalgesia was induced by injecting CFA (Sigma, St Louis, MO; 0.08 ml, 40 μg Mycobacterium tuberculosis), suspended in an 1:1 oil/saline emulsion, subcutaneously into the plantar surface of one hind paw of the rat using a 25-gauge hypodermic needle (Zhang et al. 2004). The inflammation, manifesting as redness, edema, and hyper-responsiveness to noxious stimuli, was limited to the injected paw, appeared shortly after the injection, and lasted about 2 weeks. Hyperalgesia was determined by a decrease in PWL to a noxious thermal stimulus.
2.4 Thermal hyperalgesia
The rats (n=7 per group) in experiment 2 were tested for PWL by a previously described method (Hargreaves et al. 1988; Zhang et al. 2004). They were placed under an inverted clear plastic chamber on the glass surface of a Paw Thermal Stimulator System (UCSD, San Diego) and allowed to acclimatize for 30 min before the test. A radiant heat stimulus was applied with a projector lamp bulb (CXL/CXR, 8 V, 50 W) to the plantar surface of each hind paw from underneath the glass floor. PWL to the nearest 0.1 s was automatically recorded when the rat withdrew its paw from the stimulus. Stimulus intensity was adjusted to derive an average baseline PWL of approximately 10.0 s in naive animals. Paws were alternated randomly to preclude “order” effects. A 20-s cut-off was used to prevent tissue damage.
Mean PWL was established by averaging the latency of four tests with a 5-min interval between each test. The investigator who performed the behavioral tests was blind to group assignment.
2.5 Immunofluorescence and immunohistochemistry
Rats were deeply anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and immediately perfused transcardially with 4% paraformaldehyde (Sigma) in 0.1 M phosphate buffer (PB) at pH 7.4. The lumbar 4–5 spinal cord was removed, immersed in the same fixative for 2 h at 4 °C, and transferred to 30% sucrose (w/v) in PB saline (PBS) overnight for cryoprotection. Thirty micron-thick sections were cut on a cryostat, rinsed in PBS, blocked in PBS with 10% normal donkey serum for 60 min and incubated overnight at room temperature with a mixture of rabbit polyclonal antibody against IL-1β (1:50, Endogen) and mouse monoclonal antibodies against GFAP (1:1000, Chemicon), OX-42 (1:1000, Biosource) or NeuN (1:1000, Chemicon). After three 10-min washings in PBS, sections were incubated in a mixture of CY2-conjugated donkey anti-rabbit (1:500, Jackson ImmunoResearch Laboratories) and CY3-congugated donkey anti-mouse (1:000) for 1 h at room temperature. Control sections were similarly processed, except that the primary antisera were omitted. The stained sections were mounted on gelatin-coated slides, coverslipped with aqueous mounting medium (Biomeda Corp., CA), and examined under a Nikon fluorescence microscope. IL-1β immunostained cells were magnified with a 20× objective lens and counted on 5 randomly-selected sections from each rat. The results were averaged for each individual rat and then for the group. Double labeling of IL-1RI and NR1 was performed the same way.
For immunohistochemical studies, sections were double stained for Fos and IL-1RI with the avidin-biotin-peroxidase (ABC) method. The sections were treated with 0.7% hydrogen peroxide for 30 min to remove the endogenous peroxidase (Li et al. 1987) and then incubated in 3% normal goat serum with 0.3% Triton X-100 in PBS for 1 h. They were incubated overnight in primary antiserum against Fos (Oncogene, 1:20,000) and incubated for 1 h in biotinylated goat anti-rabbit IgG (Vector, 1:400) and ABC (Vector, 1:200) solution, respectively. Finally they were developed in 0.02% diaminobenzidine (DAB) with 0.015% hydrogen peroxide and 0.02% nickel chloride in PBS, which results in a black stain. The primary and secondary antibodies were diluted in 2% normal goat serum with 0.3% Triton X-100 in PBS; the ABC was diluted in PBS alone. Tissue sections were washed 3X10 min in PBS between antibody incubations. All incubations were carried out at room temperature. In order to remove the remaining peroxidase from the Fos antibody-antigen complex of the tissue sections after DAB and hydrogen peroxide reaction, the sections were re-treated with 0.1% NaN3-0.3% H2O2 for 10 min (Li et al. 1987).
IL-1RI immunostaining was identical to the above methods, with the exception that the primary antibody was rabbit anti-IL-1RI (Santa Cruz, 1:1,000) and the peroxidase was reacted in 0.02% DAB with 0.015% hydrogen peroxide to yield a brown stain. The sections were mounted on gelatin-coated slides, dehydrated in graded ethanol, cleared in xylene, and coverslipped with DPX. As control, some sections were processed as described above but without the primary IL-1RI or c-Fos antibodies. The stained sections were analyzed under a Nikon microscope for IL-1RI-immunoreactive and Fos-immunoreactive neuron distribution within the spinal dorsal horn. Five sections were randomly selected from each animal for cell counting. The number of single-labeled Fos-immunoreactive and double-labeled Fos /IL-1RI-immunoreactive neurons were counted on laminae I-VI, averaged separately for each rat, and then averaged for the group.
2.6 Western blot
Western blot was used to examine phosphorylated NR1. After the behavioral test at 24 h post-CFA injection, the IL-1ra treated, vehicle-treated inflamed rats, and saline-injected control rats were anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and decapitated immediately. After laminectomy, the lumbar 4–5 spinal cords were removed. The ipsilateral portion of the spinal cords were homogenized in protein extraction buffer containing a cocktail of proteinase inhibitors: 50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1% NP40, 0.5% sodium dodecyl sulfate (SDS), 1% deoxycholic acid, 2.5 μg/ml aprotinin, 2 μg/ml leupetin, 2 μg/ml pepstatin A, 25 mM NaF, and 1 mM Na3VO4. After centrifuging at 14000 rpm for 10 min at 4°C, the supernatant containing the proteins was collected. Protein concentration was determined using the Bio-Rad Protein Assay. Equal amounts of protein were mixed with loading buffer. After boiling for 10 min, the proteins were fractionated on a 4–20% (w/v) SDS-PAGE and transferred onto a polyvinylidine difluoride (PVDF) membrane (Bio-Rad) with a Trans-Blot Cell System (Bio-Rad). The membrane was blocked for 1 h at room temperature with 5% BSA in PBS containing 0.1% Tween 20 and then incubated overnight at 4°C with phosphor-NR1 antiserum (Serine 896, 1:1000, Upstate). After washing with TBS buffer (20 mM Tris, 150 mM NaCl, pH 7.4), membranes were incubated for 1 h at room temperature with goat anti-rabbit horseradish peroxidase-conjugated IgG (1:3000; Upstate) diluted in 3.0% (w/v) BSA in TBS buffer. The immunoreactivity of the proteins on the membrane was visualized using the chemilluminescence detection system (ECL, Amersham). Autoradiograms were digitized, and densitometric quantification of immunoreactive bands was carried out using Scion NIH Image 1.60. The membranes were then incubated in stripping buffer (100 μM 2-mercaptoethanol, 2% SDS, 62.5 mM Tris [pH 6.7]) at 50°C for 30 min and re-probed with β-actin antibody (1:5000, Sigma) as a loading control. Those who did the tissue harvesting and western blot were blinded to the treatment.
2.7 Statistical analyses
Data from the thermal hyperalgesia tests were presented in Mean ± SE and analyzed using repeated measures analysis of variance (ANOVA) followed by post-hoc Scheffe’s multiple comparisons (Statistical Analysis System). Western blot data and immunostaining data were analyzed with one-way between-subject ANOVA followed by the Scheffé’s multiple comparison procedure. P<0.05 was set as the level of statistical significance.
3. Results
3.1 IL-1β localization in the spinal cord
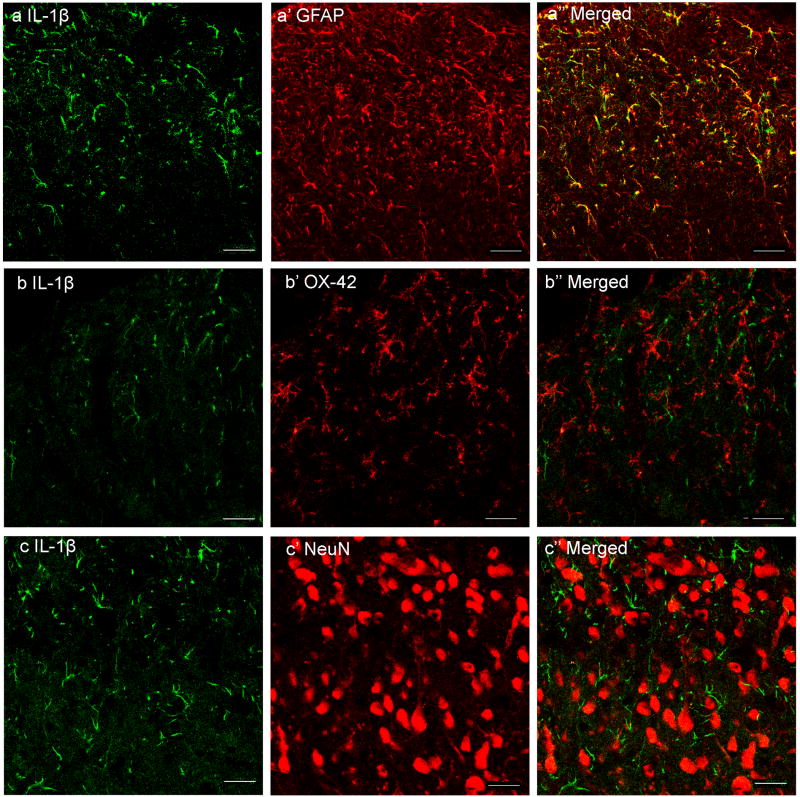
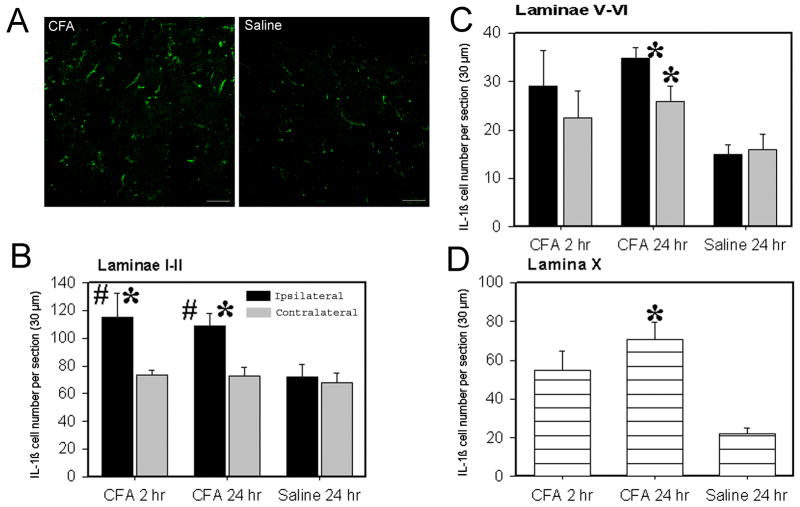
Double immunofluorescence labeling demonstrated that IL-1β immunoreactivity was co-localized with the astrocyte marker GFAP but not with the microglia marker OX-42 or the neuronal marker NeuN (Fig. 1 A-C). IL-1β-labeled glial cells were localized in laminae I-II, V-VI and X. In laminae I-II, the number of ipsilateral IL-1β immunoreactive cells 2 and 24 hr post-CFA was significantly increased compared to contralateral and saline control rat IL-1β (Fig. 2). In laminae V-VI, the values of both ipsilateral and contralateral IL-1β immunoreactive cells 24 hr but not 2 hr post-CFA was significantly increased compared to saline control rat IL-1β. The increase of IL-1β in contralateral laminae V-VI 24 hr post-CFA is not strong enough to induce mirror pain in the contralateral hind paw. In lamina X, IL-1β was also significantly increased at 24 hr but not 2 hr post-CFA compared to that of saline control rat. This implies that the IL-1β up-regulated between 2 and 24 h after inflammation was produced by astrocytes. Control sections without primary antibodies showed no specific staining.
Fig. 1.
Photomicrographs showing IL-1β expression and co-localization of IL-1β and GFAP in ipsilateral lumbar spinal superficial laminae 24 h after CFA injection into one hind paw. Sections were double-labeled with anti-IL-1β (green) and anti-GFAP, anti-OX-42 or anti-NeuN (red). The first column is IL-1β immunostaining; the second column is immunostaining of the astrocyte marker GFAP, microglia marker OX-42, and neuron marker NeuN. The third column shows the merged graphs of IL-1β with each. Note that IL-1β is localized in astrocytes, the yellow cells in a’’.
Fig. 2.
Values of IL-1β immunoreactive astrocytes in the spinal cord (Mean ± SE). A: Representative photographs showing IL-1β immunoreactive cells in superficial laminae of spinal cord 24 hr after CFA (left) or saline (right) injection. Note that CFA injection induced more IL-1β compared to saline injection. B: The number of IL-1β-immunoreactive astrocytes was significantly increased in laminae I–II at 2 and 24 h post-CFA compared to that of the contralateral side and of saline-injected rats. C-D: It also significantly increased 24 h post-CFA in both ipsilateral and contralateral spinal laminae V–VI and lamina X compared to that of saline-injected rats. * P<0.05 vs saline-injected rats; #P<0.05 vs contralateral side.
3.2 Co-localization of Fos and IL-1RI
The reddish brown reaction product for IL-1RI was localized in cytoplasm and dendrites while the dark reaction product associated with Fos was present in the nucleus, reliably distinguishing the single-labeled IL-1RI and Fos from the double-labeled IL-1RI/ Fos neurons. IL-1RI-immunoreactive neurons were evenly distributed in lumbar spinal cord. In the superficial laminae, most of these were small to medium rounded and elliptical neurons (Fig. 3A), while in lamina V, most were large and multipolar in shape with clearly stained dendrites (Fig. 3B). Immunoreactive nuclear profiles of Fos were mainly localized in ipsilateral laminae I–II and laminae V-VI, especially in the medial two-thirds of the superficial dorsal horn. Single- labeled Fos-immunoreactive and double-labeled IL-1RI/ Fos-immunoreactive neurons were counted on 5 sections from each of three rats. Most Fos-immunoreactive neurons were double-labeled with IL-1RI (77.67±3.84 per section); a few were single labeled (19.53±1.8 per section). This indicates that about 80% of the Fos immunoreactive nuclear profiles were found in IL-1RI immunoreactive neurons in laminae I–VI. No IL-1RI or Fos immunoreactive profiles were found in control sections that lacked primary antibodies.
Fig. 3.
Representative photograph showing the colocalization of immunoreactive IL-1RI and Fos in superficial laminae (A) and lamina V (B) of the spinal dorsal horn. Note that the immunostaining product for IL-1RI is localized in cytoplasm and dendrites, while that associated with Fos is present in the nucleus. Arrows indicate doubled-labeled IL-1RI/ Fos neurons. Bars = 50 μm.
3.3 Co-localization of NR1 and IL-1RI
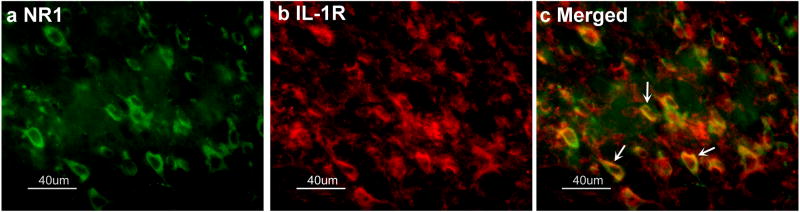
Double immunofluorescence labeling demonstrated that NR1 and IL-1RI were co-localized in the same spinal cord neurons, although some neurons were single labeled for either NR1 or IL-1R1. See Fig. 4. NR1- and IL-1RI-immunoreactive neurons were evenly distributed in the spinal cord.
Fig. 4.
Micrographs showing co-localization of NR1 and IL-1RI in lumbar spinal dorsal horn neurons. Sections were double-labeled with anti-NR1 (green) and anti-IL-1RI (red). a: NR1-immunoreactive neurons in laminae I-II; b: IL-1RI-immunoreactive neurons in laminae I–II; c: Merged graphs of a and b. Arrow indicates double-labeled NR1/IL-1RI neuron (yellow); Scale bars represent 40 μm.
3.4 IL-1ra attenuated inflammatory hyperalgesia
Before the CFA injection, overall mean baseline PWLs to noxious heat stimuli were similar in all groups of rats, and there was no significant difference in PWL between left and right hind paws. Following an injection of 0.08 ml CFA, PWL of the injected paw became significantly shorter than that of the contralateral hind paw, which was unchanged from baseline. Saline injection into the hind paw produced no changes in PWL (data not shown). IL-1ra pretreatment dosage-dependently prevented the hyperalgesia assessed by the PWL test. A 3 x 3 X 2 repeated-measures ANOVA revealed a main effect of drug treatment (F2,95) =5.51, p <0.01) and time (F(2,95) = 45.52, P<0.001) and an interaction between drug treatment and time (F(4,95) = 3.44, p< 0.05).
Post-hoc means comparisons revealed that IL-1ra at 0.01mg/ rat (i.t.) significantly (P<0.05) increased PWL at 2 and 24 hours post-CFA compared to saline control. Contralateral PWL did not change after IL-1ra treatment. The results indicated that IL-1ra at 0.01 mg/rat alleviated inflammation-induced hyperalgesia but did not affect nociception in a normal hind paw (Fig. 5).
Fig. 5.

Effects of IL-1ra on CFA-induced hyperalgesia. IL-1ra at 0.01 (n=7) mg/rat (i.t.) significantly increased PWL compared to vehicle control (n=7). *P<0.05 compared vehicle control.
3.5 IL-1ra inhibited NR1 phosphorylation in the spinal cord
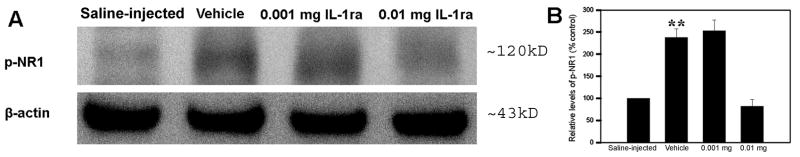
Fig. 6 shows the effects of IL-1ra treatment on NR1 phosphorylation in the spinal cord. The levels of phosphorylated NR1 were significantly higher in vehicle-treated CFA rats compared to levels in saline-injected control rats. Levels were significantly lower (P<0.01) in rats given 0.01 mg IL-1ra treatment than in those given vehicle control. This suggests that IL-1ra inhibited spinal cord NR1 phosphorylation during peripheral hind paw inflammation. In other words, up-regulated endogenous IL-1β facilitated the phosphorylation of spinal cord NR1.
Fig. 6.
A: Effects of IL-1ra on spinal cord NR1 phosphorylation. B: The values of NR1 phosphorylation in saline-injected rats were arbitrarily set at 100%. Each bar is expressed as a percentage of the saline-injected rats. CFA induced significant NR1 phosphorylation compared to saline-injected control (vehicla vs saline-injected). IL-1ra at 0.01 (n=7) mg/rat (i.t.) significantly inhibited spinal cord NR1 phosphorylation compared to vehicle control (n=7). **P<0.01 compared to saline-injected control and 0.01 mg group.
4. Discussion
The present study demonstrates that IL-1β increases significantly in the ipsilateral spinal cord during CFA-induced inflammatory pain. The data are consistent with previous reports that spinal IL-1β is up-regulated during inflammatory pain (Sweitzer et al. 1999; Samad et al. 2001; Watkins et al. 2003; Raghavendra et al. 2004). However, our data further indicate this pain-induced, up-regulated IL-1β is produced by astrocytes but not microglia. Previous studies demonstrate that both astrocytes and microglia are involved in persistent pain (Watkins and Maier 2003; Watkins et al. 2003). Our data suggest that astrocytes exert their actions by producing IL-1β, while microglia may exert their actions through cytokines other than IL-1β during inflammatory pain. Because previous studies also demonstrated that IL-1β is up-regulated during neuropathic pain (Winkelstein et al. 2001; Raghavendra et al. 2003), whether IL-1β is produced by astrocytes or microglia or both of them during neuropathic pain warrant further study.
Further, the study demonstrates that IL-1RI is co-localized with Fos in spinal neurons. It is known that IL-1RI mediates all known biological functions of IL-1β (Subramaniam et al. 2004). This suggests that IL-1β acts on the spinal nociceptive neurons, which is supported by a previous study showing that IL-1β enhances responses to C-fiber stimulation, wind-up phenomena, and post-discharge of wide-dynamic range neurons in the spinal dorsal horn of anesthetized rats (Reeve et al. 2000). Thus, during peripheral inflammation, spinal astrocytes will produce and release IL-1β, which acts on nociceptive neurons to affect pain perception.
The double immunofluorescence labeling also shows that IL-1RI and NR1 are co-localized in spinal neurons. Our previous study demonstrated that some NR1-immunoreactive neurons express Fos during peripheral inflammation-induced pain (Zhang et al. 1998b), suggesting that NMDAR-containing neurons may be nociceptive. Electrophysiological and behavioral studies showed that NMDA receptors play a critical role in transmission of noxious messages in the spinal cord (Dickenson and Sullivan 1990; Ren et al. 1992; Kim et al. 2006). MK-801 significantly attenuated thermal hyperalgesia and inhibited the background activity and noxious heat-evoked response of dorsal horn neurons in CFA-inflamed rats(Ren et al. 1992; Zhang et al. 1998a). Previous study also demonstrated that IL-1β enhances the NMDAR-mediated increase of inward current and intracellular Ca2+(Viviani et al. 2003; Yang et al. 2005). Thus, the coexistence of IL-1RI and NR1 indicates that IL-1β may act on spinal NMDA receptor-containing neurons to modulate NMDA receptors to influence pain transmission.
The main finding of the present study is that IL-1ra simultaneously attenuates inflammatory hyperalgesia and inhibits NR1 phosphorylation. The behavioral test is consistent with previous reports on neuropathic pain and lithium chloride- and lipopolysaccharide-induced pain models (Maier et al. 1993; Milligan et al. 2001; Sweitzer et al. 2001). It demonstrates that IL-1β is involved in the spinal transmission and processing of noxious inputs from the peripheral inflammatory area and that it facilitates inflammation-induced hyperalgesia.
Interestingly, IL-1ra also significantly inhibited the inflammation-induced NR1 phosphorylation, indicating that up-regulated IL-1β enhances NR1 phosphorylation. Previous studies demonstrated that NR1 phosphorylation is correlated with the presence of pain behaviors (Gao et al. 2005; Ultenius et al. 2006) and that blockage of NR1 phosphorylation significantly reversed mechanical allodynia (Gao et al. 2005). These studies demonstrate that NR1 phosphorylation plays a critical role in the transmission of noxious inputs in the spinal cord. In other words, the IL-1ra inhibition of NR1 phosphorylation is linked to the IL-1ra attenuation of inflammatory hyperalgesia, and IL-1β enhancement of NR1 phosphorylation may contribute to hyperalgesia.
Regarding the mechanisms by which IL-1β enhances NR1 phosphorylation, IL-1β may directly act on NMDAR-containing neurons as stated above. However, we do not exclude the possibility that IL-1β may influence NR1 phosphorylation indirectly. It has been demonstrated that IL-1β inhibits astrocyte glutamate uptake (Ye and Sontheimer 1996; Hu et al. 2000) and increases glutamate release (Casamenti et al. 1999). The accumulated extracellular glutamate enhances NR1 phosphorylation (Choe et al. 2006).
In conclusion, the present study demonstrates that IL-1ra attenuates inflammatory hyperalgesia and inhibits NR1 phosphorylation, which suggests that spinal IL-1β, produced by astrocytes, enhances NR1 phosphorylation to facilitate persistent inflammatory pain.
Acknowledgments
We would like to thank Dr. Lyn Lowry for her editorial support. This work was funded by NIH grant CA102383 and AT002605.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Casamenti F, Prosperi C, Scali C, Giovannelli L, Colivicchi MA, Faussone-Pellegrini MS, Pepeu G. Interleukin-1[beta] activates forebrain glial cells and increases nitric oxide production and cortical glutamate and GABA release in vivo: implications for Alzheimer’s disease. Neuroscience. 1999;91(3):831–842. doi: 10.1016/s0306-4522(98)00680-0. [DOI] [PubMed] [Google Scholar]
- Choe ES, Shin EH, Wang JQ. Regulation of phosphorylation of NMDA receptor NR1 subunits in the rat neostriatum by group I metabotropic glutamate receptors in vivo. Neuroscience Letters. 2006;394(3):246–251. doi: 10.1016/j.neulet.2005.10.072. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Differential effects of excitatory amino acid antagonists on dorsal horn nociceptive neurones in the rat. Brain Research. 1990;506(1):31–39. doi: 10.1016/0006-8993(90)91195-m. [DOI] [PubMed] [Google Scholar]
- Gao X, Kim HK, Chung JM, Chung K. Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain. 2005;116(1–2):62–72. doi: 10.1016/j.pain.2005.03.045. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Ehrlich LC, Peterson PK, Chao CC. Cytokine effects on glutamate uptake by human astrocytes. Neuroimmunomodulation. 2000;7(3):153–159. doi: 10.1159/000026433. [DOI] [PubMed] [Google Scholar]
- Kim HK, Kim JH, Gao X, Zhou JL, Lee I, Chung K, Chung JM. Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain. 2006;122(1–2):53–62. doi: 10.1016/j.pain.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Li CY, Ziesmer SC, Lazcano-Villareal O. Use of azide and hydrogen peroxide as an inhibitor for endogenous peroxidase in the immunoperoxidase method. J Histochem Cytochem. 1987;35(12):1457–1460. doi: 10.1177/35.12.2824601. [DOI] [PubMed] [Google Scholar]
- Maier SF, Wiertelak EP, Martin D, Watkins LR. Interleukin-1 mediates the behavioral hyperalgesia produced by lithium chloride and endotoxin. Brain Research. 1993;623(2):321–324. doi: 10.1016/0006-8993(93)91446-y. [DOI] [PubMed] [Google Scholar]
- Milligan ED, O’Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. Journal of Neuroscience. 2001;21(8):2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesthesia & Analgesia. 2003;97(4):1108–1116. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- Presley R, Menetrey D, Levine J, Basbaum A. Systemic morphine suppresses noxious stimulus-evoked Fos protein-like immunoreactivity in the rat spinal cord. J Neurosci. 1990;10(1):323–335. doi: 10.1523/JNEUROSCI.10-01-00323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. Journal of Pharmacology & Experimental Therapeutics. 2003;306(2):624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. European Journal of Neuroscience. 2004;20(2):467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Reeve AJ, Patel S, Fox A, Walker K, Urban L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. European Journal of Pain: Ejp. 2000;4(3):247–257. doi: 10.1053/eujp.2000.0177. [DOI] [PubMed] [Google Scholar]
- Ren K, Hylden JLK, Williams GM, Ruda MA, Dubner R. The effects of a non-competitive NMDA receptor antagonist, MK-801, on behavioral hyperalgesia and dorsal horn neuronal activity in rats with unilateral inflammation. Pain. 1992;50(3):331–344. doi: 10.1016/0304-3959(92)90039-E. [DOI] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. [see comment] Nature. 2001;410(6827):471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Subramaniam S, Stansberg C, Cunningham C. The interleukin 1 receptor family. Developmental & Comparative Immunology. 2004;28(5):415–428. doi: 10.1016/j.dci.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Sung CS, Wen ZH, Chang WK, Ho ST, Tsai SK, Chang YC, Wong CS. Intrathecal interleukin-1beta administration induces thermal hyperalgesia by activating inducible nitric oxide synthase expression in the rat spinal cord. Brain Research. 2004;1051(1–2):145–153. doi: 10.1016/j.brainres.2004.04.068. [DOI] [PubMed] [Google Scholar]
- Sweitzer S, Martin D, DeLeo JA. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience. 2001;103(2):529–539. doi: 10.1016/s0306-4522(00)00574-1. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Colburn RW, Rutkowski M, DeLeo JA. Acute peripheral inflammation induces moderate glial activation and spinal IL-1beta expression that correlates with pain behavior in the rat. Brain Research. 1999;829(1–2):209–221. doi: 10.1016/s0006-8993(99)01326-8. [DOI] [PubMed] [Google Scholar]
- Ultenius C, Linderoth B, Meyerson BA, Wallin J. Spinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the rat. Neuroscience Letters. 2006;399(1–2):85–90. doi: 10.1016/j.neulet.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. Journal of Neuroscience. 2003;23(25):8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nature Reviews Drug Discovery. 2003;2(12):973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Advances in Experimental Medicine & Biology. 2003;521:1–21. [PubMed] [Google Scholar]
- Winkelstein BA, Rutkowski MD, Sweitzer SM, Pahl JL, DeLeo JA. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. Journal of Comparative Neurology. 2001;439(2):127–139. [PubMed] [Google Scholar]
- Yang S, Liu ZW, Wen L, Qiao HFWXZ, Zhang YX. Interleukin-1β enhances NMDA receptor-mediated current but inhibits excitatory synaptic transmission. Brain Research. 2005;1034(1–2):172–179. doi: 10.1016/j.brainres.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Sontheimer H. Cytokine modulation of glial glutamate uptake: a possible involvement of nitric oxide. Neuroreport. 1996;7(13):2181–2185. doi: 10.1097/00001756-199609020-00025. [DOI] [PubMed] [Google Scholar]
- Zhang R-X, Lao L, Wang L, Liu B, Wang X, Ren K, Berman BM. Involvement of opioid receptors in electroacupuncture-produced anti-hyperalgesia in rats with peripheral inflammation. Brain Research. 2004;1020(1–2):12–17. doi: 10.1016/j.brainres.2004.05.067. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Ruda MA, Qiao JT. Pre-emptive intrathecal Mk-801, a non-competitive N-methyl-D-aspartate receptor antagonist, inhibits the up-regulation of spinal dynorphin mRNA and hyperalgesia in a rat model of chronic inflammation. Neuroscience Letters. 1998a;241(1):57–60. doi: 10.1016/s0304-3940(97)00969-5. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Wang H, Ruda M, Iadarola MJ, Qiao JT. c-Fos expression in NMDA receptor-contained neurons in spinal cord in a rat model of inflammation: a double immunocytochemical study. Brain Research. 1998b;795(1–2):282–286. doi: 10.1016/s0006-8993(98)00300-x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu J, Lei Y, Fang L, Willis WD. Protein phosphatase modulates the phosphorylation of spinal cord NMDA receptors in rats following intradermal injection of capsaicin. Molecular Brain Research. 2005;138(2):264–272. doi: 10.1016/j.molbrainres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Enhanced phosphorylation of NMDA receptor 1 subunits in spinal cord dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. Journal of Neuroscience. 2000;20(18):6989–6997. doi: 10.1523/JNEUROSCI.20-18-06989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]