Abstract
Neuroglobin (Ngb) is a newly discovered globin in the vertebrate brain that exhibits neuroprotection against hypoxic/ischemic injury. Hypoxic/ischemic brain injury is associated with accumulation of reactive oxygen species (ROS) and/or reactive nitrogen species (RNS), and antioxidants or ROS scavengers promote cell survival. Therefore, Ngb may serve as a scavenger of toxic reactive species, such as hydrogen peroxide. To examine the anti-oxidative role of neuroglobin, PC12 cells were transfected with wild-type and mutant (H64V/H96A) Ngb for 48 hours and then treated with H2O2 (0.1, 0.2 and 0.4 mM) for 6 hours. Ngb siRNA decreased the H2O2 induced Ngb expression and exacerbated H2O2 induced cell injury. Transient transfection of Ngb induced dose-dependent increases in Ngb protein expression and did not alter SOD, GPX, and catalase activities. Overexpression of wild type Ngb, but not of mutant Ngb, significantly attenuated H2O2-induced ROS/RNS accumulation and lipid peroxidation, decreased H2O2 induced mitochondrial dysfunction and apoptosis, and promoted overall cell survival. Thus, Ngb plays a protective role against oxidative stress, which appears to be primarily mediated by intrinsic Ngb antioxidant properties.
Keywords: Neuroglobin, Oxidative stress, PC12 cells
1. Introduction
Neuroglobin (Ngb) is a newly discovered globin in the vertebrate brain that displays a high affinity for oxygen (Burmester et al., 2000; Moens and Dewilde, 2000). Ngb is widely and heterotopically expressed in the CNS, and is more particularly abundant in cerebral cortex, hippocampus, thalamus, hypothalamus, and cerebellum of rat brain (Geuens et al., 2003; Reuss et al., 2002; Wystub et al., 2003). Ngb appears to play a role in neuronal protection following hypoxic and ischemic insults (Khan et al., 2006; Sun et al., 2001; Sun et al., 2003). Indeed, administration of anti-sense oligodeoxynucleotides directed against Ngb exacerbated experimental stroke in vivo (Sun et al., 2003). Furthermore, overexpression of Ngb in a transgenic mouse model reduced cerebral infarct size following middle cerebral artery occlusion (MCAO) (Khan et al., 2006), thereby lending support to the notion that Ngb may protect neurons against hypoxic-ischemic insults. However, the mechanisms underlying such Ngb-mediated neuronal protection during hypoxic/ischemic stress remain largely unknown. Several proposed mechanisms include Ngb acting as an oxygen sensor and storage molecule (Schmidt et al., 2003; Trent, III et al., 2001), operating as a guanine nucleotide dissociation inhibitor (Wakasugi et al., 2003), interacting with Na_K-ATPase (Xu et al., 2003), and even possibly by up-regulating eNOS (Khan et al., 2006). Notwithstanding such potential functional roles, a recent in vitro study suggested that Ngb could act as a scavenger of toxic reactive species, such as nitrogen monoxide, peroxynitrite and hydrogen peroxide (Herold et al., 2004), a very attractive possibility indeed, when considering our current understanding of cerebral ischemia and reperfusion injury. More recently, hydrogen peroxide concentration was found to be negatively correlated with Ngb expression level in a hypoxia/reoxygenation cell model (Fordel et al., 2007), and over-expression of neuroglobin was associated with improved cell survival following hydrogen peroxide treatment (Fordel et al., 2006). These observations further lend support to the hypothesis that Ngb may play a protective role in ROS/RNS scavenging during ischemia/reperfusion injury.
Oxidative stress has been extensively implicated in the pathophysiology of cerebral ischemia and stroke (Clemens, 2000; Gilgun-Sherki et al., 2002; Janardhan and Qureshi, 2004), and both ROS and/or RNS are over produced during ischemia/reperfusion of neural tissues (Chan, 2001; Lewen et al., 2000; Li and Jackson, 2002; Sugawara and Chan, 2003). The excessive production of ROS/RNS can cause cellular damage and subsequent cell death, because ROS and RNS will not only oxidize vital cellular components such as lipids, proteins, and DNA (Sugawara and Chan, 2003), but will also alter several signaling pathways that ultimately promote cellular damage and death during cerebral ischemia and reperfusion (Chan, 2001). For example, remarkable decreases in infarct volume were observed after permanent MCAO in SOD1-overexpressing transgenic mice (Chan et al., 1994), while SOD1-deficient mice. displayed increased cell death and brain edema after transient MCAO and global cerebral ischemia (Kondo et al., 1997). Therefore, antioxidant strategies may provide useful intervention strategies aimed at reducing ischemic brain injury.
Based on aforementioned considerations, we hypothesized that overexpression of Ngb would attenuate H2O2 induced excessive generation of ROS/RNS and protect cells from oxidative stress-induced cell injury.
2. Results
2.1. H2O2 induces Ngb protein expression in PC12 cells to promote survival
To investigate the effect of oxidative stress on Ngb expression and whether silencing Ngb expression could affect the vulnerability to oxidative stress, PC12 cells were transfected with Ngb siRNA or control siRNA for 48 h, in the presence of H2O2 (0.1mM, n=6) or same volume of H2O (Control group). Ngb protein expression was determined by western blotting and cell viability was assessed by MTT assay. Ngb protein expression was induced by H2O2 (*P<0.01 vs. control) and these increases in Ngb expression were abrogated by Ngb siRNA treatment (#P<0.01 vs. control siRNA, Fig 1A. Furthermore, H2O2 induced cell death was exacerbated by Ngb siRNA treatment (*P<0.01 vs. control, #P<0.01 vs. control siRNA, Fig 1B), indicating that blocking of Ngb expression increases in the presence of H2O2 is associated with increased susceptibility to hydrogen peroxide. These findings suggest that Ngb may play a role against oxidative stress-induced cell injury.
Figure 1. Ngb protein expression and cell viability in PC12 cells after transfection of Ngb siRNA and treatment of H2O2.
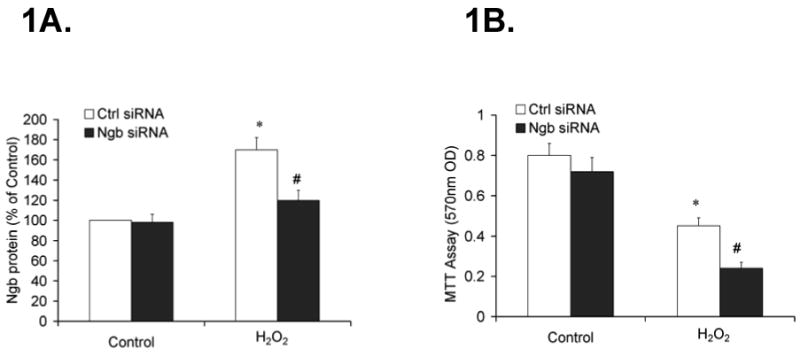
PC12 cells were transfected with Ng siRNA or control siRNA for 48 h. Following transfection, the cells were treated with H2O2 (0.1mM) or same volume of H2O (control) for 6 h. Ngb protein expression was determined by quantitative western blotting. Cell viability was assessed by MTT assay. 1A, Ngb protein expression following transfection of Ngb siRNA and treatment of H2O2. Data were expressed as a percentage of control siRNA in control group (n=6/group, *P<0.01 vs. control, #P<0.01 vs. control siRNA). 1B. Cell viability following transfection of Ngb siRNA and treatment of H2O2. Data were expressed as absolute OD values (570 nm OD) (n=6/group, *P<0.01 vs. control, #P<0.01 vs. control siRNA).
2.2 Transient Ngb transfection induces overexpression of Ngb and does not alter SOD, GPX and catalase activity in PC12 cells
A dose-dependent increase in Ngb protein expression was induced by transfection of wild type or mutant Ngb (0.5 μg, 1.0 μg and 2.0 μg/106 cells, n=6) in PC12 cells (*P<0.01 vs. vector, Fig 2A). These data suggest that transient Ngb transfections induce overexpression of Ngb protein in PC12 cells. To test whether such process could alter the endogenous antioxidant system, the enzymatic activity of SOD, GPX and catalase was measured after Ngb transfection (1.0 μg/106 cells, n=6). No significant changes in SOD, GPX and catalase occurred in PC12 cells following Ngb transfection (*P>0.05 vs. vector, Fig 2B), indicating Ngb does not interfere with the endogenous antioxidant system.
Figure 2. Ngb protein expression and SOD, GPX and Catalase activity in PC12 cells following Ngb transfection.
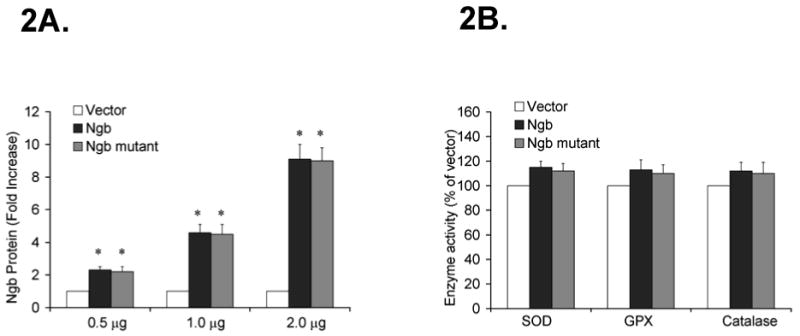
PC12 cells were transfected with 0.5 μg, 1.0 μg and 2.0 μg/106 cells of Ngb, Ngb mutant or vector DNA for 48 h. Following transfection, Ngb protein expression was determined by Western blotting. 2A, Immunoblots of Ngb protein expression following Ngb transfection. Data were expressed as a fold increase over vector transfection (n=6/group, * P<0.01 vs. vector). 2B. Enzymatic activity of SOD, GPX and Catalase following Ngb transfection. Data were expressed as absolute OD values (570 nm OD) (n=6/group, * P<0.01 vs. vector).
2.3. Overexpression of Ngb significantly attenuates H2O2–induced ROS/RNS production
ROS/RNS production in PC12 cells was measured after treatment with H2O2 (0.1, 0.2 and 0.4 mM, n=6) or same volume of H2O (control) for 6 hours. Dose dependent increases in CM-H2DCFDA and DHR 123 fluorescence occurred following H2O2 treatment (*P<0.01 vs. control, Fig 3A, 3B). Furthermore, CM-H2DCFDA fluorescence induced by H2O2 treatment (0,1 mM) was significantly decreased by transfection with wildtype Ngb (0.5, 1.0 and 2.0 μg/106 cells, n=6; *P<0.01 vs. vector). However, H2O2 induced CM-H2DCFDA fluorescence was not reduced by transfection of mutant Ngb (Fig 3C). In addition, H2O2-induced DHR123 fluorescence was also attenuated in PC12 cells by transfection of wild type Ngb, but not by transfection of mutant Ngb (*P<0.01 vs. vector, Fig 3D). These data suggest that overexpression of wildtype Ngb but not of mutant Ngb attenuates H2O2-induced total ROS/RNS production in PC12 cells and may play a role against oxidative stress.
Figure 3. Effect of Ngb overexpression on H2O2-induced ROS/RNS production in PC12 cells.
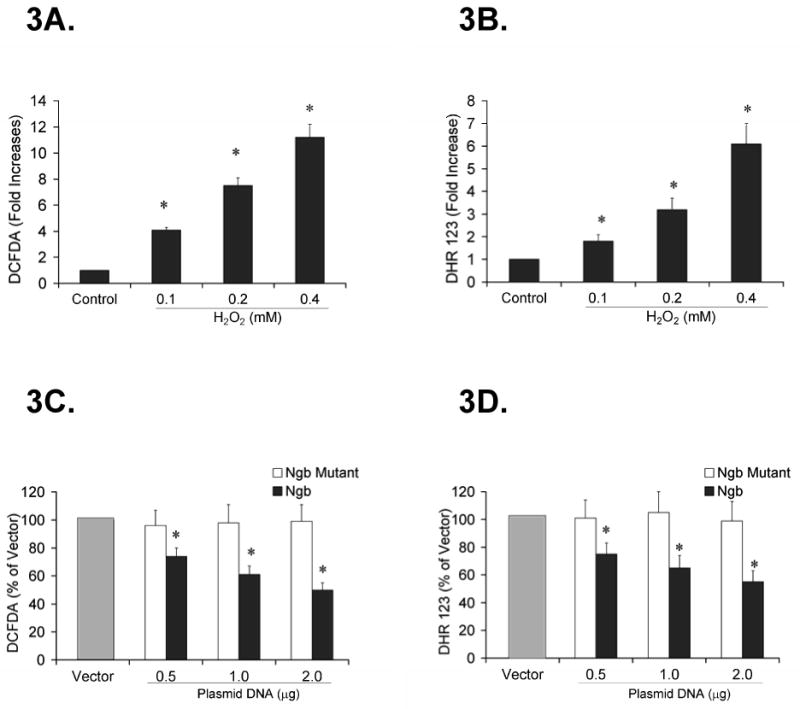
ROS/RNS production was measured with the fluorescent dyes CM-H2DCFDA and DHR 123. 3A. DCFDA fluorescence after H2O2 treatment (0.1, 0.2 and 0.4 mM) or same volume of H2O (control). Data were expressed as a fold increase over control (n=6/group, * P<0.01 vs. control). 3B. DHR123 fluorescence after H2O2 treatment. Data were expressed as fold increase over control (n=6/group, * P<0.01 vs. control). 3C. Effect of Ngb overexpression on H2O2-induced DCFDA fluorescence. Data were expressed as the percentage of vector transfection (n=6/group, * P<0.01 vs. vector). 3D. Effect of Ngb overexpression on H2O2-induced DHR123 fluorescence. Data were expressed as the percentage of vector transfection (n=6/group, * P<0.01 vs. vector).
2.4. Overexpression of Ngb markedly decreases H2O2-induced lipid peroxidation and improves mitochondrial membrane potential in PC12 cells
To determine the effects of overexpression of wildtype or mutant Ngb on lipid peroxidation and mitochondrial membrane potential in PC12 cells, cells were transfected with either wild type or mutant Ngb DNA (1.0 μg/per well) for 48 h and treated with H2O2 (0.1 mM) or same volume of H2O (control) for 6 h. Following Ngb transfection and H2O2 treatment, a marked increase in MDA formation and a decrease in JC-1 ratio occurred in the vector alone transfected cells (n=6, *P<0.01 vs. control, Fig 4A, 4B), indicating that H2O2 treatment induced lipid oxidation and mitochondrial dysfunction in PC12 cells. Furthermore, H2O2-induced MDA formation was significantly decreased by transfection of wild type Ngb, but not by transfection of mutant Ngb (n=6, *P<0.01 vs. control, #P<0.01 vs. vector, Fig 4A). The H2O2 induced decrease in JC-1 ratio was significantly improved by transfection of wild type Ngb, while mutant Ngb had no such effect (n=6, *P<0.01 vs. control, #P<0.01 vs. vector, Fig 4B), indicating that Ngb may be able to preserve mitochondrial membrane potential in the presence of H2O2. Thus, Ngb overexpression reduces H2O2 induced lipid peroxidation and mitochondrial dysfunction, and may play an important role in protecting neuronal cells against oxidative stress.
Figure 4. Effect of Ngb transfection on H2O2-induced lipid peroxidation, mitochondrial membrane potential, caspase 3/7 activity, and cell viability in PC12 cells.
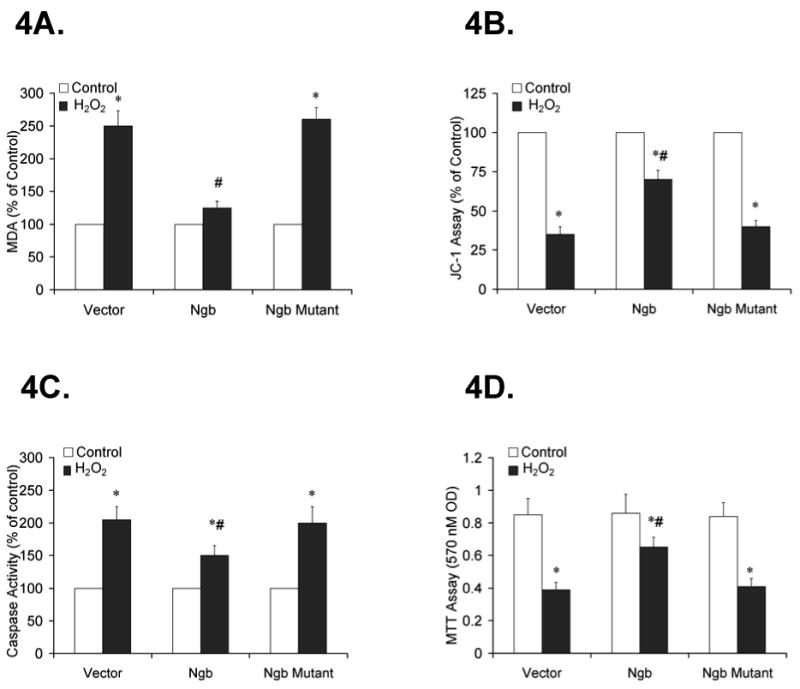
PC12 cells were transfected with vector, wild type or mutant Ngb DNA (1.0 μg/106 cell) for 48 h and treated with H2O2 (0.1mM) or same volume of H2O (control) for 6 h. 4A. Effect of Ngb transfection on H2O2-induced MDA formation. Data are expressed as percentage of control (n=6/group, * P<0.01 vs. control, # P<0.01 vs. vector). 4B. Effect of Ngb transfection on H2O2-induced mitochondrial membrane potential. Data are expressed as percentage of control (n=6/group, * P<0.01 vs. control, # P<0.01 vs. vector). 4C. Effect of Ngb transfection on H2O2-induced caspase 3/7 activity. Data are expressed as percentage of control (n=6/group, * P<0.01 vs. control, # P<0.01 vs. vehicle). 4D. Effect of Ngb transfection on H2O2-induced cell death. Data are expressed as absolute OD values (570 nm OD) (n=6/group, * P<0.01 vs. control, # P<0.01 vs. vector).
2.5. Overexpression of Ngb protects PC12 cells against H2O2-induced apoptosis and promotes cell survival
To further examine whether overexpression of Ngb could attenuate H2O2-induced apoptosis and improve cell viability, cell apoptosis and cell viability were assessed using caspase activity and MTT assays respectively following H2O2 treatment (0.1mM) or same volume of H2O (control) for 6 hours. In the vector alone treated group, increased caspase 3/7 activity and a reduction in cell viability were detected following H2O2 treatment (n=6, *P<0.01 vs. Vector, Fig 4C, 4D). In the wild type Ngb transfection group, caspase 3/7 activity was significantly decreased compared to the vector alone group (n=6, *P<0.01 vs. control, #P<0.01 vs. vector, Fig 4C). In addition, MTT reductions were also improved by transfection of wild type Ngb (n=6, *P<0.01 vs. control, #P<0.01 vs. vector, Fig 4D). However, no significant changes in caspase 3/7 activity and MTT reduction occurred in the mutant Ngb transfection group (n=6, *P<0.01 vs. vehicle, #P<0.01 vs. vehicle, Fig 4C, 4D), thereby suggesting that Ngb overexpression decreased oxidative stress-induced apoptosis and improved overall PC12 cell survival after H2O2 treatment.
3. Discussion
Previous studies support the contention that Ngb expression may be regulated upon hypoxia or ischemia exposures. Indeed, both Ngb mRNA and protein expression were increased by hypoxia in vitro and by focal cerebral ischemia in vivo (Sun et al., 2001). Furthermore, we have recently shown that exposures to environmental hypoxia will increase expression of Ngb in rodent brain (Li et al., 2006). Ngb mRNA expression was also up-regulated in response to hypoxia in HN33 cells (Zhu et al., 2002). However, no studies have explored the potential induction of Ngb expression in response to oxidative stress. To this effect, PC12 cells were treated by toxic low dose H2O2 (100 μM). We found that Ngb protein expression was moderately induced by H2O2, indicating that increased oxidative stress is a modulator of Ngb expression in addition to hypoxia or anoxia. Furthermore, Ngb siRNA not only prevented H2O2 induced Ngb expression, but also significantly exacerbated H2O2-induced cell injury, suggesting that Ngb may play a protective role against oxidative stress in PC12 cells, and as such overexpression of Ngb would convey incremental protections against H2O2. Mounting evidence has suggested that oxidative stress play an important role in hypoxia/ischemia cell injury. In the present study, we have shown that addition of H2O2 to PC12 cells in culture not only elicited ROS/RNS accumulation, but was also associated with increased lipid peroxidation, activation of caspases, decreased mitochondrial potential, and ultimately promoted cell death. Furthermore, overexpression of wild type Ngb favorably affected this deleterious cascade, while in contrast Ngb with targeted mutations within H64V and H96A residues was associated with the loss of Ngb antioxidant capacity and cellular protection, thereby indicating that these two histidine residues in the Ngb molecule are critically important for its antioxidant function and for promoting cell survival during H2O2 exposures. The major difference between Ngb and Hb is that Ngb has a six-coordinated heme. Several studies suggested that the six-coordinated heme structure plays an important role in regulating Ngb function (Dewilde et al., 2001; Nicolis et al., 2007; Nienhaus et al., 2004). The proximal and distal histidine (F8 and E7) of Ngb are two important residues to maintain the six-coordinated heme structure. Mutation of these two histidine residues will lead to a deconstructed six-coordinated heme, and will further interfere with Ngb ligand binding and its potential antioxidant properties.
Endogenous anti-oxidative defense systems will scavenge ROS/RNS induced by cerebral ischemia/reperfusion (Chan, 2001; Sugawara and Chan, 2003), and serve therefore as the initial defense line against oxidative stress damage. Since wild type Ngb overexpression decreased the excess ROS/RNS induced by H2O2 treatment in PC12 cells, it is possible that Ngb scavenging of ROS/RNS may have occurred via recruitment of the endogenous anti-oxidative system, such as induction of SOD, GPX and/or Catalase activities. However, our findings do not support this assumption, since we found that transfection with either wild type or mutant Ngb induced overexpression of Ngb, but did not alter the enzymatic activities of the anti-oxidant enzymes. Thus, the ROS scavenging capacity of Ngb appears to be independent of the classic endogenous ROS/RNS scavenging system, and may, as suggested by in vitro studies (Li et al., 2007), possess intrinsic scavenging properties, that emerge as highly beneficial in the presence of excess H2O2.
The excessive production of ROS/RNS can induce oxidation of vital cellular components such as lipids, proteins, and DNA, and eventfully either directly or indirectly lead to mitochondrial dysfunction, apoptosis and cell death (Chan, 2001; Lewen et al., 2000; Sugawara and Chan, 2003). In the present study, we found that H2O2 treatment not only induced excess ROS/RNS, but also caused mitochondrial dysfunction. During ischemia/reperfusion, mitochondrial dysfunction is a critical event that will trigger apoptosis, cause energy depletion and ultimately lead to cell death (Chan, 2001; Sugawara and Chan, 2003). The decreases in mitochondrial membrane potential found in the present study further indicate disruption of mitochondrial membrane integrity, whereby ROS produced in mitochondria may then leak to the cytoplasm, lead to oxidative stress, and initiate cell death via activation of apoptosis signaling (Budihardjo et al., 1999). Indeed, H2O2 treatment significantly increased caspase 3/7 activity and transfection of wild type Ngb attenuated this process. Considering the observation that Ngb is associated with decreases in H2O2-induced ROS production, it is likely that Ngb anti-apoptotic role may be mediated by its antioxidant properties. It has been suggested that ROS/RNS are involved in the apoptotic mechanisms triggered in ischemia/reperfusion injury (Loh et al., 2006; Ryter et al., 2007; Valko et al., 2007). Therefore, antioxidant strategies such as that afforded by increased expression of Ngb may reduce the extent of apoptotic cell death, and improve overall outcomes in CNS ischemic injury. Of note, H2O2 has been previously shown to induce apoptosis in PC12 cells, and antioxidant treatments provide protection against H2O2 induced cell injury (Cheng et al., 2007; Fujita et al., 2006; Jiang et al., 2007). Thus, antioxidant strategies may provide useful intervention strategies to reduce oxidative stress induced cell injury.
Recent studies have suggested that Ngb plays a role in the neuronal protection in response to hypoxia and ischemia. However, the mechanisms underlying such Ngb-mediated neuronal protection during hypoxic/ischemic stress remain largely unknown. For example, Ngb could act as an endogenous scavenger of toxic reactive species. In an earlier study, Ngb was able to scavenge nitrogen monoxide, peroxynitrite and hydrogen peroxide (Herold et al., 2004). More recently, hydrogen peroxide concentration was found to be inversely correlated with the level of Ngb protein expression in a hypoxia/reoxygenation cell model (Fordel et al., 2007). Furthermore, over-expression of neuroglobin was associated with improved cell survival following hydrogen peroxide exposures (Fordel et al., 2006). Ngb could be operating as a potential ROS scavenger, via reduction reactions leading to ferric Ngb conversion to ferrous Ngb by endogenous reducing enzyme systems (Trandafir et al., 2007). However, a recent study suggested that human Ngb may act as a nitrate and H2O2 scavenger with an external substrate, but that otherwise, Ngb may actually generate reactive species (Nicolis et al., 2007). Based on the present study findings and those aforementioned recent studies, it is conceivable that Ngb plays a functional role against oxidative stress and promotes cell survival during hypoxia/ischemia insults.
In conclusion, Ngb not only decreases oxidative stress-induced ROS/RNS overproduction and lipid peroxidation, but also attenuates subsequent mitochondrial dysfunction, apoptosis, and cell death. Therefore, Ngb may act as an intracellular ROS/RNS scavenger, and such antioxidant properties may play a protective role against oxidative stress-induced cell injury. Further exploration of Ngb antioxidant properties may provide opportunities for novel pharmacological interventions aiming at preventing or palliating cerebral ischemic injury.
4. Experimental procedures
4.1 Cell Culture
PC12 cells were originally obtained from American Tissue Type Cell Collection (ATCC) and were cultured on 100 mm plates coated with collagen. The collagen was diluted in 30% ethanol (1:10 dilution). The cells were grown in RPMI 1640 medium supplemented with 10% horse serum, 5% fetal calf serum, and 1% antibiotics (penicillin/streptomycin). One day before experiments, cells were seeded in 6-well culture dishes (106 per well). Following transfection of Ngb and treatment with H2O2, cells were subjected to ROS and MDA measurements, JC-1 assessments, and MTT assays.
4.2. siRNA transfection
The Ngb specific siRNA (Ambion, Austin, TX) was transfected with Lipofectamine 2000 (Life Technologies) in six-well plates. Briefly, Lipofectamine diluted in Opti-MEM (3 μl/50 μl) was incubated for 5 minutes. Control SiRNA and Ngb siRNA (100 pmol) were diluted in DMEM (50 μl) and added to the Lipofectamine mixture. The incubation was continued for an additional 20 minutes before addition to cultures and transfection. Following 48 hours transfection, the PC12 cells were treated with H2O2 (0.1mM) for 6 hours and subject to western blotting and MTT assay.
4.3. Ngb Plasmid Construct
To achieve high expression of Ngb, a cDNA encoded with human wild type Ngb was synthesized using a modification of recursive PCR strategy. Ngb cDNA was subcloned into expression vector pcDNA3.1 (Invitrogen, Carlsbad, CA) with a cytomegalovirus promoter. The C-terminal was tagged with an in-frame V5 epitope for easy detection of Ngb fusion protein. The ligation products were introduced into E. coli strain XL-10 Gold ultracompetent cells (Stratagene, La Jolla, CA). The QuikChange system (Stratagene, La Jolla, CA) was used to introduce mutations into the Ngb coding sequence in pcDNA3.1-Ngb plasmid, which directed the expression of Ngb with H64V/H96A mutation. The inserted Ngb DNA and point mutations were then verified by DNA sequencing. The functional overexpression of Ngb was confirmed by western blotting or immunohistochemistry using specific anti-Ngb (Biovedor, Heidelberg, Germany) or anti-V5 antibody (Invitrogen, Carlsbad, CA). Ngb plasmid DNA was then transfected by using Lipofectamine 2000 (Invitrogene, Carlsbad, CA). The transfection efficiency was determined by use of a plasmid encoding the ß-galactosidase (pcDNA3.1-LacZ) compared with an empty vector control (pcDNA3.1).
4.4. Western Blotting
Transfected PC12 cells were homogenized by standard procedures. Protein concentrations were determined using the Bradford protein assay. Homogenate proteins (50μg) were heated for ten minutes at 90°C and then loaded on 18% gradient PAGE gels, then transferred electrophoretically onto nitrocellulose membranes. Membranes were blocked with 5% non-fat dry milk and incubated overnight at 4°C with the Ngb polyclonal antibody (1:2500, Biovedor, Heidelberg, Germany). Ngb protein bands were detected with horseradish peroxidase-conjugated anti-chicken secondary antibodies and visualized by ECL reagents. The same membranes were also blotted with β-actin antibody (Sigma, St. Louis, MO), and Ngb blots were then normalized to β-actin. Densitometric analysis was performed with a gel scanning densitometer (Molecular Dynamics, Sunnyvale, CA). Data were expressed as fold increase of control group.
4.5. Measurement of SOD, GPX and catalase activity
PC12 cells were homogenized with a homogenizer in 10 volumes of a 50mM sodium phosphate buffer (pH 7.4) at 4 °C. Homogenates were centrifuged at 15000 g for 10 min, and the supernatant obtained was used for the following antioxidant enzyme measurements. SOD, GPX and catalase activity was determined spectrophotometrically using commercially available assay kits from Cayman Chemicals (Ann Arbor, MI). Sample protein content was measured by using the Bio-Rad protein assay kit. The enzyme activities were then normalized to the corresponding protein concentration for each sample.
4.6. Measurement of ROS/RNS Production
The fluorescent dyes CM-H2DCFDA and DHR 123 (Molecular Probe) were used to estimate total levels of ROS/RNS production in PC12 cells. PC12 cells were loaded with CM-H2DCFDA or DHR 123. After incubating with fluorescent probes for 30 min, the cells were treated with H2O2. The ROS or RNS production were determined by measurement of fluorescence derived from the fluorescent dye. The fluorescence was read with a spectrofluorometer (Victor3 1420, Perkin Elmer, Boston, MA) with appropriate excitation and emission wavelengths. Positive and negative controls were included in each measurement. Freshly prepared cells were used as negative control and cell treated with 0.4 mM H2O2 for 6 hours were used as positive control.
4.7. Lipids peroxidation assay
Malonyldialdehyde (MDA), an index of lipid peroxidation, was measured by using a commercial assay (OxisResearch, Portland, OR). This assay is based on the reaction of a chromogenic reagent, N-methyl-2-phenylindole, with MDA at 45°C, and measures specifically MDA and not other lipid peroxidation products. In brief, following 24 h H2O2 treatments, cells were washed three times with 1× PBS and homogenized in 20 mM phosphate buffer (pH 7.4) containing 0.5 mM butylated hydroxytoluene to prevent sample oxidation. The lysate was centrifuged at 1000 × g for 10 min, and a 200 μl aliquot of the supernatant was used to measure MDA levels according to the instructions of the manufacturer. An MDA standard curve was used to determine the absolute concentration of MDA in the samples using the stable MDA precursor tetramethoxypropane. Values were standardized to micrograms of protein for each sample.
4.8. Caspase-3/7 activity assay
Caspase-3/7 activity was measured using the Apo-ONE Homogeneous Caspase-3/7 Assay Kit (Promega), according to the manufacturer's instructions. Briefly, PC12 cells were rinsed twice with PBS and then lysed in 50 μL of Homogeneous Caspase-3/7 Buffer containing the caspase-3 substrate, Z-DEVD-rhodamine 110. Cell lysates were incubated for 14 h at room temperature. After incubation, the fluorescence (excitation, 480 nm and emission, 535 nm) of cell lysates (50 μL) was measured using a spectrofluorometer (Victor3 1420, Perkin Elmer).
4.9. Mitochondrial Membrane Potential
The mitochondrial membrane potential was measured using the JC-1 fluorescent dye (Molecular Probe). PC12 cells were incubated with JC-1 dye for 30 min. After incubation, cells were rinsed with PBS. The emission signals at 590 and 527 nm elicited by excitation at 485 nm were measured with a spectrofluorometer (Victor3 1420, Perkin Elmer). The ratio of the signal at 590 nm over that at 527 nm (red/green ratio) was calculated.
4.10. Cell Viability Assays
Cell viability was assessed by measuring formazan produced by the reduction of MTT. Transfected PC12 cells in 6-well culture dishes were treated with H2O2 and incubated for 6 hours at 37°C. MTT was added (5mg/ml), and the cells were incubated an additional hour. After this step, the medium was removed and cells were solubilized with dimethylsulfoxide and transferred to a 96-well plate. The formazan reduction product was measured by reading absorbance at 540nm in a plate reader.
4.11. Data analysis
Data in text and figures are expressed as mean ± SE. Two group comparisons were evaluated by paired or unpaired t tests, as appropriate. Multiple comparisons were analyzed by ANOVA and Tukey's or Newman Keuls post-hoc tests. Differences were considered statistically significant for P< 0.05.
Acknowledgments
We thank Kenneth R. Brittian and Heather B. Clair for their expert technical assistance in cell culture and immunocytochemistry. This study was supported by National Institutes of Health grants SCOR 2P50-HL-60296 (Project 2) and RO1-HL-69932, The Children's Foundation Endowment for Sleep Research, and by the Commonwealth of Kentucky Challenge for Excellence Trust Fund.
Abbreviations
- CNS
central nerve system
- eNOS
endothelial nitric oxide synthase
- GPX
glutathione peroxidase
- MCAO
middle cerebral artery occlusion
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- Ngb
Neuroglobin
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Chan PH, Epstein CJ, Kinouchi H, Kamii H, Imaizumi S, Yang G, Chen SF, Gafni J, Carlson E. SOD-1 transgenic mice as a model for studies of neuroprotection in stroke and brain trauma. Ann N Y Acad Sci. 1994;738:93–103. doi: 10.1111/j.1749-6632.1994.tb21794.x. [DOI] [PubMed] [Google Scholar]
- Cheng XR, Zhang L, Hu JJ, Sun L, Du GH. Neuroprotective effects of tetramethylpyrazine on hydrogen peroxide-induced apoptosis in PC12 cells. Cell Biol Int. 2007;31:438–443. doi: 10.1016/j.cellbi.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Clemens JA. Cerebral ischemia: gene activation, neuronal injury, and the protective role of antioxidants. Free Radic Biol Med. 2000;28:1526–1531. doi: 10.1016/s0891-5849(00)00258-6. [DOI] [PubMed] [Google Scholar]
- Dewilde S, Kiger L, Burmester T, Hankeln T, Baudin-Creuza V, Aerts T, Marden MC, Caubergs R, Moens L. Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J Biol Chem. 2001;276:38949–38955. doi: 10.1074/jbc.M106438200. [DOI] [PubMed] [Google Scholar]
- Fordel E, Thijs L, Martinet W, Lenjou M, Laufs T, Van Bockstaele D, Moens L, Dewilde S. Neuroglobin and cytoglobin overexpression protects human SH-SY5Y neuroblastoma cells against oxidative stress-induced cell death. Neurosci Lett. 2006;410:146–151. doi: 10.1016/j.neulet.2006.09.027. %20. [DOI] [PubMed] [Google Scholar]
- Fordel E, Thijs L, Moens L, Dewilde S. Neuroglobin and cytoglobin expression in mice. Evidence for a correlation with reactive oxygen species scavenging. FEBS J. 2007;274:1312–1317. doi: 10.1111/j.1742-4658.2007.05679.x. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Izawa Y, Ali N, Kanematsu Y, Tsuchiya K, Hamano S, Tamaki T, Yoshizumi M. Pramipexole protects against H2O2-induced PC12 cell death. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:257–266. doi: 10.1007/s00210-005-0025-2. [DOI] [PubMed] [Google Scholar]
- Geuens E, Brouns I, Flamez D, Dewilde S, Timmermans JP, Moens L. A globin in the nucleus! J Biol Chem. 2003;278:30417–30420. doi: 10.1074/jbc.C300203200. [DOI] [PubMed] [Google Scholar]
- Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev. 2002;54:271–284. doi: 10.1124/pr.54.2.271. [DOI] [PubMed] [Google Scholar]
- Herold S, Fago A, Weber RE, Dewilde S, Moens L. Reactivity studies of the Fe(III) and Fe(II)NO forms of human neuroglobin reveal a potential role against oxidative stress. J Biol Chem. 2004;279:22841–22847. doi: 10.1074/jbc.M313732200. [DOI] [PubMed] [Google Scholar]
- Janardhan V, Qureshi AI. Mechanisms of ischemic brain injury. Curr Cardiol Rep. 2004;6:117–123. doi: 10.1007/s11886-004-0009-8. [DOI] [PubMed] [Google Scholar]
- Jiang H, Zhang J, Zhu H, Li H, Zhang X. Nerve growth factor prevents the apoptosis-associated increase in acetylcholinesterase activity after hydrogen peroxide treatment by activating Akt. Acta Biochim Biophys Sin (Shanghai) 2007;39:46–56. doi: 10.1111/j.1745-7270.2007.00247.x. [DOI] [PubMed] [Google Scholar]
- Khan AA, Wang Y, Sun Y, Mao XO, Xie L, Miles E, Graboski J, Chen S, Ellerby LM, Jin K, Greenberg DA. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc Natl Acad Sci U S A. 2006;103:17944–17948. doi: 10.1073/pnas.0607497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Reaume AG, Huang TT, Carlson E, Murakami K, Chen SF, Hoffman EK, Scott RW, Epstein CJ, Chan PH. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci. 1997;17:4180–4189. doi: 10.1523/JNEUROSCI.17-11-04180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282:C227–C241. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- Li RC, Lee SK, Pouranfar F, Brittian KR, Clair HB, Row BW, Wang Y, Gozal D. Hypoxia differentially regulates the expression of neuroglobin and cytoglobin in rat brain. Brain Res. 2006;1096:173–179. doi: 10.1016/j.brainres.2006.04.063. [DOI] [PubMed] [Google Scholar]
- Li RC, Pouranfar F, Lee SK, Morris MW, Wang Y, Gozal D. Neuroglobin protects PC12 cells against beta-amyloid-induced cell injury. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh KP, Huang SH, De Silva R, Tan BK, Zhu YZ. Oxidative stress: apoptosis in neuronal injury. Curr Alzheimer Res. 2006;3:327–337. doi: 10.2174/156720506778249515. [DOI] [PubMed] [Google Scholar]
- Moens L, Dewilde S. Globins in the brain. Nature. 2000;407:461–462. doi: 10.1038/35035181. [DOI] [PubMed] [Google Scholar]
- Nicolis S, Monzani E, Ciaccio C, Ascenzi P, Moens L, Casella L. Does human neuroglobin act only as a scavenger? Reactivity and endogenous modification by nitrite and hydrogen peroxide. Biochem J. 2007 doi: 10.1042/BJ20070372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhaus K, Kriegl JM, Nienhaus GU. Structural dynamics in the active site of murine neuroglobin and its effects on ligand binding. J Biol Chem. 2004;279:22944–22952. doi: 10.1074/jbc.M401561200. [DOI] [PubMed] [Google Scholar]
- Reuss S, Saaler-Reinhardt S, Weich B, Wystub S, Reuss MH, Burmester T, Hankeln T. Expression analysis of neuroglobin mRNA in rodent tissues. Neuroscience. 2002;115:645–656. doi: 10.1016/s0306-4522(02)00536-5. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Giessl A, Laufs T, Hankeln T, Wolfrum U, Burmester T. How does the eye breathe? Evidence for neuroglobin-mediated oxygen supply in the mammalian retina. J Biol Chem. 2003;278:1932–1935. doi: 10.1074/jbc.M209909200. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Chan PH. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal. 2003;5:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci U S A. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Peel A, Mao XO, Xie L, Greenberg DA. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci U S A. 2003;100:3497–3500. doi: 10.1073/pnas.0637726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trandafir F, Hoogewijs D, Altieri F, Rivetti dV, Ramser K, Van Doorslaer S, Vanfleteren JR, Moens L, Dewilde S. Neuroglobin and cytoglobin as potential enzyme or substrate. Gene. 2007;398:103–113. doi: 10.1016/j.gene.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Trent JT, III, Watts RA, Hargrove MS. Human neuroglobin, a hexacoordinate hemoglobin that reversibly binds oxygen. J Biol Chem. 2001;276:30106–30110. doi: 10.1074/jbc.C100300200. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Wakasugi K, Nakano T, Morishima I. Oxidized human neuroglobin acts as a heterotrimeric Galpha protein guanine nucleotide dissociation inhibitor. J Biol Chem. 2003;278:36505–36512. doi: 10.1074/jbc.M305519200. [DOI] [PubMed] [Google Scholar]
- Wystub S, Laufs T, Schmidt M, Burmester T, Maas U, Saaler-Reinhardt S, Hankeln T, Reuss S. Localization of neuroglobin protein in the mouse brain. Neurosci Lett. 2003;346:114–116. doi: 10.1016/s0304-3940(03)00563-9. [DOI] [PubMed] [Google Scholar]
- Xu WL, Wang CL, Liao ZY, Zhang YL, Yu LH, Meng FW, Wang XX, Meng FW, Yin ZY, Qian LJ, Zhang CG. Identification of interaction and interaction domains between neuroglobin and Na(+), K(+)-ATPase beta2 subunit. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2003;35:823–828. [PubMed] [Google Scholar]
- Zhu Y, Sun Y, Jin K, Greenberg DA. Hemin induces neuroglobin expression in neural cells. Blood. 2002;100:2494–2498. doi: 10.1182/blood-2002-01-0280. [DOI] [PubMed] [Google Scholar]


