Abstract
When newly hatched Caenorhabditis elegans larvae are starved, their primordial germ cells (PGCs) arrest in the post-S phase. This starvation-induced PGC arrest is mediated by the DAF-18/PTEN–AKT-1/PKB nutrient-sensing pathway. Here, we report that the conserved spindle assembly checkpoint (SAC) component MDF-1/MAD1 is required for the PGC arrest. We identified 2 Akt kinase phosphorylation sites on MDF-1. Expression of a non-phosphorylatable mutant MDF-1 partially suppressed the defect in the starvation-induced PGC arrest in L1 larvae lacking DAF-18, suggesting that MDF-1 regulates germ cell proliferation as a downstream target of AKT-1, thereby demonstrating a functional link between cell-cycle regulation by the SAC components and nutrient sensing by DAF-18–AKT-1 during post-embryonic development. The phosphorylation status of MDF-1 affects its binding to another SAC component, MDF-2/MAD2. The loss of MDF-2 or another SAC component also caused inappropriate germ cell proliferation, but the defect was less severe than that caused by mdf-1 hemizygosity, suggesting that MDF-1 causes the PGC arrest by two mechanisms, one involving MDF-2 and another that is independent of other SAC components.
Keywords: C. elegans, nutrition signals, spindle assembly checkpoint
Introduction
Caenorhabditis elegans are conceived as a single-cell zygote and undergo a nearly invariant developmental process that requires precisely controlled cell divisions. During development, the timing and axis of every round of cell division of each embryonic cell are regulated to follow the genetically programmed pattern (Sulston et al, 1983). Most cell divisions are completed during the first half of embryogenesis, within the proliferation phase, which lasts approximately 7 h after fertilization. The precursor cells of post-embryonic lineages become quiescent and stay dormant throughout the remainder of embryogenesis; cell-cycle progression then resumes after the larvae hatch under physiologically preferable conditions. Although being quiescent, many somatic precursors are blocked in the G1 stage in a cyclin-dependent kinase inhibitor (CKI-1)-dependent manner (Hong et al, 1998; Boxem and van den Heuvel, 2001). In contrast, two primordial germ cells (PGCs), Z2 and Z3, are arrested in early prophase (i.e., chromosomes with a 4N DNA content are highly condensed, but duplicated centrosomes are not yet separated into opposite poles) in a CKI-1-independent manner (Fukuyama et al, 2003, 2006).
In addition to genetically programmed cell-cycle control, environmental conditions cause global interruption of the reproducible pattern of cell division. For instance, when eggs hatch in the absence of food, larvae arrest early in the first larval stage (L1) and do not initiate post-embryonic development until food is restored (Baugh and Sternberg, 2006; Fukuyama et al, 2006; Kao et al, 2007). This dormant state is called L1 diapause. During L1 diapause, cell-cycle arrest of all somatic cells and germ cells is sustained (Hong et al, 1998; Baugh and Sternberg, 2006; Fukuyama et al, 2006). Fukuyama et al (2006) recently reported that C. elegans Pten, DAF-18, is required for maintenance of the cell-cycle arrest of PGCs during L1 diapause. PGCs in larvae homozygous for daf-18 deletion (Δdaf-18) grown under nutritionally deprived conditions fail to arrest and continue dividing. DAF-18 is a negative regulator of insulin/insulin-like growth factor (IGF) pathway, which regulates biological processes such as dauer formation or longevity (Albert et al, 1981; Riddle et al, 1981; Dorman et al, 1995; Larsen et al, 1995; Ogg and Ruvkun, 1998; Gil et al, 1999; Rouault et al, 1999). The C. elegans insulin/IGF pathway is mediated by AGE-1/PI3K and AKT-1/-2 (Ogg and Ruvkun, 1998; Paradis and Ruvkun, 1998), as in other organisms (Vivanco and Sawyers, 2002). Consistent with DAF-18's function as a negative regulator of the insulin/IGF pathway, the inappropriate proliferation of germ cells in starved Δdaf-18 larvae can be suppressed by the loss-of-function mutant of age-1 or a deletion mutant of akt-1 (Δakt-1) (Fukuyama et al, 2006). Under nutritionally deprived conditions, DAF-18's phosphatase activity presumably antagonizes AGE-1 by dephosphorylating PIP3, which transmits intracellular signals produced by AGE-1 kinase activity (Gil et al, 1999). Increased concentrations of PIP3 activate a downstream kinase cascade that includes AKT-1/-2 (Ogg and Ruvkun, 1998; Paradis et al, 1999). DAF-18 thus regulates the germline checkpoint during L1 diapause by opposing the proliferation and growth-promoting activity of AKT-1 (Fukuyama et al, 2006). Although the structures of two C. elegans Akt kinases, AKT-1 and AKT-2, are highly related (Paradis and Ruvkun, 1998), inappropriate germ cell proliferation in starved Δdaf-18 larvae is suppressed only by the Δakt-1 mutant but not by Δakt-2 (Fukuyama et al, 2006). Moreover, although Baugh and Sternberg recently reported that DAF-16, a forkhead-family transcription factor involved in the insulin/IGF pathway controlling the dauer formation and longevity (Gottlieb and Ruvkun, 1994; Lin et al, 1997; Ogg et al, 1997), is also required for the growth arrest of starved larvae in L1 (Baugh and Sternberg, 2006), the pathway controlling germline proliferation during L1 diapause does not require DAF-16 (Fukuyama et al, 2006). Thus, nutrient signal-dependent regulation of post-embryonic germline proliferation is controlled by the pathway that shares some common components with, but is distinct from, those involved in dauer formation and longevity. The downstream target of AKT-1 in the pathway controlling the germline proliferation during L1 diapause remains unknown.
The spindle assembly checkpoint (SAC) ensures proper chromosome segregation by delaying the metaphase–anaphase transition until every pair of sister chromatids in a cell is appropriately attached to the mitotic spindle; thus, the SAC maintains the genome stability of descendants. In response to an emergency signal from an unattached or tension-free kinetochore, the SAC prevents sister chromatids from separating by inhibiting the activity of a large ubiquitin ligase complex called the anaphase-promoting complex/cyclosome (APC/CCDC20) (Musacchio and Salmon, 2007). Inactivation of APC/CCDC20 results in the accumulation of APC substrates such as the anaphase inhibitor securin (known as Pds1 in budding yeast) and cyclin B. The accumulation of securin inhibits the protease activity of separase, which is required for dissociation of sister chromatids from the centromeric region of mitotic chromosomes, whereas that of cyclin B prevents cells from exiting mitosis. As a consequence, the cell-cycle arrests take place before anaphase (Musacchio and Salmon, 2007).
SAC components Mad1-3, Bub1 and Bub3 were originally identified in budding yeast by 2 independent genetic screens (Hoyt et al, 1991; Li and Murray, 1991). Further study of these proteins revealed that they are structurally and functionally well conserved in eucaryotes (reviewed in Musacchio and Salmon, 2007).
In the SAC pathway, MAD2 binding to CDC20 is crucial, and efficient formation of the MAD2–CDC20 complex requires MAD1 (Luo et al, 2002). The molecular basis of MAD1's role in the SAC is thought to be that MAD1 forms a tight complex with a conformation of MAD2 known as closed-MAD2 (also known as C-MAD2 or N2-MAD2) and recruits a conformationally distinct MAD2, open-MAD2 (also known as O-MAD2 or N1-MAD2) to the unattached kinetochores, thereby facilitating the formation of the MAD2–CDC20 complex (Sironi et al, 2001, 2002; Luo et al, 2002). In this model, MAD1's localization to unattached kinetochores is a key step in SAC activation (Chen et al, 1998).
The C. elegans homologue of MAD1, MDF-1, has been identified as a binding partner of the C. elegans homologue of MAD2, MDF-2 (Kitagawa and Rose, 1999). Although MDF-1 has a relatively diverse amino-acid sequence, its predicted coiled-coil structure and the similarity between the short amino-acid sequence in the C-terminal domain with that of human MAD1 led us to categorize the protein as a member of the Mad1 protein family.
The mdf-1-deletion strain has various developmental defects (e.g., embryonic arrest, larval arrest, abnormal vulval development and sterility) (Kitagawa and Rose, 1999). These defects are comparable with phenotypes observed in worms depleted of either MDF-1 or MDF-2 by RNAi. Genome instability is reflected by the high incidence of males, which indicates the increased frequency of X-chromosome missegregation during meiosis. The presence of aneuploid oocytes also suggests that MDF-1 functions in the SAC. More direct evidence of the role of MDF-1 in the SAC in C. elegans is that MDF-1 is required for nocodazole-induced mitotic arrest of germ cells. Furthermore, Encalada et al (2005) recently demonstrated that, in embryonic cells, MDF-1 and MDF-2 are required for mitotic delays induced by chemical or mutational disruption of the microtubule cytoskeleton. Thus, MDF-1 and MDF-2 have an important function in the role of SAC in both embryonic cells and germ cells when mitotic spindle formation is compromised. However, depletion of MDF-1 or MDF-2 does not affect the mitotic duration under normal developmental conditions (Encalada et al, 2005), suggesting that SAC is not required for the normal timing of anaphase onset in early-stage embryos. Nevertheless, the loss of MDF-1 or MDF-2 causes severe defects in development and fertility.
Whether the role of MDF-1 in development and fertility is distinct from its function in the SAC remains unknown. However, the reduced-function mutations in APC/CCDC20 components such as emb-30(APC4) or fzy-1(CDC20) suppress the sterility caused by the loss of mdf-1 (Furuta et al, 2000; Kitagawa et al, 2002; Tarailo et al, 2007); this finding suggests that MDF-1 regulates APC/CCDC20 activity to coordinate the timing of cell division for proper development. The mechanisms that regulate MDF-1's activity during development remain unknown.
Results and discussion
Hemizygosity of mdf-1 causes inappropriate germ cell proliferation during L1 diapause
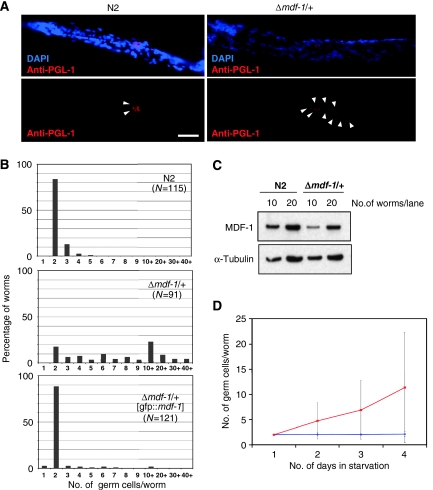
In C. elegans, the loss of MDF-1 causes infertility, most likely due to severe defects in germline development. This finding suggests that MDF-1 regulates cell-cycle progression in germ cells. On that premise, we hypothesized that MDF-1 has a role in the cell-cycle arrest of PGCs during L1 diapause. To test this hypothesis, we first analysed germ cell proliferation in mdf-1(gk2)-deletion hemizygotes (Δmdf-1/+) during L1 diapause. Newly hatched L1 larvae were starved for 4 days and then the number of PGCs per larva was counted. The body length of N2 (wild-type) worms did not increase during starvation, suggesting that their development was arrested in the L1 stage. The growth of Δmdf-1/+ larvae was also retarded during starvation (Supplementary Figure S1). This growth retardation was reversible, and larval growth resumed after they were released from starvation (Supplementary Figure S1). The body length of the Δmdf-1/+ larvae increased slightly during the first 24 h after hatching but did not change thereafter, even when the duration of starvation was extended to 5 days. Nevertheless, the majority (79%) of Δmdf-1/+ larvae starved for 4 days contained more than four PGCs (Figure 1A and B). This finding contrasts with the strict limitation of 2–3 PGCs per N2 larva in response to nutrient-deprivation conditions, suggesting that inappropriate PGC proliferation occurs when the SAC is compromised (Figure 1A and B). Although the Δmdf-1/+ strain exhibited no significant defects in development or fertility (Kitagawa and Rose, 1999) western blot analysis using anti-MDF-1 antibody (Supplementary Figure S2) revealed that the level of MDF-1 expressed in Δmdf-1/+ larvae was approximately 50% of that expressed in N2 larvae (Figure 1C). The defect in PGC arrest was also observed in starved L1 larvae that were hemizygous for sDf29, which deletes the mdf-1 locus (Supplementary Figure S3). Moreover, we demonstrated that the expression of the GFP∷MDF-1 fusion protein in Δmdf-1/+ L1 larvae restored the starvation-induced arrest of PGCs in post-S phase (Figure 1B), indicating that inappropriate PGC proliferation is specifically caused by hemizygosity of mdf-1.
Figure 1.
Hemizygosity of mdf-1 causes a defect in starvation-induced cell-cycle arrest of PGCs. (A) Fluorescence micrographs of N2 (wild-type) and Δmdf-1/+ worms in L1 diapause are shown. Newly hatched worms were starved for 4 days and then fixed. DNA was stained with DAPI (blue), and germ cell-specific P granules were stained with anti-PGL-1 antibody (red). Germ cells (PGL-1+ cells) are indicated by white arrowheads. Scale bar, 15 μm. (B) Newly hatched worms of the indicated genotypes were starved for 4 days and then fixed. DNA and germ cell-specific P granules were stained with DAPI and anti-PGL-1 antibody, respectively. The number of PGL-1+ cells per worm was counted, and the distribution of the number of germ cells per worm is plotted. (C) The level of MDF-1 expression in Δmdf-1/+ larvae. Whole-worm lysates prepared from N2 or Δmdf-1/+ larvae were separated on a gel, transferred to a nitrocellulose membrane and then probed with antibodies against MDF-1 and α-tubulin. The amount of lysate loaded into each well corresponded to that prepared from the indicated number of adult gravid worms. (D) The time course of germ cell proliferation during L1 diapause. N2 (blue plot) and Δmdf-1/+ (red plot) larvae were hatched on NGM plates with no food, and a portion of each group of worms was then fixed every day for 4 days. Fixed worms were stained with DAPI and anti-PGL-1 antibody, and the germ cells were counted. The average number of germ cells per worm is plotted.
Inappropriate germ cell proliferation was also observed in maternally rescued L1 Δmdf-1 homozygotes starved for 4 days, but the frequency of larvae with more than three germ cells was only 30% (Supplementary Figure S4A). Thus, the defect in starvation-induced cell-cycle arrest of PGCs observed in Δmdf-1 homozygotes appeared to be less severe than that observed in Δmdf-1/+ larvae. However, further characterization of PGCs arrested in Δmdf-1 homozygotes revealed that they failed to proliferate, regardless of the nutrition conditions. In contrast to N2 PGCs, which arrested during L1 diapause but resumed the cell-cycle progression when larvae were supplied with nutrients, the PGCs arrested in Δmdf-1 homozygotes remain undivided even 12 h after the larvae were released from starvation (Supplementary Figure S4B). These observations suggest that MDF-1 has a role not only in cell-cycle arrest of PGCs in response to nutrient deprivation, but also in the maintenance of the cells' ability to divide after being exposed to starvation conditions. These two roles are effective at different MDF-1 threshold levels: more MDF-1 is required for starvation-induced cell-cycle arrest than is required for germ cell proliferation. Specifically, PGCs completely devoid of MDF-1 were irreversibly arrested under starvation conditions but with incomplete penetrance, and the arrested PGCs were unable to resume cell growth, even when the larvae were put back in nutrient-replete conditions. In this study, we focused on MDF-1 function in starvation-induced cell-cycle arrest, which is reversible, and characterized the phenotype of Δmdf-1 hemizygotes (instead of homozygotes) in most of the experiments.
To determine whether Δmdf-1/+ PGCs divide during embryogenesis or after hatching, we analysed the number of PGCs in newly hatched L1 larvae and in those starved for 1, 2 or 4 days after hatching. Time-course analysis revealed that the Z2 and Z3 cells in Δmdf-1/+ embryos remained undivided during late embryogenesis but started dividing upon hatching (Figure 1D). Therefore, halving the mdf-1 gene dose did not affect the programmed entry into the post-S phase arrest during embryogenesis but did induce a defect in the ability of the larvae to sustain starvation-induced arrest of PGCs.
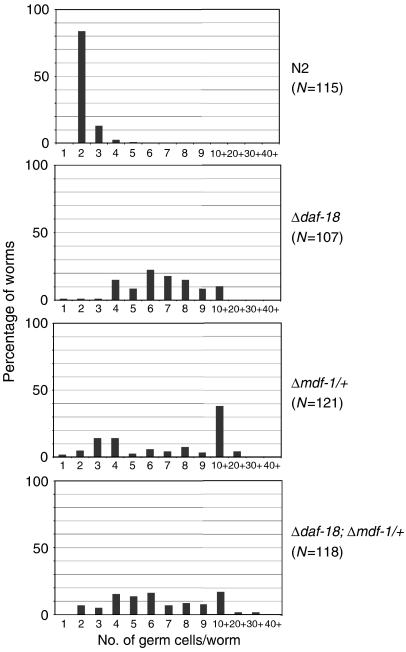
Because starvation-induced PGC arrest depends on a nutritional signal mediated by DAF-18 (Fukuyama et al, 2006), we next compared germ-line phenotypes of the Δmdf-1/+ strain with those of the strain homozygous for daf-18-deletion allele, ok480 (Δdaf-18). Consistent with the previous report, the Δdaf-18 strain exhibited defective cell-cycle arrest of PGCs (Figure 2). The Δdaf-18 strain exhibited high phenotypic penetrance, that is, 97% of PGCs divided once or twice during L1 diapause, and 70% of the worms had 4–6 PGCs after 4 days of starvation (mean number of PGCs, 6.8±2.44; variance, σ2=5.96). In contrast, starved Δmdf-1/+ L1 larvae showed substantial intraindividual variability, that is, 21% of larvae had only 2–3 PGCs, and 25% had more than 10 PGCs (mean number of PGCs, 8.5±5.31; variance, σ2=28.1).
Figure 2.
Inappropriate germ cell proliferation in Δmdf-1 hemizygotes and Δdaf-18 homozygotes. Newly hatched worms of the indicated genotypes were starved for 4 days and then fixed. DNA and germ cell-specific P granules were stained with DAPI and anti-PGL-1 antibody, respectively. The number of PGL-1+ cells per worm was counted, and the distribution of the number of germ cells per worm is plotted. Note that the plot of wild-type N2 data is a duplicate of the data shown in Figure 1B.
We also analysed the synthetic phenotype of the Δmdf-1/+–Δdaf-18 double mutant (Figure 2). The mean number of PGCs in the double-mutant larvae in L1 diapause was intermediate (7.5±5.24), and the variance σ2 was 27.45. Although our phenotypic analysis did not eliminate the possibility that MDF-1 activity is regulated independently of the DAF-18-controlled signalling pathway in response to nutritional signals, the synthetic phenotype was not additive, suggesting that MDF-1 function may not be completely independent of the DAF-18-mediated pathway.
Hypomorphic mutant fzy-1 suppresses inappropriate germ cell proliferation
In the SAC pathway, Mad1 family proteins, including MDF-1, inhibit APC/CCDC20 activity in response to defects in microtubule-kinetochore attachment. Our previous finding that the lethality of Δmdf-1 homozygotes is suppressed by hypomorphic mutations in emb-30, a component of C. elegans APC/C, or in fzy-1, a C. elegans homologue of the substrate-specific APC/C activator CDC20, suggested that MDF-1 regulates APC/CCDC20 activity during development (Furuta et al, 2000; Kitagawa et al, 2002; Tarailo et al, 2007). We then questioned whether MDF-1-mediated inhibition of APC/CCDC20 is required for starvation-induced PGC arrest.
The hypomorphic mutant allele of fzy-1, fzy-1(h1983), suppresses the lethality of the Δmdf-1 homozygotes (Kitagawa et al, 2002). This allele has a missense mutation in the fzy-1-coding region that causes a single amino-acid alteration, that is, aspartic acid at residue 433 is substituted by asparagine. The FZY-1D433N mutant protein produced from the fzy-1(h1983) allele cannot bind the APC/C substrate IFY-1 (securin) (Kitagawa et al, 2002). In fzy-1(h1983)-mutant embryonic cells, the duration of mitosis of early-stage embryos is extended, presumably due to an increased level of IFY-1 (Tarailo et al, 2007).
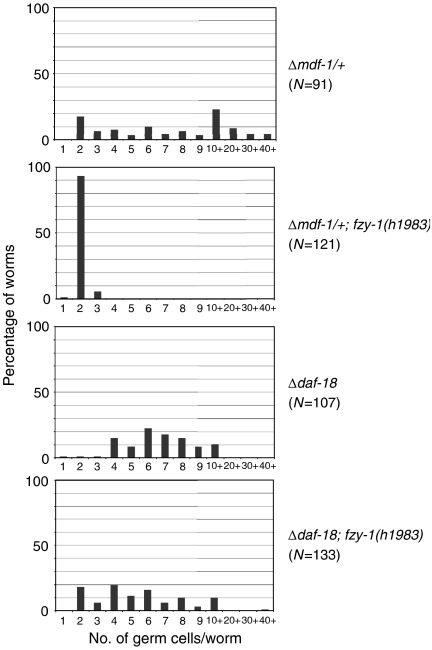
We introduced the fzy-1(h1983) mutation into the Δmdf-1/+ strain and then analysed the germ cell proliferation in worms starved in L1 for 4 days. All of the fzy-1(h1983)–Δmdf-1/+ double-mutant larvae had only two or three germ cells (Figure 3). Thus, inappropriate germ cell proliferation caused by mdf-1 hemizygosity was suppressed by the hypomorphic mutation in fzy-1. We also demonstrated that emb-30(tn377) mutation, which suppresses the lethality of the Δmdf-1 homozygotes at the permissive temperature (Furuta et al, 2000), also suppressed inappropriate germ cell proliferation caused by mdf-1 hemizygosity (Supplementary Figure S5). These results suggest that MDF-1 has an important function in starvation-induced cell-cycle arrest of PGCs via its inhibition of APC/C activity.
Figure 3.
The fzy-1(h1983) hypomorphic allele of fzy-1/CDC20 partially suppressed that caused by the loss of DAF-18. Newly hatched worms of the indicated genotypes were starved for 4 days and then fixed. DNA was stained with DAPI, and germ cell-specific P granules were stained with anti-PGL-1 antibody. The number of PGL-1+ cells per worm was counted, and the distribution of the number of germ cells per worm is plotted. Note that the charts of Δmdf-1/+ and Δdaf-18 are duplicates of the data shown in Figures 1B and 2, respectively.
If MDF-1 and FZY-1 function downstream of DAF-18 in PGCs, then the fzy-1(h1983) mutant should also suppress inappropriate germ cell proliferation in the Δdaf-18 strain. We generated fzy-1(h1983)–Δdaf-18 double mutants and analysed their germ cell proliferation during L1 diapause. The fzy-1(h1983) mutant suppressed 18% of the defect in starvation-induced cell-cycle arrest of PGCs caused by the loss of DAF-18 (Figure 3). This result suggests that MDF-1–FZY-1 is, if not the sole target, a downstream factor of the signalling pathway that is negatively regulated by DAF-18.
MDF-1 is phosphorylated by AKT-1 kinase
DAF-18 is a negative regulator of the signalling pathway mediated by AGE-1 and AKT-1. However, which cell-cycle regulators are targets of this AGE-1–AKT-1 signalling pathway controlling nutrient signal-dependent germ cell proliferation remains unknown. Although our genetic analysis did not eliminate the possibility that MDF-1 regulates the germ cell proliferation independently of DAF-18, a finding that fzy-1(h1983), which suppresses the defect in starvation-induced cell-cycle arrest of PGCs caused by hemizygosity of mdf-1, also partially suppresses the defect caused by the loss of DAF-18 suggests that MDF-1 functions, in part, downstream of the AGE-1–AKT-1 signalling pathway. Therefore, we first hypothesized that MDF-1 activity is controlled by AKT-1-mediated phosphorylation.
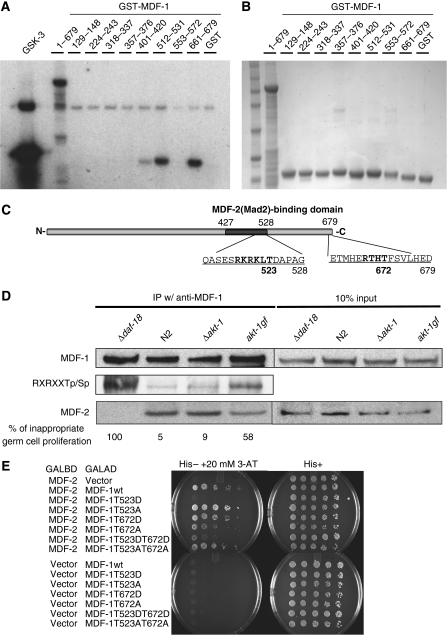
Amino-acid sequence analysis of MDF-1 revealed 12 potential AKT kinase target sites ([R/K]XX[T/S]), including a perfect match with a consensus sequence for AKT phosphorylation sites (RXRXX[T/S]) (Alessi et al, 1996). Therefore, we wanted to determine whether MDF-1 is phosphorylated by AKT kinase in vitro. Bacterially expressed glutathione-S-transferase (GST) or GST-conjugated full-length MDF-1 (GST∷MDF-1[1-679]) was incubated with purified human AKT kinase in the presence of [γ-32P]ATP. A substantial amount of [γ-32P]ATP was incorporated by GST∷MDF-1; in contrast, none was incorporated by GST (Figure 4A and B), indicating that MDF-1 was phosphorylated by AKT in vitro.
Figure 4.
MDF-1 is phosphorylated at Thr523 and Thr672 in vitro in an AKT kinase-dependent manner. (A) Bacterially expressed GST protein and recombinant proteins consisting of fusions between GST and peptides derived from various regions of MDF-1 (the positions of the amino acids corresponding to each peptide are indicated) were purified with glutathione sepharose. Indicated proteins were incubated with Akt1 kinase and [γ-32P]ATP at 30°C for 30 min, eluted with the SDS sample buffer, and separated on the gel. Incorporation of [γ-32P]ATP was analysed by autoradiography. GSK-3 is a fusion protein containing the Akt1 phosphorylation motif of hGSK-3. (B) Indicated proteins, in the amount used in the Akt1 kinase reaction, were loaded, separated on the gel and then stained with Coomassie blue dye. (C) Schematic structure of MDF-1. The predicted MDF-2-binding domain is indicated by the dark grey bar. Consensus Akt kinase phosphorylation sites ([R/K]XX[T/S] or RXRXX[T/S]) present in peptides phosphorylated by Akt1 kinase in vitro are shown in bold letters. (D) Immunoprecipitations were performed using an affinity-purified anti-MDF-1 rabbit polyclonal antibody on extracts from L1 larvae of the indicated genotypes that had been starved for 4 days. The immunoprecipitants separated on a gel were transferred to the nitrocellulose membrane and then probed with phospho-Akt substrate-specific antibody (anti-RXRXXSp/Tp) or with anti-MDF-2 antibody. The probe was stripped, and then the membrane was reprobed with anti-MDF-1 antibody. (E) The yeast two-hybrid analysis was performed to test the binding activity of wild-type (MDF-1wt) or mutated MDF-1 proteins (MDF-1T523D, MDF-1T523A) to MDF-2. The yeast strain Y190 was transformed with the indicated combination of plasmid vectors, then the growth was analysed on agar plates of synthetic media that was −Leu, −Trp, (His+) or −Leu, −Trp, −His containing 20 mM 3-AT (His−+20 mM 3-AT). Wild-type or mutant MDF-1 was cloned into the pACT vector, and MDF-2 was cloned into the pGBT9 vector. Vector: empty vector.
To determine which sites on MDF-1 are AKT-1-phosphorylation targets, we generated GST-conjugated peptides containing 20 amino-acid peptide fragments of MDF-1 surrounding the potential phosphorylation sites as substrates for in vitro AKT kinase assay. A GST–GSK3β peptide served as a positive control and was efficiently phosphorylated by AKT kinase (Figure 4A and B). This assay identified two major phosphorylation sites, Thr523 and Thr672 (Figure 4C). We confirmed that Thr523 and Thr672 were phosphorylated by demonstrating that GST-conjugated peptides in which Thr523 and Thr672 were replaced with alanine did not incorporate [γ-32P]ATP (Supplementary Figure S6).
The akt-1-null mutant suppresses inappropriate germ cell proliferation caused by the loss of DAF-18 (Fukuyama et al, 2006). This finding suggests that AKT-1 kinase is deregulated in Δdaf-18 larvae. Therefore, we next tested whether the phosphorylation status of MDF-1 during L1 diapause is altered by the loss of DAF-18. Whole-worm lysate was prepared from Δdaf-18 larvae and N2 larvae starved for 4 days in L1 diapause. The lysate was subjected to immunoprecipitation and western blot analysis. MDF-1 was immunoprecipitated with anti-MDF-1 antibody and then blotted with anti-phospho-Akt substrate antibody. The total amount of MDF-1 in Δdaf-18 and N2 lysates was indistinguishable (Figure 4D). However, substantially more phosphorylated MDF-1 was detected in the MDF-1 pull-down from the Δdaf-18 lysate than from the N2 lysate (Figure 4D).
The phosphorylation status of MDF-1 was also analysed in akt-1(ok525), an akt-1-null mutant (Δakt-1), and in akt-1(mg144), a gain-of-function mutant allele (akt-1gf). The akt-1(mg144) allele can suppress the dauer-constitutive phenotype of age-1-null mutants (Paradis and Ruvkun, 1998), suggesting that AKT-1 is constitutively active in this mutant. Consistent with the previously proposed model that AKT-1 is inactivated in starved N2 larvae arrested in L1 presumably due to negative regulation by DAF-18 (Fukuyama et al, 2006), the amount of phosphorylated MDF-1 in the Δakt-1 lysate was indistinguishable from that in the N2 lysate. On the other hand, more phosphorylated MDF-1 was detected in the akt-1gf lysate (Figure 4D). In association with the increase in phosphorylated MDF-1, akt-1gf mutant larvae exhibited inappropriate germ cell proliferation during L1 diapause (Figure 4D). These results suggest that MDF-1 is phosphorylated in an akt-1-dependent manner during L1 diapause, when the AGE-1/PI3K–AKT-1/PKB signalling pathway is deregulated. A small but detectable amount of MDF-1 was phosphorylated even in the absence of AKT-1. The amount of phosphorylated MDF-1 in Δakt-1 lysate was diminished when AKT-2 in Δakt-1 larvae was depleted by RNAi before starvation (Supplementary Figure S7), suggesting that MDF-1 is phosphorylated by both AKT-1 and AKT-2. Although AKT-2 is not required for nutrient signal-dependent germ cell proliferation control, MDF-1 may be phosphorylated by AKT-2 in other tissues.
AKT-1 deregulation hinders MDF-1 binding to MDF-2
Western blot analysis of MDF-1 immunoprecipitants with anti-MDF-2 antibody revealed that significantly less MDF-2 co-immunoprecipitated with MDF-1 in Δdaf-18 lysate, and slightly less MDF-2 co-immunoprecipitated with MDF-1 in the akt-1gf lysate (Figure 4D). Thus, the increase in the phosphorylation of MDF-1 and the reduction of MDF-1 binding to MDF-2 correlates with the defect in starvation-induced cell-cycle arrest of germ cells caused by deregulation of AKT-1 activity by either gain-of-function mutations in akt-1 or loss-of-function mutations in daf-18 (Figure 4D). Therefore, we analysed whether the phosphorylation status of MDF-1 at Thr523 and/or Thr672 affects its ability to bind MDF-2. Phosphomimetic (Thr to Asp) or phosphorylation-disabled (Thr to Ala) mutations were introduced into Thr523 and/or Thr672 on MDF-1 to generate various MDF-1 mutants. MDF-2-binding activity of those mutants was tested by the yeast two-hybrid system. Consistent with the fact that Thr523 is located in the predicted MDF-2-binding domain (Jin et al, 1998) (Figure 4C), on the selection plate containing 20 mM 3-amino-1,2,4-triazole (3-AT), only the Thr523Asp mutation but not Thr523Ala exhibited substantially reduced binding (Figure 4E), suggesting that phosphorylation at Thr523 interferes with MDF-1 binding to MDF-2. In contrast, neither Thr672Asp nor Thr672Ala affected the MDF-1–MDF-2 interaction.
Non-phosphorylatable mutant MDF-1 partially suppresses the inappropriate germ cell proliferation caused by loss of DAF-18
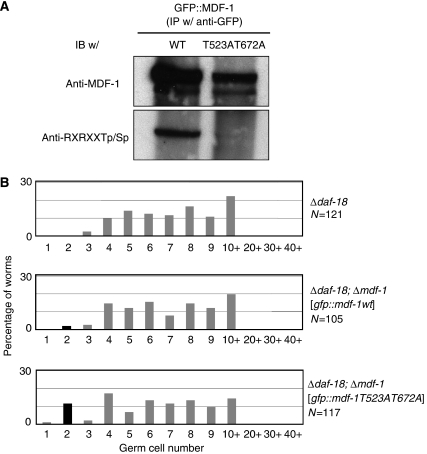
The amount of phosphorylated MDF-1 correlates with the frequency of inappropriate germ cell proliferation. This finding led us to hypothesize that, in the absence of DAF-18, AKT-1 phosphorylates MDF-1, thereby inactivating MDF-1, which in turn leads to the failure of cell-cycle arrest of PGCs. To test this hypothesis, we constructed transgenic strains expressing either wild-type MDF-1 or non-phosphorylatable mutant MDF-1 (MDF-1Thr523AlaThr672Ala, hereafter referred to as MDF-1T523AT672A) fused with GFP in the Δmdf-1–Δdaf-18 background and then analysed germ cell proliferation during L1 diapause. The expression of both wild-type and mutant proteins suppressed the lethality of the Δmdf-1 homozygotes (data not shown). GFP∷MDF-1 but not GFP∷MDF-1T523AT672A in worm lysate prepared from starved L1 larvae reacted with antibodies against the phosphorylated AKT substrate, suggesting that Thr523 and/or Thr672 are phosphorylated during L1 diapause in vivo (Figure 5A).
Figure 5.
MDF-1T523AT672A partially suppressed the inappropriate germ cell proliferation caused by the loss of DAF-18. (A) Immunoprecipitations were performed using an anti-GFP rabbit polyclonal antibody on extracts from Δdaf-18, Δmdf-1 L1 larvae expressing either GFP∷MDF-1(wild-type) or GFP∷MDF-1T523AT672A that were starved for 4 days. The immunoprecipitants separated on a gel were transferred to the nitrocellulose membrane and then probed with anti-MDF-1 antibody or phospho-Akt substrate-specific antibody (anti-RXRXXSp/Tp). (B) Newly hatched worms of the indicated genotypes were starved for 4 days and then fixed. DNA and germ cell-specific P granules were stained with DAPI and anti-PGL-1 antibody, respectively. The number of PGL-1+ cells per worm was counted, and the distribution of the number of germ cells per worm is plotted. The plots shown in black indicate the suppression of inappropriate germ cell proliferation.
The expression of GFP∷MDF-1 had little effect on the percentage of inappropriately proliferating germ cells. In contrast, the expression of GFP∷MDF-1T523AT672A restored the cell-cycle arrest of germ cells in 12% of Δdaf-18 larvae (Figure 5B). Thus, MDF-1T523AT672A partially suppressed the defect in starvation-induced PGC arrest caused by the loss of DAF-18. We can explain the incomplete suppression as follows: although the GFP fusion protein suppresses the lethality of Δmdf-1 homozygotes, it may not be fully functional, or the expression level of the GFP fusion protein by the pie-1 promoter may not be sufficient for cell-cycle regulation of PGCs. Actually, the expression level of GFP∷MDF-1T523AT672A was lower than that of wild-type GFP∷MDF-1 (Figure 5A). Alternatively, there may be other AKT-1 targets whose phosphorylation causes inappropriate germ cell proliferation. Our finding that the suppression effect of fzy-1(h1983), which completely suppresses the inappropriate germ cell proliferation caused by hemizygosity of mdf-1, on PGC proliferation in starved Δdaf-18 larvae is partial (18%) also suggests the latter possibility.
Defective starvation-induced PGC arrest caused by the loss of other SAC components was less severe than that in mdf-1 hemizygotes
To determine whether germline quiescence during L1 diapause requires other SAC components, we analysed germ cell proliferation in mdf-2(tm2190)-deletion homozygotes (Δmdf-2) and san-1(ok1580)-deletion homozygotes (Δsan-1). Unlike Δmdf-1 homozygotes, Δmdf-2 or Δsan-1 homozygous strains are maintainable (Stein et al, 2007). Both strains exhibited slightly more inappropriate germ cell proliferation than that seen in N2 wild-type larvae (Supplementary Figure S8). However, their deficiency in cell-cycle arrest was much less severe than that observed in the Δmdf-1/+ strain. Furthermore, the efficiency of the PGC arrest in the Δmdf-2/+ strain resembled that of the N2 larvae (data not shown).
We analysed germ cell proliferation in mutants carrying alterations in alleles of other SAC genes that were isolated as mutants that suppress the 1-cell, embryonic-arrest phenotype of mat-3(or180), a hypomorphic allele of the gene encoding an APC/C component (Stein et al, 2007). Some alleles showed a slight increase in the frequency of inappropriate germ cell proliferation compared with their counterparts (Supplementary Figure S8). There was no apparent correlation between the severity of the defect in cell-cycle arrest of germ cells and the efficiency of suppression of the mat-3(or180) phenotype. Thus, although these results do not exclude the possibility that MDF-2 and SAN-1 are also required for proper germ cell quiescence during L1 diapause, because the defects in all of the other mutant alleles tested were not as severe as that observed in the Δmdf-1/+ strain, we speculate that MDF-1 affects PGC proliferation by two independent mechanisms, one involving MDF-2 and another that is independent of other SAC components. However, in both cases, MDF-1 regulates the cell-cycle progression via inhibition of APC/CCDC20 activity.
APC/CCDC20 is thought to be inactivated by Emi1 during prophase for efficient accumulation of cyclins A and B, which are required for early mitotic progression (Reimann et al, 2001). Depletion of the SAC component BUB3 in Drosophila was recently shown to cause prolonged prophase (Lopes et al, 2005), suggesting that the SAC also inactivates the APC/CCDC20 for proper entry into mitosis. This finding shows the opposite outcome compared with ours in which MDF-1-mediated inhibition of the APC/C is required for starvation-induced cell-cycle arrest of germ cells at prophase (the early stage of mitosis). Therefore, different APC/CCDC20 substrates whose accumulation retains germ cells in prophase may be present. Although we found that IFY-1 (securin) accumulates in arrested PGCs (Supplementary Figure S9), whether IFY-1 regulates the G2/M transition or progression through prophase remains unknown.
MDF-1 controls germ cell proliferation in a cell-autonomous manner
We analysed the tissue-specific localization of MDF-1 in newly hatched L1-staged larvae. A substantial amount of MDF-1 was detected in PGCs (Supplementary Figure S10). MDF-1 accumulated not only in PGCs but also in intestinal cells (Supplementary Figure S10). To address whether MDF-1 functions cell autonomously or non-cell autonomously in PGCs, we performed RNAi experiments using rrf-1 and rrf-3 mutant strains. The rrf-1 gene encodes an RNA-directed RNA polymerase (RdRP) homologue. Somatic cells (but not germ cells) carrying the homozygous rrf-1(pk1417) allele are resistant to RNAi treatment. The rrf-3(pk1426) is an rrf-3-null allele, and worms homozygous for rrf-3(pk1426) are hypersensitive to RNAi. When mdf-1 was targeted by RNAi before nutrient deprivation, rrf-1(pk1417) worms as well as rrf-3(pk1426) worms exhibited more inappropriate germ cell proliferation during L1 diapause than did worms treated with control RNAi (Supplementary Figure S11). This result suggests that MDF-1 functions cell autonomously in PGCs. We also tried to resolve whether DAF-18 functions in PGCs cell autonomously or non-cell autonomously by performing the RNAi-targeting daf-18 but failed because daf-18 RNAi under our experimental condition could not phenocopy the inappropriate germ cell proliferation phenotype of Δdaf-18 strain, even in the rrf-3(pk1426) strain.
MDF-1 ensures the quality of germ cells by sustaining PGC arrest under starvation conditions
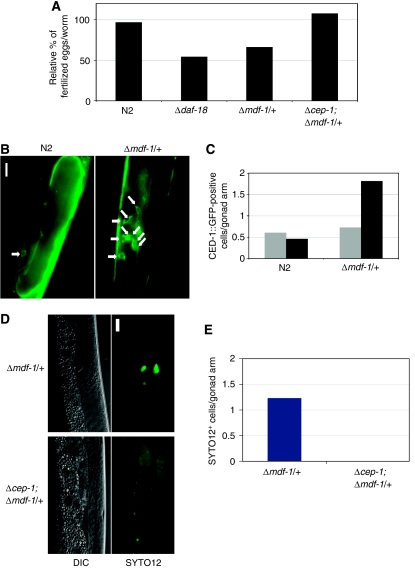
We next questioned whether Δmdf-1/+ germ cells, which inappropriately proliferated during L1 diapause, develop properly after release from starvation and resumption of larval growth. Newly hatched N2 or Δmdf-1/+ L1 larvae were starved for 4 days and then transferred to plates containing bacteria as food. N2 PGCs that had arrested in post-S phase during starvation started dividing and kept proliferating during post-embryonic development. The ratio of fertilized eggs per N2 worm released from 4 days of starvation to that of N2 worms grown under the nutritionally preferable conditions was 96.7% (Figure 6A).
Figure 6.
Germline apoptosis and reduction of brood size in mdf-1 hemizygotes released from starvation are cep-1 dependent. (A) Hermaphrodites of the indicated strains were grown in the presence of OP50 bacteria or starved for 4 days and then fed. The number of fertilized eggs laid per worm was counted for three individual worms, and the proportion of the fertilized eggs laid by temporarily starved worms compared with that laid by worms grown under normal conditions is shown. (B) Fluorescent microscope images of gonads of CED-1∷GFP-expressing strains with indicated genetic backgrounds are shown. The worms were starved for 4 days and then fed for 2 days. Apoptotic cells surrounded by CED-1∷GFP are indicated by white arrows. Scale bar, 15 μm. (C) The indicated strains were either grown for 2 days after hatching (pale grey bars) or starved for 4 days and then fed for 2 days (dark grey bars). The mean number of apoptotic cells per gonad arm of the indicated strains is plotted. (D) The differential interference contrast (DIC) microscope images (left panels) and fluorescent microscope images (right panels) of gonads of strains with the indicated genotypes stained with SYTO12 are shown. The newly hatched L1 larvae were starved for 4 days and then fed for 2 days. Apoptotic cells were positively stained with SYTO12. Scale bar, 15 μm. (E) The average numbers of SYTO12+ cells per gonad arm in worms with the indicated genotypes are plotted.
The Δmdf-1/+ germ cells that inappropriately proliferated during starvation kept proliferating after the larvae were fed. However, the ratio of fertilized eggs per Δmdf-1/+ worms released from starvation was 66.1% of that of Δmdf-1/+ worms grown under the nutritionally preferable conditions (Figure 6A). Thus, the Δmdf-1/+ germ cells that inappropriately proliferated during L1 diapause generated fewer viable zygotes, suggesting that starvation-induced cell-cycle arrest of PGCs is required for maintenance of germ cell quality. We also analysed germ cells in the Δdaf-18 strain, and the results were comparable with those seen with the Δmdf-1/+ strain (Figure 6A), thereby strengthening our argument that starvation-induced PGC arrest ensures the fertility of the animal under starvation conditions.
Mammalian embryonic cells lacking a functional SAC exhibit genomic instability and are targeted by the p53-dependent apoptotic pathway, presumably via activation of the DNA-damage checkpoint (Dobles et al, 2000; Baker et al, 2004; Baker et al, 2006). If MDF-1-dependent cell-cycle arrest of PGCs during L1 diapause is required for maintenance of the genome stability of germ cells, then Δmdf-1/+ germ cells inappropriately proliferated under starvation conditions should exhibit chromosome instability, and their descendants should be targeted as substrates for germline apoptosis.
We analysed germline apoptosis in transgenic strains expressing CED-1∷GFP in the N2 or Δmdf-1/+ background. Germ cells undergoing apoptosis were easily detected because they were surrounded by CED-1∷GFP (Zhou et al, 2001; Venegas and Zhou, 2007) (Figure 6B). Under the nutritionally preferable condition, the mean number of apoptotic cells per gonad arm of CED-1∷GFP-expressing N2 worms was 0.61, and that of Δmdf-1/+ worms was 0.73 (Figure 6C). When newly hatched N2 worms were starved for 4 days and then fed for 2 days, the mean number of apoptotic cells per gonad arm was 0.46 (Figure 6C). Thus, the 4-day starvation did not affect the frequency of germline apoptosis in the N2 background. In contrast, the mean number of apoptotic cells per gonad arm in the Δmdf-1/+ larvae maintained under the same conditions more than doubled to 1.81 (Figure 6C).
The introduction of gk138, a deletion allele of cep-1, C.elegans p53 (Δcep-1) (Schumacher et al, 2001), completely suppressed the incidence of apoptotic cells in the gonad of starved Δmdf-1/+ larvae (Figure 6D and E). Furthermore, Δcep-1 suppressed the reduction in the brood size of Δmdf-1/+ worms released from starvation (Figure 6A). This finding supports our hypothesis that inappropriate germ cell proliferation caused by hemizygosity of mdf-1 leads to genome instability in germline descendants, which are targeted as substrates for germline apoptosis, thereby leading to the reduction of viable zygotes. Thus, our results suggest that MDF-1 maintains the genome stability of germ cells by causing cell-cycle arrest in response to nutrition deprivation.
MDF-1 also has an important function in nutrient deprivation-induced somatic cell arrest
Although the body length of N2 L1 larvae remained unchanged during starvation, after a day of starvation, the body length of the Δmdf-1/+ larvae was slightly bigger than that of hatchlings (Supplementary Figure S12). This increase in body length may reflect the increased cell size or cell number in some somatic cells of post-embryonic lineage. To analyse the cell division of other somatic precursors of post-embryonic lineage, we counted the number of seam cells in transgenic strains expressing SCM∷GFP in the N2 or Δmdf-1/+ background. The epidermal seam cells consist of 10 precursors, H0-2, V1-6 and T at the hatching stage. During the L2 stage, six of these cells undergo symmetrical division, resulting in the increase in the number of seam cells from 10 at L1 to 16 at L2 and beyond (Sulston and Horvitz, 1977). One hundred per cent of N2 larvae starved for 4 days after hatching had nine or ten SCM∷GFP-positive cells. The intensity of the GFP signal in V5 cells was not sufficient for detection in some larvae; thus, nine GFP-positive cells were detected, indicating starvation-induced larval arrest in the L1 stage. We found some Δmdf-1/+ L1 larvae starved for 4 days bearing 9–10 SCM∷GFP-positive cells, but others had more (range, 12–17 SCM∷GFP-positive cells) (Supplementary Figure S12). These results suggest that the hemizygosity of mdf-1 causes extra cell division of seam cell precursors during L1 diapause with an incomplete penetrance. This result is unexpected because seam cell division during post-embryonic development is regulated at the G1-S progression in a LIN-35/Rb, FZR-1/Cdh1 and CKI-1-dependent manner (Hong et al, 1998; Boxem and van den Heuvel, 2001; Fay et al, 2002; Fukuyama et al, 2003; Xia et al, 2007). Whether MDF-1 regulates G1-S progression of seam cell precursors as a target of AKT-1 remains to be investigated.
Conclusion
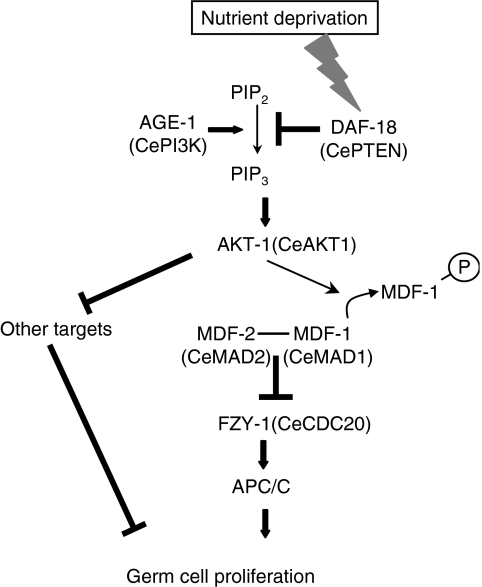
Taken together, the findings from this study have enabled us to propose a model in which PGC quiescence during L1 diapause is mediated by MDF-1 as a downstream factor in one branch of the DAF-18-regulated AGE-1–AKT-1 signalling pathway (Figure 7). Akt phosphorylation sites on MDF-1 and other MAD1 family members are surrounded by relatively diverse amino-acid sequences; a simple homology search did not reveal any corresponding sites on MAD1 proteins expressed in other species. However, two potential Akt phosphorylation sites are present on the MAD2-binding domain of human MAD1. Therefore, the mechanism by which Akt regulates MAD1 activity might be conserved in mammals. This report is the first to demonstrate that the SAC component MDF-1 is required for cell-cycle regulation of post-embryonic lineages in response to nutritional status. MDF-1-mediated inhibition of the cell-cycle in response to non-spindle/kinetochore defects should shed light on the roles of SAC components in multicellular organisms.
Figure 7.
Proposed model of the pathway that controls germ cell proliferation during L1 diapause. MDF-1 causes the starvation-induced cell-cycle arrest of PGCs by inhibiting the activity of the APC/CCDC20. MDF-1 is a downstream target of AKT-1. Phosphorylation of MDF-1 by AKT-1 reduces MDF-1's ability to bind MDF-2. DAF-18 indirectly activates MDF-1 by negatively regulating the AGE-1–AKT-1 signalling pathway. MDF-1–FZY-1 may not be the sole downstream factor of this pathway; another as yet unknown AKT-1 target may also regulate germ cell proliferation.
Materials and methods
C. elegans strains
Maintenance and cultivation of all strains were performed according to standard methods (Brenner, 1974). The strains were obtained from the Caenorhabditis Genetic Center (University of Minnesota, Minneapolis, MN) unless otherwise unregistered. Detailed description of the strains and their genotypes is available as Supplementary data at The EMBO Journal Online.
Maintaining worms in culture and counting germ cells
The preparation of starved worms and counting of germ cells were performed as described previously (Fukuyama et al, 2006), but with a minor modification. Starved worms were collected, fixed on glass slides and then stained with DAPI and anti-PGL-1 antibody (kindly provided by Susan Strome, Indiana University, Bloomington, IN). In some experiments using the mdf-1(gk2) heterozygotes (Δmdf-1/+ strains), fixed worms were stained with antibodies against MDF-1 and a component of germ cell-specific P granules to distinguish between Δmdf-1/+ (MDF-1+) and Δmdf-1-homozygous (MDF-1−) L1 larvae.
Immunoprecipitation and western blot analyses
To prepare protein lysate from L1 diapause worms starved for 4 days, we suspended the worms in lysis buffer (75 mM HEPES (pH 7.5), 300 mM KCl, 1.5 mM MgCl2, 1.5 mM EGTA, 15% glycerol, 0.05% NP-40, 1 mM DTT, protease inhibitors) and sonicated them with a Bioruptor UCD-200 (Diagenode Inc., Liege, Belgium). The lysate was cleared of worm debris by centrifugation.
MDF-1 immunoprecipitation was performed as follows: 1 μg anti-MDF-1 antibody and 10 μl wet volume of protein A-conjugated sepharose beads (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) were added to 1 mg worm protein lysate. Protein–antibody complexes were precipitated, washed with lysis buffer and eluted with 2 × SDS sample buffer. Description of antibodies used for this study is available as Supplementary data at The EMBO Journal Online.
Akt1 phosphorylation assay
To fuse GST and MDF-1 proteins, we cloned PCR-amplified full-length cDNA of mdf-1 or duplex oligonucleotides from various regions of mdf-1 cDNA into the pACT-Ptac-GST vector or pGEX4T-1 vector, expressed in BL21 cells, and purified with glutathione sepharose, as suggested by the manufacturer (GE Healthcare, UK). Purified proteins bound to sepharose beads were incubated with 400 ng Akt1 kinase (Upstate Millipore, Charlottesville, VA) and 10 μCi [γ-32P]ATP at 30°C for 30 min in 20 μl Akt1 kinase buffer (20 mM HEPES (pH 7.4), 5 mM MgCl2, 20 mM β-glycerophosphate, 1 mM EDTA, 0.1% β-mercaptoethanol). Phosphorylated proteins were eluted with 20 μl 2 × SDS sample buffer and then analysed by NuPAGE 4–12% Bis–Tris Gel (Invitrogen, Carlsbad, CA) and autoradiography. GST protein expressed in BL21 cells and purified with glutathione sepharose was used as the negative control for Akt1 kinase activity, and GSK-3 fusion protein (Cell Signaling Technology Inc., Danvers, MA) or GST–GSK-3 fusion protein generated in our laboratory was used as the positive control.
Yeast two-hybrid test
The yeast two-hybrid analysis was performed as described previously (Kitagawa and Abdulle, 2002). The yeast two-hybrid vectors pACT–MDF-1, pGBT9–MDF-2, pACT2 and pGBT9 were described previously (Kitagawa and Rose, 1999). The pACT–MDF-1T523A and pACT–MDF-1T523D were generated by replacing A1567 of mdf-1 cDNA sequence with G for MDF-1T523A, and ACA from 1567 to 1569 with GAT for MDF-1T523D by in vivo site-directed mutagenesis using a three-fragment homologous recombination system (Kitagawa and Abdulle, 2002).
Germline apoptosis assay
Eggs from the CED-1∷GFP-expressing strains, ZH231 enIs7 [Pced-1 ced-1∷gfp] and RQ263 enIs7 [Pced-1 ced-1∷gfp], and mdf-1(gk2)/nT1 (Δmdf-1/+) were isolated by the alkali/bleach method, seeded onto bacteria-free NGM plates, and incubated at room temperature for 12 h. L1 hatchlings were collected; half were transferred to NGM plates with OP50 bacteria and incubated for 2 days (not starved), and half were transferred to bacteria-free plates, incubated for 4 days, then transferred to NGM plates with OP50 bacteria and incubated for 2 days (starved). Worms were mounted under coverslips on glass slides in M9 medium containing 5 mM sodium azide. Their gonads were examined by fluorescence microscopy. Cells completely surrounded by CED-1∷GFP were considered apoptotic. Approximately 40 gonads from each genotype were analysed. SYTO12 staining was performed as described (Gumienny et al, 1999).
Supplementary Material
Supplementary Figures
Supplementary Information
Acknowledgments
Some nematode strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). These strains included deletion strains produced by the C. elegans Gene Knockout Consortium at the Oklahoma Medical Research Foundation and the University of British Columbia. We acknowledge Ann Rose, David Baillie and Zheng Zhou for providing strains, Susan Strome for kindly providing anti-PGL-1 polyclonal antibody, Andy Golden for providing strains and helpful suggestions for the manuscript, Iain Cheeseman and Arshad Desai for providing C. elegans GFP expression vector, pIC26, and Takao Fujisawa for assistance with construction of the double-mutant strains used for the genetic analysis. We thank Mary-Ann Bjornsti, Janet Partridge and Arshad Desai for their critical reading of the manuscript and members of St Jude Mitosis Club for many invaluable discussions and suggestions. This work was supported by the Cancer Center Support Grant CA021765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC).
References
- Albert PS, Brown SJ, Riddle DL (1981) Sensory control of dauer larva formation in Caenorhabditis elegans. J Comp Neurol 198: 435–451 [DOI] [PubMed] [Google Scholar]
- Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P (1996) Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett 399: 333–338 [DOI] [PubMed] [Google Scholar]
- Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, Kopecka A, Kumar R, Jenkins RB, de Groen PC, Roche P, van Deursen JM (2004) BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet 36: 744–749 [DOI] [PubMed] [Google Scholar]
- Baker DJ, Jeganathan KB, Malureanu L, Perez-Terzic C, Terzic A, van Deursen JM (2006) Early aging-associated phenotypes in Bub3/Rae1 haploinsufficient mice. J Cell Biol 172: 529–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh LR, Sternberg PW (2006) DAF-16/FOXO regulates transcription of cki-1/Cip/Kip and repression of lin-4 during C. elegans L1 arrest. Curr Biol 16: 780–785 [DOI] [PubMed] [Google Scholar]
- Boxem M, van den Heuvel S (2001) lin-35 Rb and cki-1 Cip/Kip cooperate in developmental regulation of G1 progression in C. elegans. Development 128: 4349–4359 [DOI] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Shevchenko A, Mann M, Murray AW (1998) Spindle checkpoint protein Xmad1 recruits Xmad2 to unattached kinetochores. J Cell Biol 143: 283–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobles M, Liberal V, Scott ML, Benezra R, Sorger PK (2000) Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell 101: 635–645 [DOI] [PubMed] [Google Scholar]
- Dorman JB, Albinder B, Shroyer T, Kenyon C (1995) The age-1 and daf-2 genes function in a common pathway to control the lifespan of Caenorhabditis elegans. Genetics 141: 1399–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encalada SE, Willis J, Lyczak R, Bowerman B (2005) A spindle checkpoint functions during mitosis in the early Caenorhabditis elegans embryo. Mol Biol Cell 16: 1056–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay DS, Keenan S, Han M (2002) fzr-1 and lin-35/Rb function redundantly to control cell proliferation in C. elegans as revealed by a nonbiased synthetic screen. Genes Dev 16: 503–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama M, Gendreau SB, Derry WB, Rothman JH (2003) Essential embryonic roles of the CKI-1 cyclin-dependent kinase inhibitor in cell-cycle exit and morphogenesis in C. elegans. Dev Biol 260: 273–286 [DOI] [PubMed] [Google Scholar]
- Fukuyama M, Rougvie AE, Rothman JH (2006) C. elegans DAF-18/PTEN mediates nutrient-dependent arrest of cell cycle and growth in the germline. Curr Biol 16: 773–779 [DOI] [PubMed] [Google Scholar]
- Furuta T, Tuck S, Kirchner J, Koch B, Auty R, Kitagawa R, Rose AM, Greenstein D (2000) EMB-30: an APC4 homologue required for metaphase-to-anaphase transitions during meiosis and mitosis in Caenorhabditis elegans. Mol Biol Cell 11: 1401–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil EB, Malone Link E, Liu LX, Johnson CD, Lees JA (1999) Regulation of the insulin-like developmental pathway of Caenorhabditis elegans by a homolog of the PTEN tumor suppressor gene. Proc Natl Acad Sci USA 96: 2925–2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S, Ruvkun G (1994) daf-2, daf-16 and daf-23: genetically interacting genes controlling Dauer formation in Caenorhabditis elegans. Genetics 137: 107–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumienny TL, Lambie E, Hartwieg E, Horvitz HR, Hengartner MO (1999) Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development 126: 1011–1022 [DOI] [PubMed] [Google Scholar]
- Hong Y, Roy R, Ambros V (1998) Developmental regulation of a cyclin-dependent kinase inhibitor controls postembryonic cell cycle progression in Caenorhabditis elegans. Development 125: 3585–3597 [DOI] [PubMed] [Google Scholar]
- Hoyt MA, Totis L, Roberts BT (1991) S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell 66: 507–517 [DOI] [PubMed] [Google Scholar]
- Jin DY, Spencer F, Jeang KT (1998) Human T cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell 93: 81–91 [DOI] [PubMed] [Google Scholar]
- Kao G, Nordenson C, Still M, Ronnlund A, Tuck S, Naredi P (2007) ASNA-1 positively regulates insulin secretion in C. elegans and mammalian cells. Cell 128: 577–587 [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Abdulle R (2002) In vivo site-directed mutagenesis of yeast plasmids using a three-fragment homologous recombination system. Biotechniques 33: 288, 290, 292 passim [DOI] [PubMed] [Google Scholar]
- Kitagawa R, Law E, Tang L, Rose AM (2002) The Cdc20 homolog, FZY-1, and its interacting protein, IFY-1, are required for proper chromosome segregation in Caenorhabditis elegans. Curr Biol 12: 2118–2123 [DOI] [PubMed] [Google Scholar]
- Kitagawa R, Rose AM (1999) Components of the spindle-assembly checkpoint are essential in Caenorhabditis elegans. Nat Cell Biol 1: 514–521 [DOI] [PubMed] [Google Scholar]
- Larsen PL, Albert PS, Riddle DL (1995) Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics 139: 1567–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Murray AW (1991) Feedback control of mitosis in budding yeast. Cell 66: 519–531 [DOI] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C (1997) daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278: 1319–1322 [DOI] [PubMed] [Google Scholar]
- Lopes CS, Sampaio P, Williams B, Goldberg M, Sunkel CE (2005) The Drosophila Bub3 protein is required for the mitotic checkpoint and for normal accumulation of cyclins during G2 and early stages of mitosis. J Cell Sci 118: 187–198 [DOI] [PubMed] [Google Scholar]
- Luo X, Tang Z, Rizo J, Yu H (2002) The Mad2 spindle checkpoint protein undergoes similar major conformational changes upon binding to either Mad1 or Cdc20. Mol Cell 9: 59–71 [DOI] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED (2007) The spindle-assembly checkpoint in space and time. Nat Rev 8: 379–393 [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G (1997) The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389: 994–999 [DOI] [PubMed] [Google Scholar]
- Ogg S, Ruvkun G (1998) The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway. Mol Cell 2: 887–893 [DOI] [PubMed] [Google Scholar]
- Paradis S, Ailion M, Toker A, Thomas JH, Ruvkun G (1999) A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev 13: 1438–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis S, Ruvkun G (1998) Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev 12: 2488–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann JD, Freed E, Hsu JY, Kramer ER, Peters JM, Jackson PK (2001) Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 105: 645–655 [DOI] [PubMed] [Google Scholar]
- Riddle DL, Swanson MM, Albert PS (1981) Interacting genes in nematode dauer larva formation. Nature 290: 668–671 [DOI] [PubMed] [Google Scholar]
- Rouault JP, Kuwabara PE, Sinilnikova OM, Duret L, Thierry-Mieg D, Billaud M (1999) Regulation of dauer larva development in Caenorhabditis elegans by daf-18, a homologue of the tumour suppressor PTEN. Curr Biol 9: 329–332 [DOI] [PubMed] [Google Scholar]
- Schumacher B, Hofmann K, Boulton S, Gartner A (2001) The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Cutr Biol 11: 1722–1727 [DOI] [PubMed] [Google Scholar]
- Sironi L, Mapelli M, Knapp S, De Antoni A, Jeang KT, Musacchio A (2002) Crystal structure of the tetrameric Mad1–Mad2 core complex: implications of a ‘safety belt' binding mechanism for the spindle checkpoint. EMBO J 21: 2496–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi L, Melixetian M, Faretta M, Prosperini E, Helin K, Musacchio A (2001) Mad2 binding to Mad1 and Cdc20, rather than oligomerization, is required for the spindle checkpoint. EMBO J 20: 6371–6382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein KK, Davis ES, Hays T, Golden A (2007) Components of the spindle assembly checkpoint regulate the anaphase-promoting complex during meiosis in Caenorhabditis elegans. Genetics 175: 107–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR (1977) Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol 56: 110–156 [DOI] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN (1983) The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 100: 64–119 [DOI] [PubMed] [Google Scholar]
- Tarailo M, Kitagawa R, Rose AM (2007) Suppressors of spindle checkpoint defect (such) mutants identify new mdf-1/MAD1 interactors in Caenorhabditis elegans. Genetics 175: 1665–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas V, Zhou Z (2007) Two alternative mechanisms that regulate the presentation of apoptotic cell engulfment signal in Caenorhabditis elegans. Mol Biol Cell 18: 3180–3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2: 489–501 [DOI] [PubMed] [Google Scholar]
- Xia D, Zhang Y, Huang X, Sun Y, Zhang H (2007) The C. elegans CBFbeta homolog, BRO-1, regulates the proliferation, differentiation and specification of the stem cell-like seam cell lineages. Dev Biol 309: 259–272 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Hartwieg E, Horvitz HR (2001) CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell 104: 43–56 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures
Supplementary Information