Abstract
The Golgi apparatus occupies a central position within the secretory pathway, but the molecular mechanisms responsible for its assembly and organization remain poorly understood. We report here the identification of zinc finger protein-like 1 (ZFPL1) as a novel structural component of the Golgi apparatus. ZFPL1 is a conserved and widely expressed integral membrane protein with two predicted zinc fingers at the N-terminus, the second of which is a likely ring domain. ZFPL1 directly interacts with the cis-Golgi matrix protein GM130. Depletion of ZFPL1 results in the accumulation of cis-Golgi matrix proteins in the intermediate compartment (IC) and the tubulation of cis-Golgi and IC membranes. Loss of ZFPL1 function also impairs cis-Golgi assembly following brefeldin A washout and slows the rate of cargo trafficking into the Golgi apparatus. Effects upon Golgi matrix protein localization and cis-Golgi structure can be rescued by wild-type ZFPL1 but not mutants defective in GM130 binding. Together, these data suggest that ZFPL1 has an important function in maintaining the integrity of the cis-Golgi and that it does so through interactions with GM130.
Keywords: GM130, Golgi apparatus, Golgi matrix, intermediate compartment, ZFPL1
Introduction
The Golgi apparatus occupies a central position within the secretory pathway, receiving newly synthesized material from the endoplasmic reticulum (ER), then processing and sorting this material into appropriate carriers for delivery to its final destination. In higher eucaryotes, cargo passes through the intermediate compartment (IC), a pleiomorphic collection of tubulo-vesicular membranes, on its way from the ER to the Golgi apparatus (Schweizer et al, 1990; Saraste and Svensson, 1991; Bannykh et al, 1998; Appenzeller-Herzog and Hauri, 2006). How cargo delivery from the IC to the cis-Golgi occurs is poorly understood at present. Transport intermediates, which can be tubular or vesicular in nature, may form at stable IC elements and subsequently fuse with a pre-existing cis-Golgi (Ben-Tekaya et al, 2005; Appenzeller-Herzog and Hauri, 2006). Alternatively, the IC may exist as a dynamic, maturing structure that undergoes homotypic fusion with other IC elements to bring about the de novo formation of a new cis-Golgi compartment (Presley et al, 1997; Scales et al, 1997; Ladinsky et al, 1999; Glick, 2000; Marra et al, 2007). In this scenario, the integrity of the cis-Golgi is dependent upon the continual input of material from the IC.
The cis-Golgi matrix protein GM130 is a long coiled-coil protein of the golgin family that is localized to the cytoplasmic face of cis-Golgi membranes (Nakamura et al, 1995). GM130 is anchored to the membrane through binding of its C-terminus to the Golgi reassembly and stacking protein of 65 kDa (GRASP65), a protein originally identified as a Golgi stacking factor (Barr et al, 1998; Barr and Short, 2003). The N-terminus of GM130 binds to the golgin p115, which may contribute to the tethering of Golgi membranes before membrane fusion (Nakamura et al, 1997). Several functions have been proposed for GM130, including trafficking from the IC to the Golgi apparatus. GM130 can cycle to the IC, where it appears to have an important function in the homotypic tethering and fusion of IC elements (Marra et al, 2001, 2007). This is thought to be important for the incorporation of the IC into the Golgi apparatus, which in turn helps to maintain the structural integrity of this organelle (Marra et al, 2007). Consistent with this view, a number of studies have found that ER-to-Golgi trafficking occurs less efficiently in the absence of GM130 (Seemann et al, 2000b; Alvarez et al, 2001; Marra et al, 2001, 2007; Diao et al, 2007). GM130 may also contribute to Golgi structure independently of trafficking, for example, by laterally tethering Golgi membranes to promote the lengthening of cisternae and ultimately the formation of the Golgi ribbon (Puthenveedu and Linstedt, 2004; Kondylis et al, 2005; Puthenveedu et al, 2006). Finally, GM130 has been implicated in the stacking of Golgi cisternae, which can be thought of as tethering without subsequent membrane fusion, and in regulation of centrosome function (Shorter and Warren, 1999; Kodani and Sutterlin, 2007).
During mitosis, the mammalian Golgi apparatus undergoes extensive fragmentation, a process driven by phosphorylation of key Golgi targets by mitotic kinases (Shorter and Warren, 2002). We have used a proteomics-based approach to identify new mitotic Golgi phosphoproteins with the rationale that these proteins will correspond to important structural and/or trafficking components at the Golgi apparatus (Diao et al, 2003). Here, we describe zinc finger protein-like 1 (ZFPL1) as a novel mitotic Golgi phosphoprotein. ZFPL1 is a widely expressed integral membrane protein with two predicted zinc fingers at its N-terminus. It behaves as a cis-Golgi matrix protein and directly binds GM130. Depletion of ZFPL1 causes GM130 to accumulate in a more tubular IC, slows the rate of cargo delivery into the Golgi apparatus and impairs cis-Golgi assembly. Together, our findings support a role for ZFPL1 in maintaining the structural and functional integrity of the cis-Golgi.
Results
Identification of ZFPL1 as a Golgi integral membrane protein
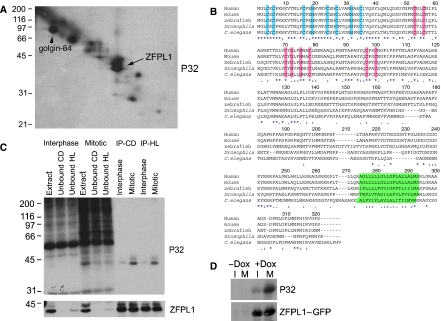
We exploited a previously described proteomics-based approach to identify Golgi proteins that undergo mitotic phosphorylation in vitro (Diao et al, 2003). Carbonate-resistant mitotically phosphorylated proteins were resolved by 16-BAC/SDS–PAGE and the prominent spots running as a doublet at ∼40 kDa excised and analysed by mass spectrometry (Figure 1A, arrow). Both spots corresponded to a previously reported but uncharacterized protein called zinc finger protein-like 1 (ZFPL1) (Hoppener et al, 1998). Human ZFPL1 has a predicted molecular weight of 34 kDa, with two putative zinc finger domains (ZFDs) in the N-terminus, and a region near the C-terminus that may correspond to a transmembrane domain (Figure 1B). The second ZFD conforms to the consensus for a ring finger (Freemont, 1993; Borden, 2000). Unusually, there is aspartic acid at the fourth predicted zinc coordinating residue in vertebrate ZFPL1 rather than cysteine or histidine. The predicted zinc coordinating residues of both putative ZFDs are highly conserved between species, with the exception of aspartic acid, which is histidine in Drosophila melonogaster, Caenorhabditis elegans and Arabidopsis thaliana (Figure 1B and Supplementary Figure S1). ZFPL1 appears to be present in all metazoans but is apparently absent from yeast (Figure 1B). Analysis of the EST database indicates expression of ZFPL1 in all major tissues (http://www.ncbi.nlm.nih.gov/UniGene/ESTProfileViewer.cgi?uglist=Hs.656314).
Figure 1.
Identification of ZFPL1 as a mitotic Golgi phosphoprotein. (A) Rat liver Golgi membranes were incubated with mitotic HeLa cytosol in the presence of [γ-32P]ATP and radiolabelled proteins analysed by 16-BAC/SDS–PAGE two-dimensional electrophoresis followed by autoradiography. The arrow indicates the position of the doublet identified as ZFPL1 by Q-TOF mass spectrometry. The position of the known phosphoprotein golgin-84 is also indicated. (B) Amino-acid sequence of human ZFPL1 (Acc. No. NP_006773) aligned with orthologues from mouse (Mus musculus, Acc. No. NP_077193), zebrafish (Danio rerio, Acc. No. NP_956967), Drosophila melanogaster (Acc. No. NP_732731) and Caenorhabditis elegans (Acc. No. NP_503731). Conserved residues in the N-terminus predicted to coordinate zinc within a novel type of zinc finger are in blue, whereas residues predicted to coordinate zinc within a ring finger are in pink. The predicted transmembrane domains are in green. Identical residues are indicated with an asterisk, whereas weakly and strongly similar residues are marked with one or two dots, respectively. (C) ZFPL1 was immunoprecipitated from denatured extracts prepared from Golgi membranes incubated with interphase or mitotic cytosol and [γ-32P]ATP. Total extracts, unbound fractions and proteins immunoprecipitated with antibodies to ZFPL1 cytoplasmic domain (CD) or hydrophilic region (HL) were analysed by SDS–PAGE followed by autoradiography (P32) and western blotting with antibodies to ZFPL1. (D) HEK293 cells stably expressing ZFPL1–GFP under the control of a Tet repressor were left uninduced (−Dox) or induced with doxycycline (+Dox) for 24 h in the absence (I) or presence of nocodazole (M) to enrich for mitotic cells. 32P-orthophosphate was included for the last 4 h and ZFPL1–GFP was immunoprecipitated with anti-GFP antibodies before analysis by autoradiography (P32) and western blotting with anti-ZFPL1 antibodies.
To confirm that ZFPl1 is mitotically phosphorylated, antibodies were raised to the protein and used for immunoprecipitation from mitotically treated Golgi membranes. Both ZFPL1 antibodies that were raised detected a doublet running at ∼40 kDa on western blots corresponding to the position of the major mitotic 32P-labelled bands at this position on a 1D gel (Figure 1C). Both antibodies quantitatively precipitated ZFPL1, which was highly labelled with 32P in the mitotic but not interphase samples, confirming it is phosphorylated by mitotic cytosol. To determine whether ZFPL1 is also phosphorylated in vivo, cells stably expressing inducible C-terminally GFP-tagged ZFPL1 were synchronized and labelled with 32P and subjected to immunoprecipitation with anti-GFP antibodies. ZFPL1–GFP incorporated 32P in mitotic but not interphase cells, indicating mitotic phsophorylation of the protein in vivo (Figure 1D).
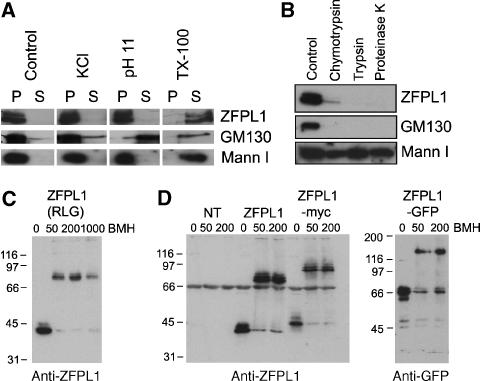
Our initial proteomic analysis suggested that ZFPL1 is an integral membrane protein (only proteins resistant to carbonate extraction were analysed). To further assess this possibility, rat liver Golgi membranes were extracted under various conditions and ZFPL1 detected by western blotting. As shown in Figure 2A, ZFPL1 was resistant to extraction with both 1 M KCl and sodium carbonate pH 11, which effectively removed the tightly bound peripheral protein GM130. ZFPL1 was however extracted by the detergent Triton X-100. These findings indicate that ZFPL1 is an integral membrane protein. To assess ZFPL1 topology, Golgi membranes were incubated with proteases in the absence of detergent. ZFPL1, similarly to GM130, was completely digested by proteases, whereas the predominantly luminal protein mannosidase I was resistant to digestion (Figure 2B), indicating that the majority of ZFPL1 is on the cytoplasmic face of the membrane.
Figure 2.
ZFPL1 is a type II integral membrane protein that forms dimers. (A) Rat liver Golgi membranes were extracted with buffer alone (control), buffer containing 1 M KCl, 0.1 M sodium carbonate in H2O (pH 11) or buffer containing 0.5% Triton X-100 (TX-100), re-isolated by centrifugation and analysed by western blotting with antibodies to ZFPL1, GM130 or mannosidase I (Mann I). (B) Golgi membranes were incubated in buffer alone (Control) or buffer containing 10 μg/ml chymotrypsin, trypsin or 1 μg/ml proteinase K before re-isolation by centrifugation and analysis by western blotting with the indicated antibodies. (C) Golgi membranes were incubated in buffer without cross-linker (0) or in the presence of 50, 200 or 1000 μM BMH before analysis by western blotting with anti-ZFPL1 antibodies. (D) Membranes prepared from non-transfected (NT) HeLa cells or cells transiently expressing untagged ZFPL1 (ZFPL1), myc-tagged (ZFPL1–myc) or GFP-tagged (ZFPL1–GFP) ZFPL1 were incubated in buffer alone (0) or buffer containing 50 or 200 μM BMH before analysis by western blotting with antibodies to ZFPL1 (left) or GFP (right). Molecular mass standards are in kDa.
A number of Golgi proteins exist as dimers, or higher-order oligomers, including the cis-Golgi matrix proteins GM130 and GRASP65 (Barr et al, 1998; Wang et al, 2005). We decided to analyse whether ZFPL1 may also exist in a higher-order structure by using covalent cross-linking. Treatment of rat liver Golgi membranes with the thiol-specific cross-linker bismaleimidohexane (BMH) resulted in the quantitative shift of ZFPL1 from ∼40 kDa to ∼80 kDa on SDS–PAGE gels (Figure 2C). To determine whether this product is a ZFPL1 dimer, versions of the protein with differently sized tags were expressed in cells, and the membranes isolated and incubated with cross-linker. In each case, the cross-linked product was double the mass of the non-cross-linked protein, indicating that ZFPL1 exists as a dimer in the membrane (Figure 2D).
ZFPL1 is localized to the cis-Golgi and behaves as a matrix protein
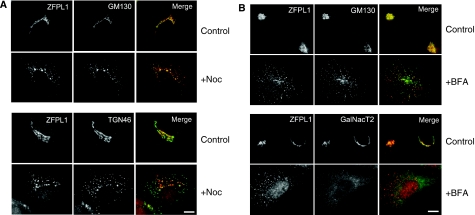
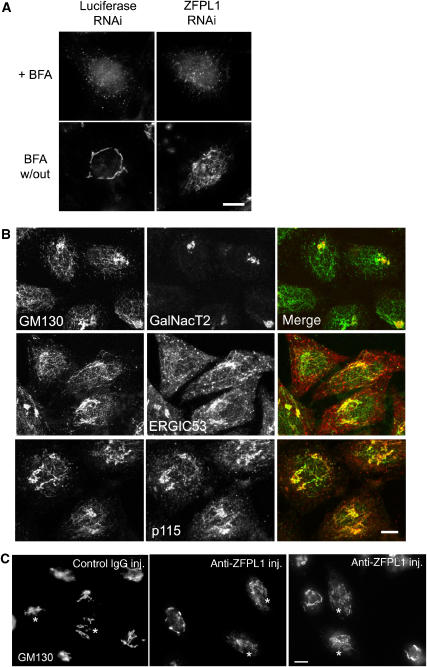
To determine the cellular localization of ZFPL1, we performed immunofluorescence microscopy with anti-ZFPL1 antibodies. ZFPL1 was localized to the Golgi apparatus, where it exhibited a high degree of overlap with the cis-Golgi matrix protein GM130, but only limited overlap with the trans-Golgi network protein TGN46 (Figure 3A). Excellent co-localization of ZFPL1 and GM130 was also observed after nocodazole-induced fragmentation of the Golgi ribbon, whereas ZFPL1 and TGN46 were clearly distinct. These results suggest that ZFPL1 is restricted to the cis-side of the Golgi apparatus. Components of the cis-Golgi matrix relocate to punctate cytoplasmic structures, sometimes referred to as remnants, which appear close to ER exit sites upon treatment of cells with brefeldin A (BFA), rather than to the ER network, as is the case for Golgi enzymes (Lippincott-Schwartz et al, 1990; Seemann et al, 2000a; Mardones et al, 2006). ZFPL1 was also relocated to the cytoplasmic remnants upon BFA treatment, where it co-localized with GM130 (Figure 3B). As expected, the Golgi enzyme GalNacT2 was in the ER after BFA treatment and did not co-localize with the ZFPL1-containing remnants. ZFPL1 therefore behaves as a cis-Golgi matrix protein after BFA treatment.
Figure 3.
ZFPL1 is localized to the cis-Golgi and behaves as a matrix protein. (A) Epifluorescence microscopy of Vero cells left untreated (control) or treated with 5 μg/ml nocodazole for 2 h at 37°C (+Noc) and double-labelled with antibodies to ZFPL1 (red) and either GM130 or TGN46 (green). (B) Vero cells were treated with 5 μg/ml BFA for 1 h at 37°C (+BFA) and double-labelled with antibodies to ZFPL1 (red) and either GM130 or GalNacT2 (green). Scale bar, 10 μm.
ZFPL1 directly interacts with GM130
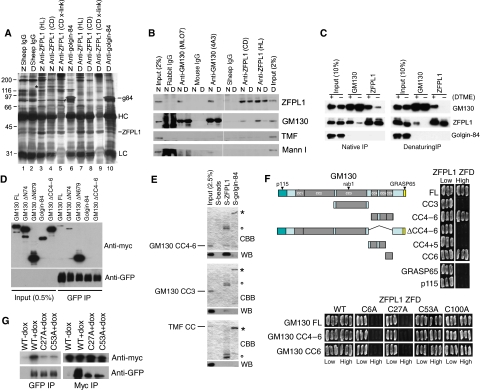
To identify interaction partners for ZFPL1, we performed co-immunoprecipitation experiments from Golgi membrane extracts under native conditions. As shown in Figure 4A, ZFPL1 antibodies co-precipitated a protein of ∼130 kDa under native, but not denaturing, conditions (marked with an asterisk). This protein was not present in control immunoprecipitations performed with sheep IgG or antibodies to golgin-84. Mass spectrometry identified the ∼130 kDa protein as GM130. Western blotting confirmed that GM130 was co-precipitated with two different ZFPL1 antibodies under native, but not denaturing, conditions and not with various control antibodies (Figure 4B). The golgin TATA-modulatory factor (TMF) and mannosidase I were also not present in the ZFPL1 precipitates. Reciprocal co-immunoprecipitation of ZFPL1 with GM130 antibodies was observed, supporting the idea that ZFPL1 and GM130 specifically associate with each other in Golgi membranes. In line with this, we found that the GM130–ZFPL1 interaction could be stabilized by cross-linking before membrane extraction and immunoprecipitation under native or denaturing conditions (Figure 4C).
Figure 4.
Interaction of ZFPL1 with the cis-Golgi matrix protein GM130. (A) Rat liver Golgi membranes were extracted under native (N) or denaturing (D) conditions and subjected to immunoprecipitation with sheep IgG or affinity-purified antibodies to ZFPL1 or golgin-84 and analysed by silver staining. X-link indicates samples where the ZFPL1 antibody was covalently coupled to beads before use. The positions of immunoprecipitated ZFPL1 (in lanes 3–5 and 7–9) and golgin-84 (lanes 6 and 10) are indicated as are the IgG heavy (HC) and light chains (LC). The asterisk marks the position of an ∼130 kDa protein that co-precipitates with ZFPL1 under native conditions. Twelve peptides isolated from this band matched GM130 using MALDI-TOF mass spectrometry. (B) Rat liver Golgi membranes were extracted under denaturing (D) or native (N) conditions and subjected to immunoprecipitation with control IgGs or antibodies to GM130 (rabbit MLO7 or mouse 4A3) or ZFPL1 (sheep anti-cytoplasmic domain (CD) or anti-hydrophilic region (HL)). Extract (2% input) and immunoprecipitated proteins were analysed by western blotting with antibodies to ZFPL1, GM130, TMF or Mannosidase I (Mann I) as indicated. (C) Golgi membranes were incubated in the absence (−) or presence (+) of DTME cross-linker before extraction and immunoprecipitation under native or denaturing conditions with antibodies to GM130 or ZFPL1. Samples were subjected to western blotting with antibodies to GM130, ZFPL1 or golgin-84. (D) HeLa cells stably expressing full-length ZFPL1–GFP were transiently transfected with plasmids encoding myc-tagged full-length GM130, GM130 lacking the N-terminal 74 (ΔN74) or 679 (ΔN679) residues or coiled-coils 4–6 (ΔCC4–6), or myc-tagged full-length golgin-84. Native immunoprecipitations were carried out with anti-GFP antibodies and samples blotted with antibodies to Myc or GFP. (E) Purified protein S-tagged full-length ZFPL1 or golgin-84 cytoplasmic domain coupled to S-beads or beads alone were incubated with constructs encoding His-tagged GM130 coiled-coils 4–6 or coiled-coil 3, or the entire coiled-coil region of TMF fused to GST and bound proteins analysed by Coomassie blue staining (CBB) or western blotting with anti-His or anti-GST antibodies (WB). The positions of S-tagged ZFPL1 and golgin-84 bait proteins are indicated with a circle and an asterisk respectively. (F) top, The indicated GM130 truncation constructs or full-length p115 or GRASP65 were tested for interaction with a ZFPL1 ZFD construct (residues 1–112) in the yeast two-hybrid system. (F) bottom, The indicated GM130 truncation constructs were tested for interaction with wild-type or the indicated point mutant ZFPL1 ZFD constructs in the yeast two-hybrid system. Interaction is indicated by growth on high selection. (G) HeLa cells stably expressing full-length wild-type ZFPL1–GFP or the indicated point mutants were transiently transfected with myc-tagged GM130 lacking the N-terminal 679 residues and subjected to native immunoprecipitation with antibodies to Myc or GFP followed by western blotting with the same antibodies.
To map the region of GM130 important for binding to ZFPL1, truncated versions of the protein were co-expressed in HeLa cells stably expressing full-length ZFPL1 with a C-terminal GFP tag and proteins immunoprecipitated with anti-GFP antibodies. Full-length GM130 and a construct lacking the N-terminal 74 residues bound weakly to ZFPL1–GFP (Figure 4D). A C-terminal construct containing coiled-coils 4–6 (ΔN679) bound strongly to ZFPL1–GFP, whereas no binding was detected for a construct lacking this region or the negative control golgin-84. To determine whether the interaction between ZFPL1 and GM130 is direct, binding was performed with purified recombinant proteins. As shown in Figure 4E, purified full-length ZFPL1 bound directly to His-tagged GM130 coiled-coils 4–6 but not coiled-coil 3 of GM130 or the coiled-coil region of TMF. To further corroborate these findings, binding was analysed in the yeast two-hybrid system. Full-length GM130, but not the control proteins GRASP65, p115, TMF or golgin-84 bound to a truncated ZFPL1 construct containing the two predicted N-terminal ZFDs (Figure 4F and data not shown). This indicates that the N-terminal region of ZFPL1 contains the GM130-binding site. GM130 containing coiled-coils 4–6 or coiled-coil 6 alone bound to ZFPL1 ZFD, whereas a construct containing only coiled-coils 4–5 of GM130 failed to interact, suggesting coiled-coil 6 is the region of GM130 responsible for interaction with ZFPL1. To determine which part of the ZFPL1 N-terminus binds GM130, predicted zinc-coordinating residues within each of the putative ZFDs were mutated to alanine and binding assessed. As shown in Figure 4F, mutation of the first zinc finger motif abolished GM130 binding, whereas mutation of the ring domain had no effect, suggesting the first zinc finger motif is the GM130-binding site. In co-immunoprecipitation experiments, which are generally more stringent than the yeast two-hybrid assay, mutation of either ZFD significantly reduced binding to GM130 (Figure 4G). This suggests that although the first ZFD is likely the primary binding site for GM130, both ZFDs are required for stable or high-affinity binding.
Depletion of ZFPL1 causes GM130 to accumulate in a more tubular IC
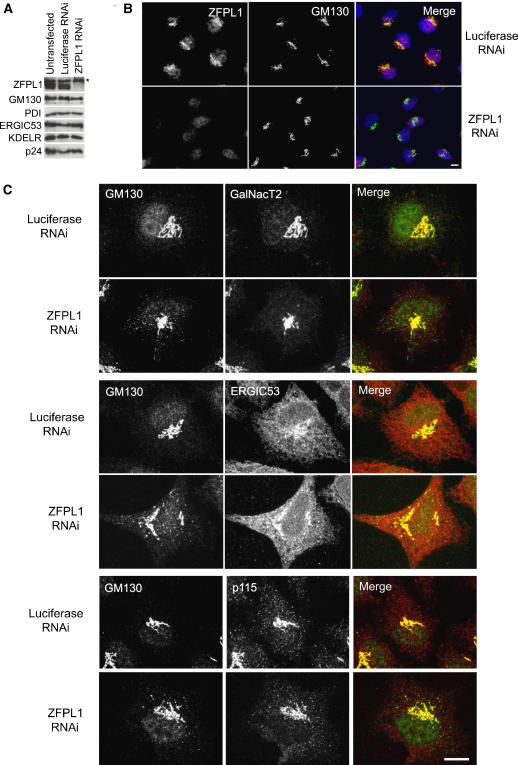
To assess ZFPL1 function, RNA interference was used to deplete the protein from HeLa cells. Efficient depletion of ZFPL1 was observed at 72 h post-transfection (Figure 5A). This was seen with three independent oligos (only results with oligo 2 are presented). All three oligos also gave the same effect upon GM130 distribution, IC morphology and cargo trafficking, as described below (again, only results with oligo 2 are presented). Depletion of ZFPL1 did not affect levels of other Golgi and ER proteins analysed, including GM130 (Figure 5A). Depletion of ZFPL1 could also be monitored by immunofluorescence microscopy (Figure 5B). The Golgi ribbon was not dramatically affected by depletion of ZFPL1 (Figure 5B and figures therein). At the electron microscopy level, Golgi stacks were present in ZFPL1-depleted cells and exhibited a similar gross morphology to controls (Supplementary Figure S2). Quantitation revealed that the number of cisternae per stack was unaffected by ZFPL1 depletion, but the average length of cisternae within the stack was slightly reduced (Supplementary Figure S2).
Figure 5.
Depletion of ZFPL1 alters the steady-state distribution of GM130. (A) HeLa cells were transfected with control (luciferase) siRNA or siRNA targeting ZFPL1, incubated for 72 h and analysed by western blotting with antibodies to the indicated proteins. The asterisk indicates a protein that cross-reacts with the anti-ZFPL1 antibody by western blotting. (B) SiRNA-treated cells were fixed 72 h post-transfection, double-labelled with antibodies to ZFPL1 (red) and GM130 (green) and visualized by epifluorescence microscopy. DNA is blue. (C) SiRNA-treated cells were analysed 72 h post-transfection by confocal microscopy. Cells were double-labelled with antibodies to GM130 (green) and either GalNacT2 (red, top panel), ERGIC53 (red, middle panel) or p115 (red, bottom panel). Scale bar, 10 μm.
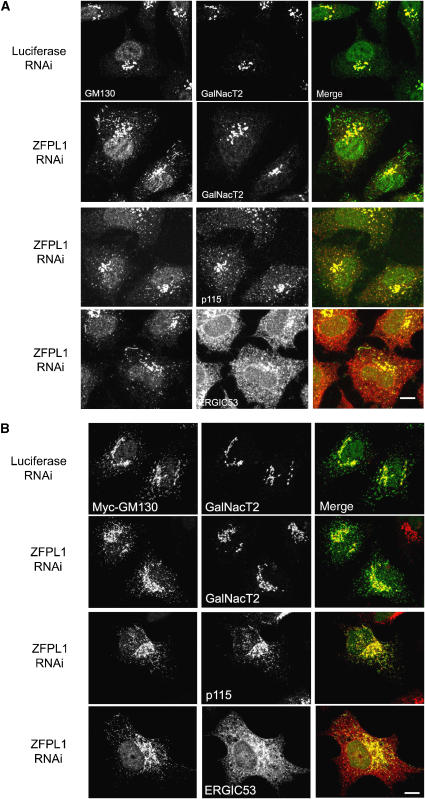
Close inspection of ZFPL1-depleted cells using immunofluorescence microscopy indicated that the steady-state distribution of GM130 was altered compared with that in control cells. GM130 was present in cytoplasmic punctae that sometimes resembled short tubules in ZFPL1-depleted cells, whereas in controls it was restricted to the Golgi ribbon (Figure 5C). GRASP65, which is tightly bound to GM130, exhibited a similar redistribution in ZFPL1-depleted cells (Supplementary Figure S3). The GM130- and GRASP65-positive structures lacked detectable levels of the Golgi stack protein GalNacT2 and the TGN marker golgin-97 (Figure 5C and Supplementary Figure S3). However, they contain the IC marker ERGIC53 as well as p115 and KDEL receptor, which are typically localized to the IC in addition to the cis-Golgi.
To further characterize effects of ZFPL1 depletion upon GM130 distribution, we analysed cells that had undergone prolonged incubation at 15°C, a condition where transport from the IC to the Golgi is arrested, resulting in swelling of the IC, and that has been used previously to increase levels of IC-associated GM130 (Marra et al, 2001). In control cells, some GM130 was detected in IC elements, as expected (Figure 6A). However, in cells depleted of ZFPL1, there was a dramatic increase in the amount of GM130 in IC elements (Figure 6A). This was also true for GRASP65 (Supplementary Figure S3). The GM130 and GRASP65-positive structures contained p115, KDEL receptor and ERGIC53, but lacked GalNacT2 and golgin-97 (Figure 6A and Supplementary Figure S3). Interestingly, many of the GM130-positive structures were tubular in nature, and in some cases the tubules were extremely long (up to 20 μm in length). The tubules sometimes emanated from the Golgi region. In other cases, they emanated from the GM130-positive punctae towards the Golgi region or appeared to connect individual punctae.
Figure 6.
GM130 tubules are exaggerated upon ZFPL1 depletion. (A) HeLa cells were transfected with control (luciferase) siRNA or siRNA targeting ZFPL1 as indicated, incubated at 37°C for 72 h, then shifted to 15°C for 3 h before fixation and double labelling with antibodies to GM130 (green) and GalNacT2, ERGIC53 or p115 (red). Cells were visualized by confocal microscopy. (B) SiRNA-treated cells were incubated for 48 h at 37°C, then transiently transfected with myc-tagged GM130 and incubated for a further 24 before fixation and double labelling with antibodies to myc (green) and GalNacT2, ERGIC53 or p115 (red). Cells were visualized by confocal microscopy. Scale bar, 10 μm.
When ectopically expressed in HeLa cells at 37°C, myc-tagged GM130 is readily detectable in the IC (GalNacT2-negative, p115-positive structures), which often looks tubular in appearance (Figure 6B). When expressed in ZFPL1-depleted cells, there is an increase in myc–GM130 associated with the IC, and these structures are now more tubulated (Figure 6B). Therefore, in all conditions analysed, depletion of ZFPL1 causes an increase in the amount of GM130 associated with the IC and also leads to an increased tubulation of GM130-positive compartments.
GM130-containing punctae and tubules formed upon ZFPL1 depletion contain anterograde cargo
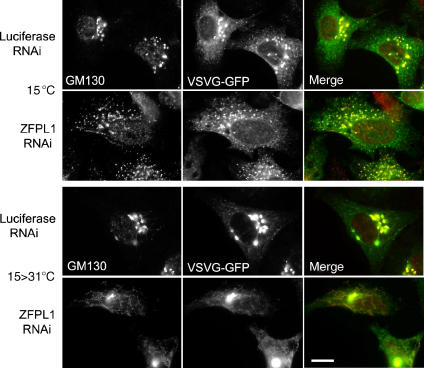
We next wanted to assess whether the punctate and tubular GM130-containing structures observed after ZFPL1 depletion contain anterograde cargo, as has been seen for GM130-containing IC in non-perturbed cells (Marra et al, 2001, 2007). For this purpose, RNAi-treated cells were infected with an adenovirus encoding a GFP-tagged version of the well-characterized anterograde cargo protein temperature-sensitive mutant viral glycoprotein ts-O45 VSV-G. This protein is retained in the ER at 40°C but undergoes synchronized transport along the secretory pathway when shifted to the permissive temperature of 31°C. When control cells were incubated at 15°C for 3 h, the majority of VSV-G exited the ER and accumulated in the IC, as expected (Figure 7). In ZFPL1-depleted cells, VSV-G also exited the ER and accumulated in the IC, but in this case, there was significantly more GM130 present in the peripheral VSV-G structures compared with controls, and they were more tubular in appearance (Figure 7). Upon shifting the ZFPL1-depletd cells from 15 to 31°C to induce transport carrier formation, there was a dramatic tubulation of GM130-containing membranes, and significantly, VSV-G was present within these tubular structures (Figure 7). These results indicate that the GM130-containing structures formed in ZFPL1-depleted cells contain anterograde cargo. These may correspond to transport intermediates operating between the IC and Golgi apparatus, tubulated cis-Golgi or both.
Figure 7.
GM130 punctae and tubules formed upon ZFPL1 depletion contain anterograde cargo. HeLa cells were transfected with control (luciferase) siRNA or siRNA targeting ZFPL1, incubated for 48 h, then infected with adenovirus encoding ts-O45 VSV-G–GFP (green) and incubated at 40°C overnight. Cells were shifted to 15°C for 3 h and either fixed directly (top) or transferred to 31°C for a further 10 min before fixation (bottom). Cells were labelled with anti-GM130 antibodies (red) and visualized by epifluorescence microscopy. Scale bar, 10 μm.
ZFPL1 is required for efficient ER to Golgi transport
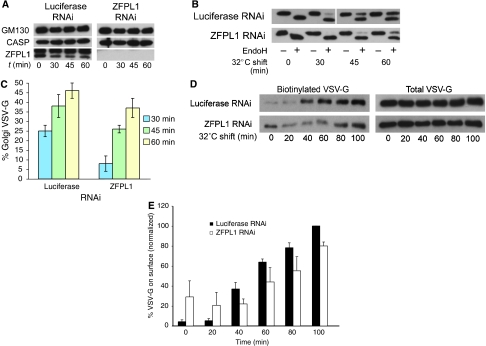
To analyse whether ZFPL1 can regulate trafficking between the ER and Golgi apparatus, untagged VSV-G was expressed in ZFPL1-depleted cells, which were shifted from 40 to 31°C and delivery to the medial-Golgi monitored by acquisition of endoglycosidase H (EndoH) resistance. In cells depleted of ZFPL1 (Figure 8A), there was a reduction in the amount of EndoH-resistant VSV-G compared to controls, indicating an inhibition of trafficking to the Golgi apparatus (Figure 8B and C). The effect was apparent at all times, but most noticeable at the earlier time points, suggestive of a transport delay in the ZFPL1-depleted cells. To further corroborate these results, VSV-G trafficking was studied with a second assay in which delivery of VSV-G to the plasma membrane was monitored using cell-surface biotinylation. This method is independent of the glycosylation status of VSV-G, thereby avoiding possible complications arising from changes in Golgi enzyme distribution, or Golgi ribbon integrity that may alter VSV-G processing rather than its trafficking per se (Puthenveedu et al, 2006). As shown in Figure 8D, delivery of VSV-G to the cell surface was reduced in the ZFPL1-depleted cells compared with the luciferase control. We conclude from these experiments that ZFPL1 is required for efficient trafficking of VSV-G from the ER to the Golgi apparatus.
Figure 8.
ZFPL1 depletion impairs ER-to-Golgi trafficking. (A) HeLa cells were transfected with control (luciferase) siRNA or siRNA targeting ZFPL1, incubated for 48 h, infected with adenovirus encoding ts-O45 VSV-G and incubated at 40°C overnight. Cells were shifted to 31°C for various times as indicated before lysis and analysis by western blotting with antibodies to GM130, CASP or ZFPL. (B) Cell lysates were treated with or without endoglycosidase-H (Endo-H) before western blotting with P5D4 anti-VSVG antibody. (C) Quantitation of VSV-G trafficking to the medial Golgi (Endo-H resistance). Results are shown as the mean±s.d. for three experiments. (D) RNAi-treated VSV-G-expressing cells were shifted to 31°C for the indicated times and appearance of VSV-G at the cell surface was monitored by surface biotinylation. Total and surface (biotinylated) VSV-G were detected by western blotting with P5D4 antibody. (E) Quantitation of VSV-G trafficking to the cell surface was monitored by biotinylation. Results are shown as the mean±s.d. for three experiments.
ZFPL1 is required for assembly of the cis-Golgi
We next wanted to test whether ZFPL1 has an important function in Golgi assembly. To test this hypothesis, ZFPL1-depleted cells were treated with BFA to induce Golgi disassembly and the drug subsequently washed out to allow Golgi reformation. Disassembly of the Golgi apparatus induced by BFA was not affected by ZFPL1 depletion (Figure 9A and data not shown). However, when BFA was removed, Golgi assembly was inhibited compared with controls. GM130 was present in an irregular tubular network extending throughout the cytoplasm in ZFPL1-depleted cells rather than the compact Golgi ribbon seen in control cells (Figure 9A). The GM130 tubules contained GRASP65 and proteins found in the IC, including p115, ERGIC53 and KDEL receptor (Figure 9B and Supplementary Figure S4). Interestingly, the GM130 tubules were devoid of GalNacT2 and golgin-97, markers of the Golgi stack and TGN, respectively (Figure 9B and Supplementary Figure S4), which accumulated in large perinuclear structures that likely correspond to Golgi fragments. Electron microscopy revealed the presence of Golgi stacks in many ZFPL1-depleted cells following BFA washout (Supplementary Figure S5). Together, these results suggest that ZFPL1 is required for the assembly of the cis-Golgi or its structural integrity post-assembly, but not that of the Golgi stack itself.
Figure 9.
Assembly of the cis-Golgi is impaired by ZFPL1 depletion or microinjection of ZFPL1 antibodies. (A) HeLa cells were treated with control (Luciferase) siRNA or siRNA targeting ZFPL1 for 72 h, incubated with 5 μg/ml BFA for 1 h at 37°C and either fixed directly or the drug washed out for an additional 2 h. Cells were labelled with antibodies to GM130 and visualized by epifluorescence microscopy. (B) SiRNA-treated cells were incubated with 5 μg/ml BFA for 1 h, and the drug was washed out for a further 2 h before double-labelling with antibodies to GM130 (green) and GalNacT2, ERGIC53 or p115 (red) and visualization by confocal microscopy. (C) HeLa cells were treated with 5 μg/ml BFA for 1 h, micro-injected in the presence of BFA with rabbit IgG (control) or affinity-purified antibodies to ZFPL1 (CD), washed to remove the drug and incubated for a further 2 h before fixation and labelling with antibodies to GM130. Injected cells are marked with an asterisk. Scale bar, 10 μm.
To confirm these findings, we used a different approach to perturb ZFPL1 function, namely antibody microinjection. Affinity-purified anti-ZFPL1 antibodies were microinjected into cells that had been incubated with BFA for 1 h to induce Golgi disassembly, and the drug was removed and assembly of the cis-Golgi followed using GM130. As shown in Figure 9C, microinjection of control antibodies had no effect on cis-Golgi reassembly. However, injection of anti-ZFPL1 antibodies dramatically inhibited the assembly process, with GM130 accumulating in extensive cytoplasmic tubules (Figure 9C), similar to the phenotype observed in ZFPL1-depleted cells.
Both ZFDs of ZFPL1 are required for cis-Golgi assembly
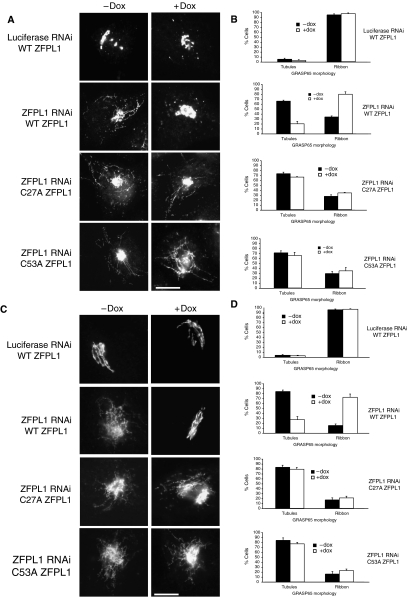
To confirm the specificity of the phenotypes described above and study the mechanism of ZFPL1 function, RNAi rescue experiments were performed. The rescue construct was full-length human ZFPL1 containing three silent mismatch mutations in the oligo2 siRNA sequence and a C-terminal GFP tag. HeLa cells stably expressing this construct under the control of a tetracycline-inducible promoter were generated and RNAi performed without or with ZFPL1–GFP induction. The predicted ZFDs of ZFPL1 are the most conserved parts of the protein (Figure 1 and Supplementary Figure S1), suggesting that they are functionally important. The first domain contains the GM130-binding site, whereas the second domain, which is a predicted ring finger, may also stabilize this interaction (Figure 4F and G). To determine the functional importance of the two ZFDs, and therefore the interaction of ZFPL1 with GM130, RNAi rescue constructs containing point mutations in predicted zinc-coordinating residues in either the first or second ZFDs were also generated. Importantly, both zinc finger mutants and the wild-type ZFPL1–GFP constructs were correctly targeted to the Golgi apparatus (Supplementary Figure S6).
As shown in Figure 10A, GRASP65 was present in peripheral puncta and cytoplasmic tubules when ZFPL1-depleted cells were incubated at 15°C in the absence of ZFPL1–GFP expression. As expected, these structures also contained GM130, p115 and ERGIC53 (data not shown). Expression of wild-type ZFPL1–GFP rescued this phenotype, with GRASP65 returning to a predominantly ribbon-like appearance with a few remaining cytoplasmic puncta, as seen in the luciferase control RNAi cells (Figure 10A and B). In contrast, expression of ZFPL1–GFP with mutations in either the first or second (ring) ZFDs was unable to rescue the phenotype, with GRASP65 remaining in highly tubulated structures that also contained GM130, p115, ERGIC53 and KDEL receptor, as well as the mutant ZFPL1–GFP protein (Figure 10A and B and data not shown). The functional role of the ZFDs was further investigated by studying cis-Golgi assembly following BFA washout. In the absence of ZFPL1–GFP expression, GRASP65 and GM130 (not shown) were found in extensive cytoplasmic tubules in ZFPL1-depleted cells (Figure 10C and D). Expression of wild-type ZFPL1–GFP restored GRASP65 and GM130 (not shown) localization to a compact ribbon organization similar to that seen in the luciferase control cells, whereas expression of either mutant had no effect on either protein, which remained in tubules (Figure 10C and D). We conclude from these experiments that both the predicted ZFDs of ZFPL1 have an important function in assembling the cis-Golgi and that interaction between ZFPL1 and GM130 is required for this process.
Figure 10.
Both ZFDs of ZFPL1 are required for cis-Golgi assembly. (A) Luciferase or ZFPL1 siRNA-treated HeLa cells stably expressing full-length wild-type ZFPL1–GFP or the C27A or C53A point mutants under the control of a Tet repressor were left uninduced (−Dox) or induced (+Dox) before incubation at 15°C for 3 h. Cells were fixed and labelled with antibodies to GRASP65 before visualization by epifluorescence microscopy. Scale bar, 10 μm. (B) Quantitation of the GRASP65 phenotype. Cells were scored for the presence of GRASP65 in cytoplasmic tubules. Results are shown as the mean percentage of cells with GRASP65 in tubules or intact ribbon±s.d. Results were obtained from two experiments, counting 200 cells per condition in each experiment. (C) SiRNA-treated cells were left uninduced (−Dox) or induced (+Dox) for ZFPL–GFP expression and incubated with 5 μg/ml BFA for 1 h at 37°C. The drug was washed out and cells were allowed to recover for 2 h before labelling with antibodies to GRASP65 and visualization by epifluorescence microscopy. Scale bar, 10 μm. (D) Quantitation of cis-Golgi assembly. Cells were scored for the presence of GRASP65 exclusively in an intact ribbon or also in cytoplasmic tubules. Results are shown as the mean percentage of cells with GRASP65 in tubules or intact ribbon±s.d. Results were obtained from three experiments, counting 200 cells per condition in each experiment.
Discussion
In this study, we have identified ZFPL1 in a screen for mitotic Golgi phosphoproteins. ZFPL1 was previously described as a likely transcription factor based on the presence of two putative zinc fingers at the N-terminus and a region at the C-terminus that was proposed to exist as a leucine zipper (Hoppener et al, 1998). We now show that this region is likely a trans-membrane domain, and that ZFPL1 is restricted to the Golgi apparatus, with the majority of the protein including the putative zinc fingers on the cytoplasmic side of the membrane. Northern blotting previously suggested that ZFPL1 was only expressed in the pancreas (Hoppener et al, 1998), yet we have been able to detect the protein by western blotting in all cell types analysed (data not shown), and inspection of the EST databases indicates that ZFPL1 transcripts are present in all major tissues. ZFPL1 is conserved among metazoa, with the highest homology in the N-terminus, but apparently absent from yeast, suggesting that its function is dispensable in yeast.
ZFPL1 has the properties of a Golgi matrix protein in vivo, and directly binds GM130, a golgin implicated in membrane tethering reactions at the IC and cis-Golgi (Seemann et al, 2000b; Alvarez et al, 2001; Marra et al, 2001, 2007; Diao et al, 2007). Depletion of ZFPL1 leads to a number of effects, including mislocalization of GM130 to the IC, increased tubulation of cis-Golgi and IC membranes and inhibition of cis-Golgi assembly following BFA washout. The GM130-positive tubules seen in these experiments often emanate from the Golgi region and contain cis-Golgi proteins, but lack markers of later Golgi compartments. These observations suggest that ZFPL1 is involved in maintaining the structural integrity of the cis-Golgi. Defects in cis-Golgi integrity could arise from impaired assembly of Golgi matrix proteins such as GM130 and GRASP65 and associated factors into a structural scaffold onto which the cis-Golgi membranes can assemble (Slusarewicz et al, 1994; Wang et al, 2003). The appearance of matrix proteins in the IC in ZFPL1-depleted cells is consistent with their reduced assembly and retention in the cis-Golgi. A structurally impaired cis-Golgi under these conditions might be less able to accept incoming IC carriers, explaining the reduction in trafficking observed when ZFPL1 is depleted. Another not mutually exclusive scenario is that ZFPL1 directly participates in trafficking from the IC to the cis-Golgi. It has been shown that GM130 participates in the tethering and fusion of IC elements that is required for their incorporation into the cis-Golgi (Marra et al, 2007). ZFPL1 may also have an important function in this process, explaining the reduced cargo trafficking in ZFPL1-depleted cells. However, any role here is likely not a major one, given the assembly of later Golgi compartments we observe upon BFA washout in ZFPL1-depleted cells.
What is the mechanism of ZFPL1 action? RNAi rescue experiments indicate that the N-terminal ZFDs of ZFPL1 are required for cis-Golgi integrity. These domains are required for interaction with GM130, indicating that the ZFPL1–GM130 interaction is important for its function in vivo. ZFPL1 may exert an effect as a tethering factor itself, linking to GM130 on an apposing membrane, although this does not fit well with binding to the membrane-proximal C-terminal region of GM130. Alternatively, ZFPL1 may regulate tethering indirectly, either by retaining GM130 in the correct place to facilitate cross-bridging of membranes or through regulating GM130 interactions with itself or other interacting proteins (binding of GM130 to p115 and GRASP65 is unaffected in ZFPL1-depleted cells; Frost and Lowe, unpublished data). A third possibility is that ZFPL1 promotes the lateral association of GM130-containing complexes on the same membrane. This could allow the formation of a large planar structure onto which membranes flatten out to form cisternae. As ZFPL1 is a dimer, it is capable of cross-linking GM130, itself a dimer, into higher-order oligomers.
The tubulation of cis-Golgi and IC membranes seen upon ZFPL1 depletion is reminiscent of the effects seen upon BFA treatment or depletion of the ARF exchange factor GBF1, which both remove COPI from membranes (Lippincott-Schwartz et al, 1990; Szul et al, 2007). However, we fail to observe any loss of COPI from the Golgi or IC when ZFPL1 is depleted (Supplementary Figure S7). We also fail to see changes in COPII membrane association, or in microtubule integrity, excluding these as possible causes for the phenotypes observed in our experiments (Supplementary Figure S7). Rather, we favour the idea that effects upon Golgi matrix proteins are responsible, as described above. Interestingly, over-expression of GM130 can induce cis-Golgi and IC tubulation (Marra et al, 2001 and Frost and Lowe, unpublished data), suggesting that the matrix proteins themselves have the capacity to drive tubulation under certain conditions.
In summary, we have identified ZFPL1 as a novel structural component of the Golgi apparatus. ZFPL1 interacts with the cis-Golgi matrix protein GM130 and is important both for the integrity of the cis-Golgi and optimum trafficking efficiency into this compartment. Future studies will concentrate on identifying additional interacting partners for ZFPL1 and determining how these proteins exert an effect in conjunction with ZFPL1 and Golgi matrix proteins to ensure the correct structural and functional organization of the early Golgi apparatus.
Materials and methods
Yeast two-hybrid analysis
Yeast two-hybrid experiments were performed as described previously (Diao et al, 2003).
In vitro phosphorylation of Golgi membranes and 16-BAC gel electrophoresis
Preparation of rat liver Golgi membranes, interphase and mitotic HeLa cytosols and in vitro phosphorylation of Golgi membranes was performed according to Diao et al (2003). Phosphorylated membranes were extracted with 0.1 M sodium carbonate (pH 11) and subjected to 2D 16-BAC/SDS–PAGE (Hartinger et al, 1996), followed by Coomassie blue staining and autoradiography.
Cell culture and transfection
HeLa and Vero cells were grown at 37°C and 5% CO2 in Dulbecco's modified Eagle medium (DMEM), supplemented with 10% foetal bovine serum (FBS). For nocodazole treatment, cells were first placed on ice for 5 min before incubation at 37°C for 2 h in the presence of 5 μg/ml nocodazole. For BFA treatment, cells were incubated with 5 μg/ml BFA for 1 h at 37°C. In washout experiments, the drug was removed by five washes in fresh medium before incubation for a further 2 h at 37°C. In some experiments, BFA-treated cells were micro-injected with 3 mg/ml sheep IgG or anti-ZFPL1 (CD) antibodies before washout. Transient transfections were performed using FUGENE 6 (Roche) according to the manufacturer's instructions. HEK293 or HeLa cells stably expressing C-terminal GFP-tagged WT or point mutant ZFPL1 were made using Flp-In TRex-293 (Invitrogen) or Flp-In TRex-HeLa cells (Stephen Taylor, University of Manchester, UK) with pOG44, a plasmid encoding the Flp recombinase. After selection in 150 μg/ml hygromycin and 10 μg/ml blasticidin (TRex-293) or 200 μg/ml hygromycin and 4 μg/ml blasticidin (TRex-HeLa), colonies were pooled. Cells were maintained in 50 μg/ml hygromycin and 10 μg/ml blasticidin (TRex-293) or 200 μg/ml hygromycin and 4 μg/ml blasticidin (TRex-HeLa) and ZFPL1–GFP expression was induced by adding 1 μg/ml doxycycline for 16–24 h. Transient transfections of stable cells were performed at the same time as induction. Radio-labelling of HEK ZFPL1–GFP cells was performed by incubating in the presence or absence of 1 μg/ml doxycycline for 5 h, then adding 200 ng/ml nocodazole for 12–14 h to induce mitotic arrest. Nocodazole was omitted for interphase cultures. Cells were then incubated with 200 μCi/ml 32P-orthophosphate in the presence or absence of doxycycline and nocodazole for a further 4 h before lysis.
RNA interference
RNA intereference was performed on HeLa cells using Oligofectamne (Invitrogen) and siRNA oligos (Dharmacon Research) as described previously (Diao et al, 2003). Lamin A (target sequence AACUGGACUUCCAGAAGAACA) or luciferase (GL2, Eurogentec) was used as negative control. ZFPL1 was targeted with oligo 1 (CGACCCGCCUUGUCUGCUA), oligo 2 (CCUAGGAAGGUGUAUGAUA) or oligo 4 (GCUCCAAGAUAGCGACTAC). Unless indicated otherwise, the results shown were obtained using oligo 2. Similar results were obtained with all three oligos. Cells were analysed 72 h after transfection. For rescue experiments, TRex–HeLa cells were induced 48 h after transfection and cultured for an additional 48 h before analysis.
VSVG trafficking
Adenovirus encoding ts-O45 VSV-G-GFP was kindly provided by Dr Nobuhiro Nakamura (Kanazawa University, Japan). High-titre adenovirus encoding untagged ts-O45 VSV-G was described previously (Diao et al, 2007). Cells were grown in 3 cm dishes and transfected with siRNA as appropriate. Cells were washed and incubated in 400 μl DMEM supplemented with 5% FBS containing 0.5 μl high-titre adenovirus for 1 h at 37°C in a humidified incubator. Pre-warmed DMEM supplemented with 10% FBS (2 ml) was added and the cells were transferred to 40°C overnight. Cells were rapidly transferred to 15 or 31°C by changing the medium to fresh carbonate-free medium containing 0.1 mg/ml cycloheximide pre-cooled to either 15 or 31°C and incubating in a water bath at this temperature for the appropriate time. Cells were then either fixed and analysed by immunofluorescence microscopy or processed for surface biotinylation or EndoH digestion according to Diao et al (2007).
Membrane extraction and protease protection
Ten micrograms of rat liver Golgi membranes were incubated in 100 μl of KPO4 buffer (0.1 M KPO4, pH 6.7, 5 mM MgCl2), KPO4 buffer containing 1 M KCl or 0.5% Triton X-100, or in 0.1 M sodium carbonate diluted in water (pH 11) for 30 min on ice. Protease protection was performed by incubating 10 μg Golgi membranes in 50 μl KPO4 buffer containing 0.2 M sucrose and 10 μg/ml trypsin, 10 μg/ml chymotrypsin or 1 μg/ml proteinase K for 15 min on ice. Membranes were re-isolated by centrifugation through a 0.4 M sucrose cushion (in KPO4 buffer) for 10 min at 50 K r.p.m. in a TLA55 rotor (Beckman Instruments) and analysed by SDS–PAGE followed by western blotting.
Cross-linking
Rat liver Golgi membranes (10 μg) were incubated in 50 μl PBS containing 0 μM, 50 μM, 200 μM or 1 mM BMH on ice for 30 min. Alternatively, membranes were prepared from HeLa transiently expressing ZFPL1. Cells were scraped in PBS from a 10 cm dish, swollen in 5 ml of 0.4 × HKS (1 × is 20 mM HEPES, pH 7.4, 0.1 M KCl, 0.2 M sucrose) for 5 min on ice, then broken in 1 ml of HKS containing protease inhibitors by passing 20 × through a 25-gauge needle. A post-nuclear supernatant was made by centrifugation at 800 g for 10 min, and membranes isolated from this by spinning for 30 min at 50 K r.p.m. in a TLA55 rotor. Membranes were resuspended in 200 μl HKS and split into 50 μl aliquots for cross-linking as above. After cross-linking, an equal volume of 2 × SDS–PAGE sample buffer was added, and the samples were boiled and analysed by western blotting. Cross-linking of Golgi membranes with dithio-bismaleimidoethane (DTME) was performed according to Diao et al (2007).
Immunoprecipitation
Rat liver Golgi membranes (100 μg) were extracted in 200 μl of HKMT (20 mM HEPES, pH 7.4, 0.1 M KCl, 5 mM MgCl2, 0.5% Triton X-100) for 15 min on ice (native conditions) or 200 μl HKMS (HKMT with 1% SDS instead of Triton X-100) for 5 min at room temperature followed by boiling for 5 min (denaturing conditions) and addition of 200 μl of 4% Triton X-100. Extracts were prepared from ZFPL1–GFP-expressing cells firstly by washing the cells in PBS, followed by extraction in 20 mM Hepes, pH 7.4, 0.1 M NaCl, 1 mM DTT, 0.5% Triton X-100 (300 μl per 10 cm dish) for 15 min on ice. Golgi and cell extracts were clarified by centrifugation at 14 K r.p.m. for 15 min at 4°C in a microfuge The appropriate purified IgG (2 μg) was added and samples were incubated for 1–4 h at 4°C followed by 20 μl protein G-Sepharose (50% slurry, Zymed) or protein A-Sepharose (50% slurry, Amersham) for a further 1 h at 4°C.
Pull-down experiments
S-tagged ZFPL1 or golgin-84 (5 μg) were coupled to S protein agarose beads (10 μl) (Novagen) for 1 h at 4°C and incubated with purified recombinant proteins (5 μg) in 100 μl of 20 mM HEPES, pH 7.4, 0.1 M NaCl, 1 mM DTT, 0.5% Triton X-100 for 2 h at 4°C. Beads were washed three times with binding buffer and eluted in SDS sample buffer before analysis by SDS–PAGE and Coomassie blue staining or western blotting with appropriate antibodies.
Supplementary Material
Supplementary Material
Acknowledgments
We thank our colleagues for generously providing reagents as noted above. We are grateful to the University of Manchester Faculty of Life Sciences Biomolecular analysis core facility for performing mass spectrometry. This work was supported by an MRC Senior Non-Clinical Research Fellowship (G117/494) and a Wellcome Trust project grant (068196) awarded to ML and an MRC collaborative grant (G0501725) awarded to ML and Philip Woodman, Stephen High and Viki Allan at the University of Manchester, UK.
References
- Alvarez C, Garcia-Mata R, Hauri HP, Sztul E (2001) The p115-interactive proteins GM130 and giantin participate in endoplasmic reticulum–Golgi traffic. J Biol Chem 276: 2693–2700 [DOI] [PubMed] [Google Scholar]
- Appenzeller-Herzog C, Hauri HP (2006) The ER–Golgi intermediate compartment (ERGIC): in search of its identity and function. J Cell Sci 119: 2173–2183 [DOI] [PubMed] [Google Scholar]
- Bannykh SI, Nishimura N, Balch WE (1998) Getting into the Golgi. Trends Cell Biol 8: 21–25 [DOI] [PubMed] [Google Scholar]
- Barr FA, Nakamura N, Warren G (1998) Mapping the interaction between GRASP65 and GM130, components of a protein complex involved in the stacking of Golgi cisternae. EMBO J 17: 3258–3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr FA, Short B (2003) Golgins in the structure and dynamics of the Golgi apparatus. Curr Opin Cell Biol 15: 405–413 [DOI] [PubMed] [Google Scholar]
- Ben-Tekaya H, Miura K, Pepperkok R, Hauri HP (2005) Live imaging of bidirectional traffic from the ERGIC. J Cell Sci 118: 357–367 [DOI] [PubMed] [Google Scholar]
- Borden KL (2000) RING domains: master builders of molecular scaffolds? J Mol Biol 295: 1103–1112 [DOI] [PubMed] [Google Scholar]
- Diao A, Frost L, Morohashi Y, Lowe M (2007) Coordination of golgin tethering and SNARE assembly: GM130 binds syntaxin 5 in a p115-regulated manner. J Biol Chem; e-pub ahead of print, 31 December 2007 [DOI] [PubMed] [Google Scholar]
- Diao A, Rahman D, Pappin DJ, Lucocq J, Lowe M (2003) The coiled-coil membrane protein golgin-84 is a novel rab effector required for Golgi ribbon formation. J Cell Biol 160: 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemont PS (1993) The RING finger. A novel protein sequence motif related to the zinc finger. Ann NY Acad Sci 684: 174–192 [DOI] [PubMed] [Google Scholar]
- Glick BS (2000) Organization of the Golgi apparatus. Curr Opin Cell Biol 12: 450–456 [DOI] [PubMed] [Google Scholar]
- Hartinger J, Stenius K, Hogemann D, Jahn R (1996) 16-BAC/SDS–PAGE: a two-dimensional gel electrophoresis system suitable for the separation of integral membrane proteins. Anal Biochem 240: 126–133 [DOI] [PubMed] [Google Scholar]
- Hoppener JW, De Wit MJ, Simarro-Doorten AY, Roijers JF, van Herrewaarden HM, Lips CJ, Parente F, Quincey D, Gaudray P, Khodaei S, Weber G, Teh B, Farnebo F, Larsson C, Zhang CX, Calender A, Pannett AA, Forbes SA, Bassett JH, Thakker RV et al. (1998) A putative human zinc-finger gene (ZFPL1) on 11q13, highly conserved in the mouse and expressed in exocrine pancreas. The European Consortium on MEN 1. Genomics 50: 251–259 [DOI] [PubMed] [Google Scholar]
- Kodani A, Sutterlin C (2007) The Golgi protein GM130 regulates centrosome morphology and function. Mol Biol Cell 19: 745–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondylis V, Spoorendonk KM, Rabouille C (2005) dGRASP localization and function in the early exocytic pathway in Drosophila S2 cells. Mol Biol Cell 16: 4061–4072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladinsky MS, Mastronarde DN, McIntosh JR, Howell KE, Staehelin LA (1999) Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J Cell Biol 144: 1135–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Donaldson JG, Schweizer A, Berger EG, Hauri HP, Yuan LC, Klausner RD (1990) Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell 60: 821–836 [DOI] [PubMed] [Google Scholar]
- Mardones GA, Snyder CM, Howell KE (2006) Cis-Golgi matrix proteins move directly to endoplasmic reticulum exit sites by association with tubules. Mol Biol Cell 17: 525–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra P, Maffucci T, Daniele T, Tullio GD, Ikehara Y, Chan EK, Luini A, Beznoussenko G, Mironov A, De Matteis MA (2001) The GM130 and GRASP65 Golgi proteins cycle through and define a subdomain of the intermediate compartment. Nat Cell Biol 3: 1101–1113 [DOI] [PubMed] [Google Scholar]
- Marra P, Salvatore L, Mironov A Jr, Di Campli A, Di Tullio G, Trucco A, Beznoussenko G, Mironov A, De Matteis MA (2007) The biogenesis of the Golgi ribbon: the roles of membrane input from the ER and of GM130. Mol Biol Cell 18: 1595–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Lowe M, Levine TP, Rabouille C, Warren G (1997) The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell 89: 445–455 [DOI] [PubMed] [Google Scholar]
- Nakamura N, Rabouille C, Watson R, Nilsson T, Hui N, Slusarewicz P, Kreis TE, Warren G (1995) Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol 131: 1715–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJ, Lippincott-Schwartz J (1997) ER-to-Golgi transport visualized in living cells. Nature 389: 81–85 [DOI] [PubMed] [Google Scholar]
- Puthenveedu MA, Bachert C, Puri S, Lanni F, Linstedt AD (2006) GM130 and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat Cell Biol 8: 238–248 [DOI] [PubMed] [Google Scholar]
- Puthenveedu MA, Linstedt AD (2004) Gene replacement reveals that p115/SNARE interactions are essential for Golgi biogenesis. Proc Natl Acad Sci USA 101: 1253–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J, Svensson K (1991) Distribution of the intermediate elements operating in ER to Golgi transport. J Cell Sci 100 (Part 3): 415–430 [DOI] [PubMed] [Google Scholar]
- Scales SJ, Pepperkok R, Kreis TE (1997) Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell 90: 1137–1148 [DOI] [PubMed] [Google Scholar]
- Schweizer A, Fransen JA, Matter K, Kreis TE, Ginsel L, Hauri HP (1990) Identification of an intermediate compartment involved in protein transport from endoplasmic reticulum to Golgi apparatus. Eur J Cell Biol 53: 185–196 [PubMed] [Google Scholar]
- Seemann J, Jokitalo E, Pypaert M, Warren G (2000a) Matrix proteins can generate the higher order architecture of the Golgi apparatus. Nature 407: 1022–1026 [DOI] [PubMed] [Google Scholar]
- Seemann J, Jokitalo EJ, Warren G (2000b) The role of the tethering proteins p115 and GM130 in transport through the Golgi apparatus in vivo. Mol Biol Cell 11: 635–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter J, Warren G (1999) A role for the vesicle tethering protein, p115, in the post-mitotic stacking of reassembling Golgi cisternae in a cell-free system. J Cell Biol 146: 57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter J, Warren G (2002) Golgi architecture and inheritance. Annu Rev Cell Dev Biol 18: 379–420 [DOI] [PubMed] [Google Scholar]
- Slusarewicz P, Nilsson T, Hui N, Watson R, Warren G (1994) Isolation of a matrix that binds medial Golgi enzymes. J Cell Biol 124: 405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szul T, Grabski R, Lyons S, Morohashi Y, Shestopal S, Lowe M, Sztul E (2007) Dissecting the role of the ARF guanine nucleotide exchange factor GBF1 in Golgi biogenesis and protein trafficking. J Cell Sci 120: 3929–3940 [DOI] [PubMed] [Google Scholar]
- Wang Y, Satoh A, Warren G (2005) Mapping the functional domains of the Golgi stacking factor GRASP65. J Biol Chem 280: 4921–4928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Seemann J, Pypaert M, Shorter J, Warren G (2003) A direct role for GRASP65 as a mitotically regulated Golgi stacking factor. EMBO J 22: 3279–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material