Abstract
Histone deacetylase 3 (Hdac3) is an enzymatic component of transcriptional repression complexes recruited by the nuclear hormone receptors. Inactivation of Hdac3 in cancer cell lines triggered apoptosis, and removal of Hdac3 in the germ line of mice caused embryonic lethality. Therefore, we deleted Hdac3 in the postnatal mouse liver. These mice developed hepatomegaly, which was the result of hepatocyte hypertrophy, and these morphological changes coincided with significant imbalances between carbohydrate and lipid metabolism. Loss of Hdac3 triggered changes in gene expression consistent with inactivation of repression mediated by nuclear hormone receptors. Loss of Hdac3 also increased the levels of Pparγ2, and treatment of these mice with a Pparγ antagonist partially reversed the lipid accumulation in the liver. In addition, gene expression analysis identified mammalian target of rapamycin signalling as being activated after deletion of Hdac3, and inhibition by rapamycin affected the accumulation of neutral lipids in Hdac3-null livers. Thus, Hdac3 regulates metabolism through multiple signalling pathways in the liver, and deletion of Hdac3 disrupts normal metabolic homeostasis.
Keywords: chromatin, Hdac3, histone acetylation, metabolism
Introduction
Histone deacetylases (HDACs) are classified based on sequence similarity to yeast homologues. Class I HDACs are most similar to yeast Rpd3 and are ubiquitously expressed in many tissues. These enzymes localize primarily to the nucleus and include HDAC 1, 2, 3, and 8. Class II HDACs, which include HDACs 4–7 and 9–11, are related to yeast Hda1 and are expressed in a more tissue-specific manner (de Ruijter et al, 2003). Although the putative catalytic domain of class II HDACs is highly homologous to that of class I enzymes, the vertebrate class IIa HDACs (4, 5, 7, and 9) have little intrinsic enzymatic activity, but associate with HDAC3 (Fischle et al, 2002; Gallinari et al, 2007; Lahm et al, 2007). Sirtuins (Sir) are a third class of HDACs, homologous to the yeast Sir2 enzyme, and differ from the first two classes of HDACs in their requirement for NAD+ for both deacetylase and ADP-ribosylation activities (Gartenberg, 2000; Imai et al, 2000).
Gene deletion studies have begun to define the individual physiological roles of class I HDACs. Targeted deletion of Hdac1 in mice led to embryonic lethality by E10.5, due to proliferation defects, and these defects were also found in cultured embryonic stem cells (Lagger et al, 2002; Zupkovitz et al, 2006). Inactivation of Hdac2 in mice resulted in a low percentage of lethality during embryogenesis, but almost half of Hdac2-null pups died within a month after birth due to a defect in heart development. Hdac2-null mice that survived to a later age had an apparent proliferation defect in the heart tissue and failed to respond normally to hypertrophic induction (Trivedi et al, 2007). These genetic models demonstrate the importance of understanding the function of individual HDACs, as well as the tissue-specific requirements for each enzyme.
HDAC3 is an enzymatic component of the nuclear receptor co-repressor complexes that contain N-CoR (nuclear hormone co-repressor) and SMRT (silencing mediator for retinoid and thyroid receptors), which are recruited by nuclear hormone receptors to regulate transcription in the absence of hormone (Li et al, 2000; Guenther et al, 2001; Yoon et al, 2003). Thus, HDAC3 may be required for the normal physiological action of many nuclear hormone receptors and is the critical catalytic component when nuclear hormone receptor functions are disrupted in cancer. For example, the retinoic acid receptor is disrupted by the t(15;17) in acute promyelocytic leukaemia (Alcalay et al, 1991) and the action of HDAC3 may be required for leukaemogenesis (Grignani et al, 1998; Guidez et al, 1998; He et al, 1998; Lin et al, 1998). Similarly, other leukaemia-related factors such as the t(12;21) and t(8;21) fusion proteins recruit HDAC3, suggesting that this enzyme might be a key therapeutic target in paediatric B-cell acute lymphocytic leukaemia and acute myeloid leukaemia (Chakrabarti and Nucifora, 1999; Fenrick et al, 1999, 2000; Guidez et al, 2000; Amann et al, 2001; Wang and Hiebert, 2001). Indeed, both natural and synthetic histone deacetylase inhibitors (HDIs) are being tested clinically as anticancer agents for the treatment of leukaemia and a variety of solid tumours (Santini et al, 2007).
Disruption of HDAC3 function in cell lines, by either gene deletion or RNAi in tumour cell lines, suggested that HDAC3 was required for maintaining cell viability (Takami and Nakayama, 2000; Glaser et al, 2003; Li et al, 2006). By contrast, primary cells treated with HDIs arrested cell cycle progression, but were more resistant to apoptosis (Papeleu et al, 2003). Germline deletion of Hdac3 triggered early embryonic lethality (Bhaskara et al, 2008); therefore, we genetically engineered mice to conditionally delete Hdac3 in the liver using transgenic mice expressing the interferon-inducible Mx1-Cre recombinase (Mx1-Cre) or the liver-specific albumin-Cre recombinase (Alb-Cre). Although there was no dramatic increase in either apoptosis or proliferation due to the initial loss of Hdac3, the liver became enlarged over time, which appeared to be due to hepatocyte hypertrophy. In addition, genes that regulate lipid and cholesterol biosynthesis were de-repressed, which in turn resulted in a dramatic increase in both liver and serum levels of triglycerides and cholesterol. Many of these genes are controlled by nuclear hormone receptors, such as peroxisome proliferator-activated receptor gamma (PPARγ) and thyroid hormone receptor (THR), which utilize N-CoR or SMRT and Hdac3 to repress transcription. Multiple signalling pathways contribute to the abnormal metabolic phenotype, as demonstrated by inhibition of Pparγ or mammalian target of rapamycin (mTOR) in vivo. Thus, Hdac3 is required for regulating liver metabolic homeostasis.
Results
Liver-specific deletion of Hdac3 results in organ hypertrophy
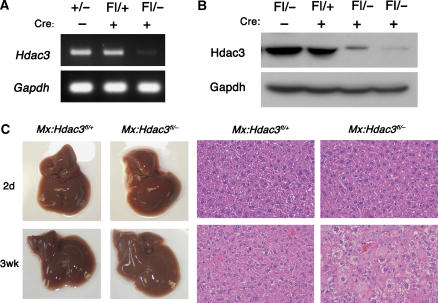
Deletion of Hdac3 in the germ line caused early embryonic lethality (Bhaskara et al, 2008). Therefore, to understand the physiological role of Hdac3 in adult tissue homeostasis, we conditionally deleted Hdac3 using Cre recombinase-expressing transgenic mice and a ‘floxed' allele of Hdac3 (Supplementary Figure S1). Initially, Hdac3 heterozygous (+/−) and floxed (fl/+) mice were crossbred with transgenic Mx1-Cre mice. To generate tissue-specific heterozygous (Mx1-Cre; Hdac3fl/+, hereafter referred to as Mx:Hdac3fl/+) or null (Mx1-Cre; Hdac3fl/−, hereafter referred to as Mx:Hdac3fl/−) mice, Mx1-Cre expression was stimulated by injecting synthetic double-stranded RNA (polyinosinic-polycytidylic acid (pIpC)) to induce interferon (Kuhn et al, 1995). Two days after the final injection of pIpC, RNA was extracted from the liver tissue of Mx:Hdac3fl/− mice and analysed by RT–PCR to confirm inactivation of Hdac3 (Figure 1A). Nuclear lysates from liver samples demonstrated a dramatic decrease in Hdac3 levels in Mx:Hdac3fl/− mice (Figure 1B). Two weeks after the final pIpC injection, an increase in liver size and weight of up to two-fold was noted in Mx:Hdacfl/− mice (Figure 1C). Histological analysis of Mx:Hdac3fl/− livers revealed little change within 2 days of the last injection, but there was a gradual increase in a mosaic pattern of hypertrophic hepatocytes with grainy cytoplasm, which peaked within 2 weeks of the last pIpC injection (Figure 1C).
Figure 1.
Inducible deletion of Hdac3 using Mx1-Cre. (A) RT–PCR was used to detect Hdac3 mRNA levels in whole-liver extracts and (B) western blot analysis detected Hdac3 protein in whole-cell lysates of livers from mice of the indicated genotypes 2 days after the final injection of pIpC. (C) Gross morphology (left-hand panels) of control and Hdac3-null livers at 2 days and 3 weeks after pIpC injection. The right-hand panels show H&E-stained sections ( × 400) from the livers shown in the left-hand panels.
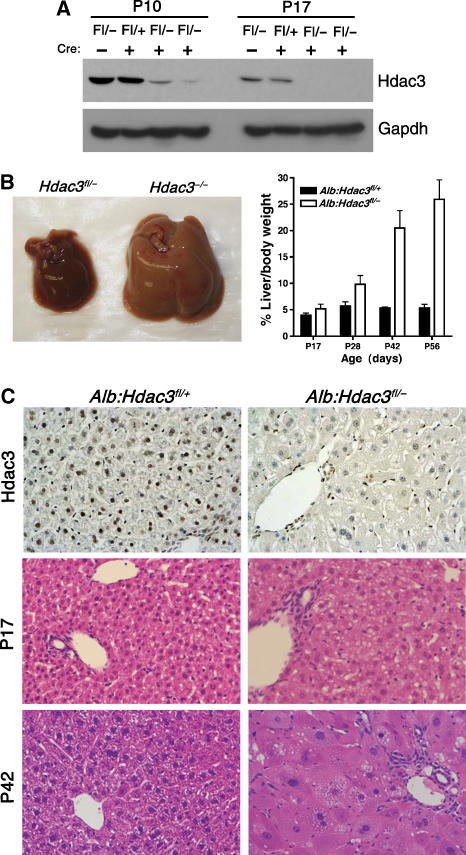
To complement the analysis with the Mx1-Cre model and to avoid possible side effects of interferon signalling affecting liver function, we crossbred Hdac3fl/fl and Hdac3+/− mice with mice expressing Cre recombinase under the control of the albumin promoter (Alb-Cre) to generate heterozygous (Alb-Cre; Hdac3fl/+, hereafter referred to as Alb:Hdac3fl/+) and null (Alb-Cre; Hdac3fl/−, hereafter referred to as Alb:Hdac3fl/−) offspring. Alb-Cre is expressed in parenchymal liver cells, resulting in roughly 40% recombination in hepatocytes at birth and almost complete recombination by 2 weeks after birth (Postic et al, 1999). Although Hdac3 is required for cell viability in vitro, Alb:Hdac3fl/− mice were viable, even with observable decreases in Hdac3 mRNA and protein levels occurring as early as postnatal day 4 (P4; data not shown). Recombination was confirmed by PCR to detect the floxed and null alleles (data not shown). Western blot analysis indicated that Hdac3 levels were dramatically decreased 10 days after birth and were undetectable by P17 in Alb:Hdac3fl/− mice (Figure 2A). Western blot and RT–PCR analysis also indicated that the levels of Hdac1 or Hdac2 were not upregulated to compensate for the loss of Hdac3 in Alb:Hdac3fl/− livers (data not shown).
Figure 2.
Constitutive deletion of Hdac3 using liver-specific Alb-Cre. (A) Hdac3 western blot analysis of liver extracts of 10- or 17-day-old mice of the indicated genotypes. (B) Deletion of Hdac3 results in liver hypertrophy over time. Representative photographs of P42 control and Hdac3-null livers are shown. The graph depicts the ratio of liver weight to gross animal weight between 17 and 56 days of age (P17, P=0.0001; P28, P=0.0001; P42, P=0.0013; P56, P=0.0001). Statistics were generated using the Student's t-test. (C) Immunohistochemistry for Hdac3 in P28 liver tissue ( × 200) is shown in the upper panels, and histological analysis using H&E-stained sections ( × 400) of Hdac3-null livers isolated from mice of the indicated ages is shown in the lower panels. Littermate controls are depicted in the left-hand panels.
At P17 the livers appeared normal, but by P28 the livers were pale and hypertrophic, and this trend continued into adulthood. Although the pups were weaned at P21, it is unlikely that the altered morphology was due to the weaning transition, as similar changes were observed in the Mx1-Cre model (Figure 1C). The ratio of liver weight to body weight increased from roughly 5% in the heterozygous mice to 25–30% in Alb:Hdac3fl/− mice (Figure 2B), and immunohistochemical staining demonstrated complete loss of Hdac3 in hepatocytes (Figure 2C, upper panels). Haematoxylin and eosin (H&E)-stained liver sections indicated that, similar to the Mx1-Cre model (Figure 1), Alb:Hdac3fl/− livers contained hepatocytes with abnormal cytoplasm starting at P17, and later became hypertrophic (Figure 2C, lower panels).
Deletion of Hdac3 leads to increased hepatocellular damage
The increase in hepatocyte size correlated with the increased organ size, suggesting that altered cell size rather than increased cell number was the basis of the hepatomegaly. To test this model, we examined cell death and proliferation in Alb:Hdac3fl/− mice. At P17, there was no significant increase in the number of proliferating cells, as measured by Ki67 immunohistochemistry (Supplementary Figure S2A) or BrdU incorporation (Supplementary Figure S2B). At 6–8 weeks after birth when the number of cycling cells in the livers of control mice had decreased to ∼1%, Hdac3-null livers retained increased numbers of BrdU-positive hepatocytes (Supplementary Figure S2B). To assess the amount of cellular death and damage occurring at P17, we used TUNEL to detect apoptosis and serum alanine transaminase (ALT) levels to detect hepatotoxicity. The numbers of TUNEL-positive cells were uniformly low and no increase was detected in the P17 (Supplementary Figure S2C) or P28 (data not shown) Hdac3-null livers. By contrast, ALT levels were already elevated at P17 and remained high over time (Supplementary Figure S2D), which indicated cellular damage. This may suggest that the increase in cycling cells detected at P28 in Hdac3-null livers was a response to cellular damage.
Loss of Hdac3 disrupts metabolic homeostasis
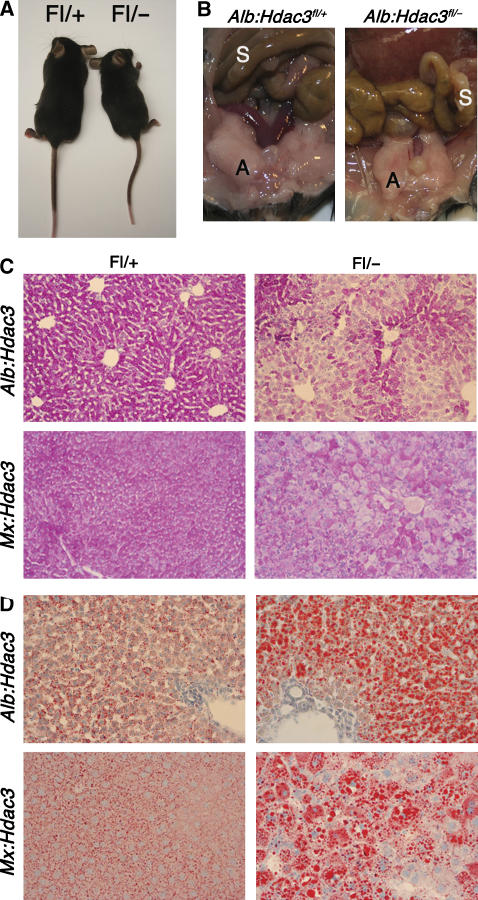
By 4–6 weeks of age, Alb:Hdac3fl/− mice were smaller than their littermates and this general growth defect was maintained into adulthood, suggesting a possible defect in metabolism (Figure 3A). By 6–8 weeks of age, Alb:Hdac3fl/− mice were visibly leaner, and their visceral adipose tissue was substantially reduced (Figure 3B). Histological analysis of liver sections for glycogen storage using periodic acid Schiff (PAS) demonstrated a dramatic depletion of glycogen in both Alb:Hdac3fl/− and Mx:Hdac3fl/− hepatocytes compared to heterozygous littermates of the same age (Figure 3C). By contrast, when liver sections from these mice were stained for neutral lipids using Oil Red O, there was a significant increase in the amount of lipid in both Alb:Hdac3fl/− and Mx:Hdac3fl/− mice compared to control livers (Figure 3D), demonstrating an imbalance between carbohydrate and lipid metabolism.
Figure 3.
Loss of Hdac3 significantly alters metabolism. (A) Alb:Hdac3fl/− mice are smaller than littermate controls, starting at 4 weeks of age. Control animal is depicted to the left in the photo. (B) Representation of the decrease in visceral adipose tissue in 15-week-old female Alb:Hdac3fl/− mice (right panel). S, small intestine; A, visceral adipose tissue. Note that the liver in the Alb:Hdac3fl/− mouse extends into the photo whereas the normal-sized liver in the wild type is not visible in this frame. (C) Decrease in glycogen as detected by PAS stain and (D) accumulation of lipid droplets as detected by Oil Red O stain of Alb:Hdac3fl/− (P17) and Mx:Hdac3fl/− (2 weeks after final pIpC injection) livers ( × 100). Littermate controls are shown in the left-hand panels.
Metabolism was further examined by quantifying the levels of triglycerides, cholesterol, free fatty acids, HDL, and LDL in both the liver and the serum of Alb:Hdac3fl/− mice by mass spectrometry. By P17, the levels of triglycerides in Alb:Hdac3fl/− liver samples were dramatically increased (up to 17-fold) compared to controls, and remained continuously high as the mice aged (Table I). Total tissue cholesterol gradually increased in Hdac3-null livers, accumulating to levels two-fold higher than in control mice, including unesterified cholesterol (data not shown) and cholesterol esters (Table I). Likewise, there was a very significant increase in serum triglycerides, total serum cholesterol, and LDL in Alb:Hdac3fl/− mice as they aged (Table II). Thus, the export of lipids and cholesterol from the liver was not inhibited due to the loss of Hdac3, and the synthesis of lipids and cholesterol in the liver was increased, leading to their accumulation in the tissue and serum.
Table 1.
Quantification of metabolic parameters in Alb:Hdac3 liver tissue
| Parameter | Age | Alb:Hdac3fl/+ | Alb:Hdac3fl/− | P-value |
|---|---|---|---|---|
| TG | P17 | 3.634±0.821 | 57.674±29.710 | 0.0036 |
| P28 | 3.03±0.996 | 22.6275±9.343 | 0.017 | |
| P42 | 6.03±3.156 | 20.75±5.836 | 0.018 | |
| P56 | 3.3025±1.352 | 15.05±2.813 | 0.0003 | |
| FFA | P17 | 1.29±0.057 | 1.32±0.099 | NC |
| P28 | 0.737±0.345 | 1.3025±0.229 | 0.046 | |
| P42 | 0.463±0.153 | 0.633±0.248 | 0.37 | |
| P56 | 0.895±0.295 | 1.2175±0.392 | 0.24 | |
| T. Chol. | P17 | 3.34±1.300 | 1.706±0.472 | 0.03 |
| P28 | 1.203±0.188 | 1.5825±0.068 | 0.012 | |
| P42 | 1.823±0.485 | 2.786±0.710 | 0.12 | |
| P56 | 1.3325±0.106 | 2.81±0.314 | 0.0001 | |
| CE | P17 | 1.365±0.403 | 0.61±0.226 | 0.0071 |
| P28 | 0.453±0.153 | 0.695±0.223 | 0.17 | |
| P42 | 0.476±0.227 | 1.016±0.235 | 0.046 | |
| P56 | 0.3375±0.075 | 1.5±0.195 | 0.0001 | |
| Averages were calculated (n=at least 4) and include standard deviations for indicated time points. All values are expressed in units of μg/mg. TG, triglycerides; FFA, free fatty acids; T. Chol., total cholesterol; CE, cholesterol esters; NC, no change; P-values were calculated using Student's t-test. | ||||
Table 2.
Quantification of metabolic parameters in Alb:Hdac3 serum
| Parameter | Age | Alb:Hdac3fl/+ | Alb:Hdac3fl/− | P-value |
|---|---|---|---|---|
| TG | P17 | 58.333±6.501 | 145.2±42.833 | 0.0021 |
| P28 | 73±15.133 | 90±38 | 0.51 | |
| P42 | 87.333±23.714 | 234.667±131.5 | 0.13 | |
| P56 | 108.75±25.395 | 403±139.817 | 0.0061 | |
| T. Chol. | P17 | 144.5±12.582 | 146.4±32.083 | 0.9 |
| P28 | 93.333±13.051 | 182.667±24.007 | 0.0048 | |
| P42 | 95.667±14.224 | 236.333±31.005 | 0.002 | |
| P56 | 109.25±14.268 | 327±32.424 | 0.0001 | |
| HDL | P17 | 55.667±7.638 | 33.667±11.93 | 0.055 |
| P28 | 36±5.568 | 53.333±8.145 | 0.038 | |
| P56 | 42.25±4.193 | 60a | ||
| LDL | P17 | 84.333±14.572 | 100.333±21.825 | 0.35 |
| P28 | 42.667±10.97 | 111.333±12.858 | 0.0021 | |
| P56 | 45.25±10.404 | 260a | ||
| >Averages were calculated (n=at least 4) and include standard deviations for indicated time points. All values are expressed in units of mg/dl. TG, triglycerides; T. Chol., total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein. | ||||
| aTG level over 400 mg/dl invalidates calculations for LDL and HDL, so only select mice could be used in the analysis. | ||||
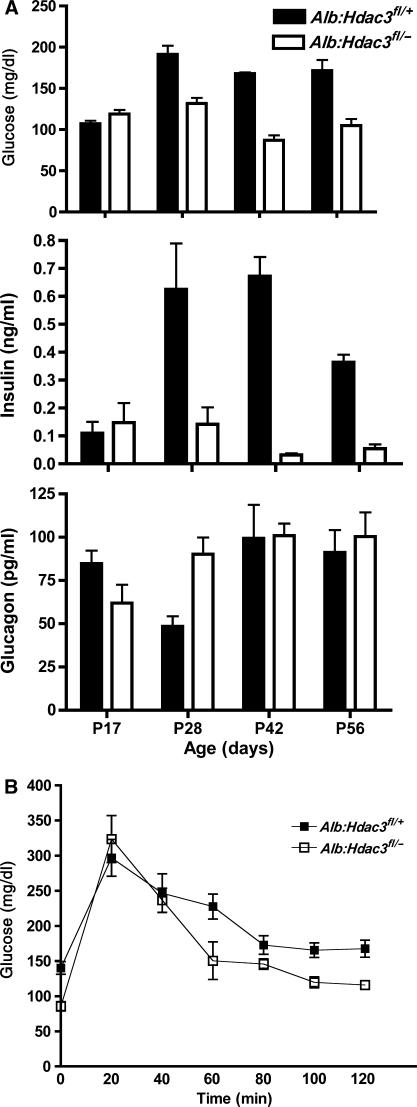
Because of the increase in serum triglycerides and LDL starting in mice only 17 days old, we investigated the effects these increases would have on the levels of glucose and its regulatory hormones. In P17 animals, we found similar levels of glucose and insulin in the serum of control and Alb:Hdac3fl/− mice, and slightly lower glucagon levels in Hdac3 liver-specific knockout mice. However, by P28, glucose levels had decreased in Alb:Hdac3fl/− mice and, by 6 weeks of age, the mice were hypoglycaemic with only half of the normal serum glucose levels. The hypoglycaemia was accompanied by dramatically lower levels of insulin beginning at P28 that persisted as Alb:Hdac3fl/− mice aged. Glucagon levels increased by almost two-fold in the P28 Alb:Hdac3fl/− animals, but returned to levels similar to control animals by 6 weeks of age, even though the glucose and insulin levels were still significantly lower than normal levels (Figure 4A).
Figure 4.
Alb:Hdac3fl/− mice are hypoglycaemic and are insulin sensitive. (A) Graphical representation of whole-blood measurements of glucose (P17, P=0.065; P28, P=0.0001; P42, P=0.0002; P56, P=0.0006) and the serum levels of insulin (P17, P=0.63; P28, P=0.02; P42, P=0.0001; P56, P=0.0001) and glucagon (P17, P=0.062; P28, P=0.021; P42, P=0.9; P56, P=0.66) at the indicated time points in Alb:Hdac3 mice. (B) The glucose tolerance test was performed in 10-week-old Alb:Hdac3 mice, which were fasted for 6 h before a dose of 1.5 mg/g glucose. Glucose levels were measured at the indicated time points over a 2-h period. (0′, P=0.0012; 20′, P=0.54; 40′, P=0.78; 60′, P=0.054; 80′, P=0.14; 100′, P=0.013; 120′, P=0.0082). Statistics were generated using the Student's t-test.
The hypoglycaemia and the low insulin levels suggested that Alb:Hdac3fl/− mice were hypersensitive to insulin. Therefore, we performed a glucose tolerance test on both control and Alb:Hdac3fl/− mice. In 10-week-old animals, the fasting glucose levels in Hdac3 liver-specific null mice were almost two-fold lower than that in littermate control mice (Figure 4B, 0 min time point). Twenty minutes after the injection of glucose, the levels of glucose peaked in a similar fashion in both control and Alb:Hdac3fl/− animals, and returned to near-basal levels within 2 h, with a subtly quicker response in Alb:Hdac3fl/− mice (Figure 4B). Thus, despite hypoglycaemia and the high levels of triglycerides and cholesterol in Alb:Hdac3fl/− mice, these animals responded normally to an insulin-dependent glucose challenge.
Lipid and cholesterol biosynthesis regulatory genes are de-repressed after inactivation of Hdac3
A major function of HDACs is the regulation of transcription through deacetylation of histones and non-histone proteins. Therefore, to define the mechanism by which inactivation of Hdac3 affected liver function, we compared gene expression in the livers of Alb:Hdac3+/+, Alb:Hdac3fl/+, and Alb:Hdac3fl/− mice. At P17, Hdac3-null livers showed only modest alterations in gross organ morphology, and the liver was no longer populated by Hdac3-expressing haematopoietic progenitor cells, whereas P28 mice displayed more morphological changes. Thus, we used cDNA microarray analysis at these two time points to address primary and secondary changes in gene expression patterns.
At P17, the major classes of de-regulated genes were involved in metabolism including lipid, fatty acid, steroid, and carbohydrate production, metabolic transport, and cytochromes P450 (Table III). Q-RT–PCR of alternate mRNA samples showed excellent correspondence between the changes observed in the microarray assays and manual validation (Supplementary Figure S4). A large number of these genes are regulated by nuclear hormone receptors, such as PPARs, THR, liver X receptor, and retinoid X receptor. Of significance, a number of key rate-limiting enzymes that control metabolic functions were upregulated. For example, acetyl CoA carboxylase (Acacb) regulates the amount of fatty acids in the cell (Widmer et al, 1996), and its expression was increased ∼21-fold. Squalene epoxidase (Sqle, ∼173-fold increase) regulates one of the final catalytic reactions of cholesterol biosynthesis (Xu et al, 2005; Clapham and Arch, 2007). The final step in the synthesis of cholesterol is catalysed by sterol 14 α-demethylase (Debeljak et al, 2003) (also known as Cyp51), and the mRNA levels for Cyp51 were ∼22-fold higher in Hdac3-null livers (Table III). Thus, Hdac3 is required for the regulation of genes that control key steps in lipid and cholesterol biosynthesis early in the postnatal liver.
Table 3.
Transcription changes in Alb:Hdac3fl/− mice at P17 and P28
| Biological process | Gene name (gene symbol) | Average fold change | |
|---|---|---|---|
| P17 | P28 | ||
| Metabolism | Acetyl-Coenzyme A Carboxylase beta (Acacb) | 21.5850 | 2.8755 |
| Lipid/fatty acid | Enoyl-Coenzyme A hydratase/3-hydroxyacyl Co-A dehydrogenase (Ehhadh) | 3.6582 | 12.4253 |
| Stearoyl-Coenzyme A desaturase 1 (Scd1) | 3.2483 | 9.1621 | |
| Acyl-Coenzyme A oxidase 1, palmitoyl (Acox1) | 3.8679 | 2.6733 | |
| Adipose differentiation related protein (Adfp) | 3.9698 | 9.2127 | |
| Apolipoprotein A-IV (Apoa4) | 11.6171 | 32.6144 | |
| Sterol-C4-methyl oxidase-like (Sc4mol) | 24.2809 | −2.130 | |
| CD36 antigen (Cd36) | 1.8733 | 5.6965 | |
| ELOVL family member 6, elongation of long chain fatty acids | 9.6841 | 5.6540 | |
| Fatty acid desaturase 2 (Fads2) | 2.6065 | 3.3344 | |
| Fatty acid synthase (Fasn) | 5.4032 | 4.3510 | |
| Phospholipase A2, group VI (Pla2g6) | 4.1585 | 3.6305 | |
| Acetoacetyl-CoA synthetase (Aacs) | 5.9526 | 1.0360 | |
| Low density lipoprotein receptor (Ldlr) | 2.4232 | −1.2550 | |
| Very low density lipoprotein receptor (Vldlr) | 6.4971 | 5.2987 | |
| StAR-related lipid transfer (START) domain containing 4 (Stard4) | 3.2569 | 1.1132 | |
| Cytochrome P450, family 4, subfamily a, polypeptide 12 (Cyp4a12) | 27.7789 | 6.9124 | |
| Cytochrome P450, family 4, subfamily a, polypeptide 14 (Cyp4a14) | 3.0869 | 2.7802 | |
| Cytochrome P450, family 7, subfamily a, polypeptide 1 (Cyp7a1) | 2.5207 | 3.9425 | |
| Cholesterol | 3-Hydroxy-3-methylglutaryl-Coenzyme A synthase (Hmgcs) | 7.9894 | 1.2339 |
| Isopentenyl-diphosphate delta isomerase (Idi1) | 18.2946 | −2.2900 | |
| Farnesyl diphosphate farnesyl transferase 1 (Fdft1) | 5.4985 | −1.0576 | |
| Farnesyl diphosphate synthetase (Fdps) | 13.7628 | 1.7472 | |
| Squalene epoxidase (Sqle) | 172.9337 | −1.5849 | |
| Cytochrome P450, 51 (Cyp51) | 21.9741 | −1.8527 | |
| Other | Aldolase 1 (Aldo1) | 2.7904 | |
| Glucokinase (Gck) | 2.6046 | 2.7178 | |
| Hexokinase 2 (Hk2) | 7.1749 | ||
| Insulin induced gene 1 (Insig1) | 4.7532 | −1.4560 | |
| Insulin-like 6 (Insl6) | 2.5668 | 3.3368 | |
| Aldehyde oxidase 1 (Aox1) | 3.1535 | 1.4061 | |
| Xanthine dehydrogenase (Xdh) | 2.1399 | 1.0452 | |
| Aldehyde dehydrogenase family 3, subfamily A2 (Aldh3a2) | 2.5317 | 3.3068 | |
| Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) (345274) | 2.1606 | 2.6829 | |
| Nuclear receptor-related | Serum/glucocorticoid regulated kinase 2 (Sgk2) | 3.8111 | 2.6817 |
| Thyroid hormone responsive SPOT14 homolog (Thrsp) | 33.7453 | 2.4455 | |
| Retinoic acid early transcript gamma (Raet1b) | 6.4309 | 3.6549 | |
| Retinoic acid early transcript delta (Raet1d) | 6.4457 | 3.4374 | |
| Glucocorticoid modulatory element binding protein 1 (Gmeb1) | 2.1215 | ||
| Glucocorticoid induced gene 1 (Gig1) | 2.7644 | ||
| Dopa decarboxylase (Ddc) | 3.2737 | 3.1596 | |
| Peroxisome proliferator activated receptor gamma (Pparγ) | 4.7764 | ||
| Cellular regulation | Dual-specificity tyrosine-(Y)-phosphorylation regulated kinase 3 (Dyrk3) | 3.2766 | 7.4758 |
| Inositol 1,3,4-triphosphate 5/6 kinase (Itpk1) | 2.8298 | 5.1727 | |
| Dual specificity phosphatase 8 (Dusp8) | 2.1698 | 3.9070 | |
| Protein kinase C, alpha (Prkca) | 3.3297 | ||
| Ras homolog gene family, member B (Rhob) | 3.4098 | ||
| Insulin-like growth factor 2 (Igf2) | 6.9211 | ||
| Mitogen activated protein kinase 3 (Mapk3) | 2.3059 | ||
| Cell cycle | Cyclin A2 (Ccna2) | 2.6210 | |
| Cyclin-dependent kinase 6 (Cdk6) | 3.2699 | 2.4362 | |
| Cyclin-dependent kinase inhibitor 1A/P21 (Cdkn1a) | 12.1251 | ||
| Cyclin-dependent kinase inhibitor 2A/P16 (Cdkn2a) | 3.8940 | ||
| Myelocytomatosis oncogene (Myc) | 4.0527 | ||
| Rous sarcoma oncogene (Src) | 4.7890 | ||
| Jun oncogene (Jun) | 3.2893 | ||
| Jun proto-oncogene related gene d1(Jund1) | 2.7052 | 3.0491 | |
| Polo-like kinase 2 (Plk2) | 2.5635 | ||
| Polo-like kinase 3 (Plk3) | 3.1039 | 5.1568 | |
| Polo-like kinase 4 (Plk4) | 2.5163 | ||
| Topoisomerase (DNA) II alpha (Top2a) | 7.1831 | ||
| Cytoskeletal/structural | Keratin complex 1, acidic, gene 18 (Krt1-18) | 4.3301 | 10.0402 |
| Keratin complex 2, basic, gene 8 (Krt2-8) | 4.3515 | 9.1991 | |
| Procollagen, type I, alpha 1 (Col1a1) | 4.9802 | 16.6567 | |
| Periplakin (Ppl) | 3.0304 | 3.0085 | |
| Tubulin, alpha 8 (Tuba8) | 3.3211 | 3.4593 | |
| Tubulin, beta 2 (Tubb2) | 1.9632 | 32.2436 | |
| Actin, gamma, cytoplasmic (Actg) | 2.5746 | ||
Although the majority of genes that were upregulated at P17 are related to metabolism, a number of transcription factors, including nuclear hormone receptor gene targets, and cell signalling regulatory molecules were also de-regulated (Table III). In addition, genes that contribute to the PI3K/Akt and MAP/ERK pathways, which are linked to cellular stress and cell size regulation, increased from P17 to P28. Genes encoding structural proteins, such as actin and keratin, became increasingly upregulated at p28 (Table III), which correlated with increased cell size, and these changes in gene expression were more likely due to the necessary compensation of the cell to support its abnormal growth than due to transcriptional control by Hdac3. Thus, the microarray data demonstrate that Hdac3 is not only a key regulator of metabolism in the liver, but may also be required for cross-talk between multiple pathways, which leads to complex secondary changes in hepatocellular gene expression over time.
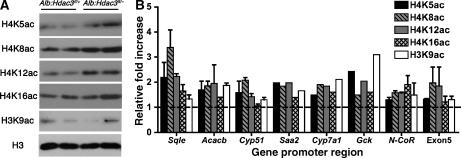
Hdac3 inactivation affects histone acetylation
To further probe the primary molecular mechanism underlying the altered liver function in Alb:Hdac3fl/− mice, we examined global histone acetylation in vivo by western blot analysis. At 17 days after birth when there is complete depletion of Hdac3 and dramatic changes in gene expression profiles, we found modest increases in the acetylation of histone H4K5, H4K8, and H4K12, but variable changes in H3K9 (Figure 5A). Similar results were found using chromatin immunoprecipitation (ChIP) of promoter regions of genes upregulated in the microarray analysis (Figure 5B). Although some N-terminal histone residues have been identified as preferential substrates for Hdac3 (Hartman et al, 2005), the promoter regions of de-repressed genes, such as Sqle and Acacb, had a general increase in acetylated histone residues. There was also increased acetylation in the coding region of Cyp51 (exon 5) and at the promoter of N-CoR, which was not transcriptionally activated in the absence of Hdac3 (Figure 5B). General increases in acetylation at both the global level and at specific genomic regions suggest that altered histone acetylation contributes to the changes in gene expression in Hdac3-null livers, although hyperacetylation of other proteins may also be a contributing factor.
Figure 5.
Histone acetylation increases in Alb:Hdac3fl/− mice. (A) Analysis of histone modifications in P17 liver nuclear lysates with the specified genotypes using the indicated antibodies. (B) ChIP of promoters in livers of genes identified as upregulated through microarray analysis and non-transcriptionally activated sequences using antibodies to the indicated acetylated histone residues. Exon5 refers to sequences in exon 5 of Cyp51. Data are shown relative to the levels of histone H3, and the increase in Alb:Hdac3fl/− mice over controls is denoted by the dashed line.
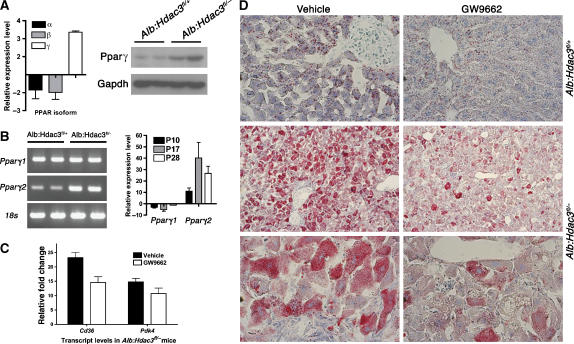
Loss of Hdac3 increases Pparγ expression and activity in hepatocytes
Gene expression analysis identified multiple transcriptional networks that were disrupted at both the P17 and P28 time points and are regulated by nuclear hormone receptors that recruit Hdac3. At P17, one of these networks was centered around Pparγ, which is not the predominant Ppar family member normally expressed in the liver (Zhu et al, 1993). By P28, an even greater number of Pparγ targets were upregulated, as part of a larger network regulated by nuclear hormone receptors (Supplementary Figure 3). Pparγ is a key regulator of metabolic homeostasis, specifically in adipocytes, and this hormone receptor recruits the Hdac3/N-CoR repression complex (Fajas et al, 2002; Guan et al, 2005). Although not normally highly expressed in the liver, the transcript level of Pparγ, but not Pparα or Pparβ, was increased by ∼3-fold, and Pparγ protein levels were also increased in Alb:Hdac3fl/− mice (Figure 6A). In addition, Pparγ was upregulated four-fold in Mx:Hdac3fl/− mice up to 2 weeks after the last injection of pIpC (data not shown). Pparγ exists in two isoforms (Pparγ1 and Pparγ2), which differ in their N-termini (Zhu et al, 1993) and have differential functions and activities (Werman et al, 1997; Ren et al, 2002). Using oligonucleotide primers specific to Pparγ1 and Pparγ2 for Q-RT–PCR, we found that Pparγ2 expression was specifically induced as early as P10 in Alb:Hdac3fl/− livers (Figure 6B), which coincided with increased levels of Pparγ-regulated genes, such as Cd36 (Table III).
Figure 6.
Pparγ is upregulated in the absence of Hdac3. (A) Detection of Ppar isoforms (Pparα, Pparβ, and Pparγ) in P17 Hdac3-null livers by Q-RT–PCR, and western blot analysis of Pparγ in liver whole-cell lysates at P28. Heterozygous littermates were used as controls. (B) Differential regulation of the two different isoforms of Pparγ in 17-day Alb:Hdac3 mice is shown in the left-hand panel. Q-RT–PCR of Pparγ1 and Pparγ2 levels in Alb:Hdac3fl/− mice at the indicated time points is shown in the right-hand panel. (C) Q-RT–PCR of the Pparγ target genes, Cd36 and Pdk4, in Alb:Hdac3fl/− vehicle- and GW9662-treated mice (P=0.029 and P=0.208, respectively, Student's t-test). (D) Cohorts of Alb:Hdac3 mice were treated with vehicle or GW9662, an inhibitor of Pparγ, for 4 weeks and liver tissue was stained with Oil-Red O to detect lipid. The upper panels depict control mice ( × 400 magnification) and middle ( × 100 magnification) and lower panels ( × 400 magnification) depict Alb:Hdac3fl/− mice.
To determine the level of contribution of Pparγ to the Hdac3-null liver phenotype, the Pparγ inhibitor GW9662 was injected into a cohort of mice daily for 4 weeks. Pparγ inhibition correlated with reduced expression of Cd36 and Pdk4 (Figure 6C). Hepatocyte structure was not affected by inhibition of Pparγ, but Oil Red O staining detected a decrease in lipid accumulation in Alb:Hdac3fl/− mice treated with GW9662 (Figure 6D, lower right-hand panels). Thus, inhibition of Pparγ partially reversed lipid accumulation in Hdac3-null livers, indicating that Pparγ contributes to the increase in lipids upon inactivation of Hdac3.
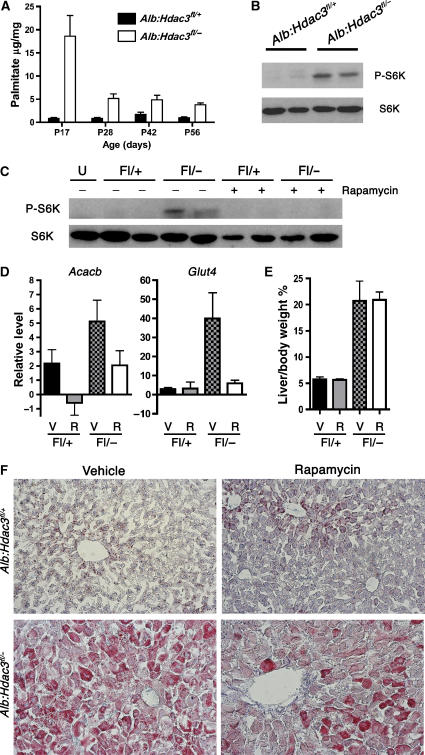
Inhibition of mTOR affects lipid levels in Alb:Hdac3fl/− mice
Activation of the PI3K/Akt pathway is involved in cellular homeostasis and metabolic control (Fingar and Blenis, 2004; Hay and Sonenberg, 2004), and increased levels of palmitate activated mTOR, a downstream target of Akt signalling, in primary hepatocytes (Mordier and Iynedjian, 2007). Mass spectrometry analysis uncovered a marked increase in palmitate levels in Hdac3-null liver tissue at P17, which remained high through P56 (Figure 7A). This increase was accompanied by increased phosphorylation of ribosomal S6 kinase (S6K), an mTOR substrate (Figure 7B). Therefore, we injected 3-week-old mice with rapamycin, a well-characterized inhibitor of mTOR, daily for 3 weeks. This regimen inhibited mTOR function in vivo, as evidenced by the lack of S6K phosphorylation in Alb:Hdac3fl/− mice (Figure 7C). The expression levels of transcripts that are regulated by mTOR signalling, such as Acacb (Brown et al, 2007) and Glut4 (Hernandez et al, 2001), were also downregulated by 2.5- and 8-fold, respectively (Figure 7D). Inhibition of mTOR stunted the growth of both control and Hdac3-null mice, but it did not dramatically affect the liver hypertrophy, as rapamycin-treated Alb:Hdac3fl/− mice had comparable liver weight/body weight ratios as vehicle-treated Alb:Hdac3fl/− mice (Figure 7E). However, treatment with rapamycin decreased the accumulation of neutral lipid in Alb:Hdac3fl/− mice (Figure 7F). Thus, in addition to inactivation of nuclear hormone receptor-mediated transcriptional repression, mTOR activation contributes to the phenotypes observed in Hdac3-null livers.
Figure 7.
Loss of Hdac3 activates mTOR, which contributes to disrupted metabolism. (A) Palmitate levels in Hdac3:Alb mice at P17–P56. (B) Western blot analysis of P28 liver whole-cell lysates indicating levels of S6K and phosphorylated S6K (P-S6K). (C) Cohorts of Alb:Hdac3fl/+ and Alb:Hdac3fl/− mice were treated with vehicle or rapamycin for 3 weeks, and a representative western blot analysis of S6K and P-S6K indicates inhibition of mTOR after rapamycin treatment. (D) Q-RT–PCR of known mRNAs regulated by mTOR in the indicated groups of mice (Acacb, P=0.151, and Glut4, P=0.015, using Student's t-test). V, vehicle treated; R, rapamycin treated. (E) Graphical representation of liver weight/body weight ratio of vehicle-treated, or rapamycin-treated Alb:Hdac3 mice. V, vehicle treated; R, rapamycin treated. (F) Oil-Red O staining of liver tissue from the indicated genotypes, treated with vehicle or rapamycin. The upper panels depict control livers, whereas the lower panels depict Hdac3-null livers ( × 200 magnification).
Discussion
The genetic analysis of Hdac3 in postnatal mice, using two separate mouse models of Cre recombinase, indicates that it is an important regulatory component of molecular complexes that control gene expression, which in turn controls metabolic functions in the liver. Loss of Hdac3 disrupted liver cholesterol and lipid homeostasis by de-repressing genes that regulate lipid and steroid metabolism, allowing the accumulation of lipids at the expense of glycogen storage. Although HDACs have many non-histone protein targets for deacetylation (Juan et al, 2000; Luo et al, 2000; Ashburner et al, 2001; Hubbert et al, 2002; Shimazu et al, 2006; Gregoire et al, 2007), the disruption of liver metabolism was evident at the level of transcription and was accompanied by increases in histone acetylation. The regulation of many of these genes can be directly traced to nuclear hormone receptors such as the THR and Pparγ that recruit Hdac3.
Pparγ is the hub of a large gene network that is de-repressed upon deletion of Hdac3. The Pparγ2 isoform was specifically upregulated in Hdac3-null livers, potentially through nuclear hormone receptor-mediated regulation (Tontonoz et al, 1994; Picard et al, 2004). Enforced expression of Pparγ2 in fibroblasts induced lipogenic gene transcription and activated a differentiation response towards adipocytes (Tontonoz et al, 1994). In addition, liver-specific induction of Pparγ2 in a mouse model of obesity triggered a lipogenic gene programme and hepatic steatosis that is reminiscent of inactivation of Hdac3 (Yu et al, 2003; Zhang et al, 2006). Moreover, Alb:Hdac3fl/− mice had low levels of insulin and were hypoglycaemic, which is similar to the effects of administration of Pparγ agonists (Lehmann et al, 1995). Inhibition of Pparγ with GW9662 decreased lipid accumulation by 20–50% in Hdac3-null livers, but it did not consistently affect blood glucose levels (data not shown). Thus, although aspects of the Hdac3-null liver phenotype are consistent with phenotypes induced by activation of Pparγ, loss of Hdac3 disrupts additional metabolic cues that may be regulated by other nuclear hormone receptors or other signalling pathways (Table III).
Alb:Hdac3fl/− livers also contained increased levels of palmitate, which can lead to oxidative stress and trigger lipid-responsive signalling pathways (Mordier and Iynedjian, 2007). Indeed, these pathways were activated as demonstrated by the expression of cellular stress-responsive genes (Table III) and the phosphorylation of S6K (Figure 7). Although constitutively active Akt induced hepatomegaly in mice (Ono et al, 2003; Haga et al, 2005), rapamycin did not affect cell size in Hdac3-null livers (Figure 7). Thus, lipid metabolism, but not hepatocyte hypertrophy, was at least partially dependent on the mTOR/raptor complex, which is sensitive to rapamycin (Loewith et al, 2002; Jacinto et al, 2004).
The primary targets of HDIs in clinical trials are class I HDACs (Marks et al, 2001). Alb:Hdac3fl/− phenotypes have major implications for drugs targeting these enzymes. Liver function was dramatically disrupted when Hdac3 was constitutively removed (Alb-Cre mice), yet the mice survived and were leaner, albeit with high levels of cholesterol, high triglycerides, and low blood sugar. This indicates that HDIs may transiently affect liver metabolism. Most of these compounds have relatively short half-lives in vivo, which may be a major benefit, as liver function would only be partially impaired. In addition, the Mx1-Cre model indicated that any toxic side effects of disruption of Hdac3 function are likely reversible. That is, it appeared that 4–5 weeks after Cre induction, Hdac3 expression and liver morphology normalized (SK Knutson, unpublished data). Thus, even if complete inactivation of Hdac3 could be achieved by continual infusion of an HDI, by terminating therapy the liver would be expected to recover, and pulse therapy would be expected to be relatively safe. Nevertheless, in dire cases where HDI therapy may have a major impact on the survival of the patient, even continuous infusion may be tolerated for several weeks. However, the changes in Alb:Hdac3fl/− livers are similar to the initiating events in non-alcoholic fatty liver disease, which include increases in lipid accumulation and cellular hypertrophy (reviewed by Sanyal, 2005). Therefore, long-term treatment with this regimen may ultimately have severe side effects, similar to those observed in Alb:Hdac3fl/− mice.
Materials and methods
Description of mice
Mice harbouring a conditional allele (fl) or a null allele (−) of Hdac3 were crossed to transgenic mice expressing Mx1-Cre (Kuhn et al, 1995) or Alb-Cre (Postic et al, 1999; Postic and Magnuson, 2000). The offspring from these mice were then bred to yield mice with a conditional allele in conjunction with either a wild-type (fl/+) or null allele (fl/−), and Cre. To induce Cre expression in Mx1-Cre-expressing mice, 5-week-old animals received an intraperitoneal (i.p.) injection of 500 μg pIpC in PBS every other day for 13 days, for a total of seven injections, with the last day of injection being denoted as day 0. Mice were killed at 1 day, 2 days, and 1, 2, 3, 4, and 5 weeks after the last injection.
Three-week-old Alb:Hdac3 mice were used in both GW9662 (Cayman Chemicals) and rapamycin (LC Laboratories) studies. For GW9662 experiments, mice were injected with 2 mg/kg, daily for 4 weeks. For rapamycin experiments, mice were injected with 10 mg/kg, daily for 3 weeks.
Preparation of liver lysates and western blot analysis
Whole-cell lysates were made by homogenizing in PBS plus 0.5% Triton X-100, 0.1% DOC, and 0.1% SDS, sonicated and cleared by centrifugation. Lysates were subjected to 10 or 12% SDS–PAGE, transferred to a PVDF membrane (Millipore), blocked with 5% non-fat dry milk for 1 h, incubated with primary antibody at 4°C and secondary antibody for 1–3 h at room temperature, and developed using SuperSignal West Pico reagents (Pierce).
Antibodies
The following antibodies were obtained from Upstate Cell Signaling: histone H3 (05-928), histone H4 (07-108), acetyl-histone H4 (Lys5) (07-327), acetyl-histone H4 (Lys8) (06-760), acetyl-histone H4 (Lys12) (06-761), acetyl-histone H4 (Lys16) (07-329), and acetyl-histone H3 (Lys9) (06-942). The antibodies for Hdac3 (ab-32369-100 and ab3279), histone H4K5ac (ab51997), histone H4K12ac (ab1761), and GAPDH (clone 6C5, ab8245) were obtained from Abcam. The antibody for Pparγ (sc-7196) was obtained from Santa Cruz. The p70 S6 Kinase (#2708) and phospho-p70 S6 Kinase (Thr398; #9206) antibodies were obtained from Cell Signaling Technology.
Histology and immunohistochemistry
The tissue was fixed in 4% paraformaldehyde at 4°C, dehydrated, and embedded in paraffin. Sections (5 μm) were stained with H&E and PAS. Apoptotic cells were detected by TUNEL using the ApopTag Plus Kit (Chemicon International). Cycling cells were detected by injecting the mice i.p. with 100 μg/g BrdU in PBS 1 h before they were killed. BrdU was detected using anti-BrdU for immunohistochemistry and BrdU-positive cells were counted per 100 nuclei in control and Hdac3-null liver sections. Frozen sections were prepared by fixing the tissue in 4% paraformaldehyde at 4°C for 2 h followed by 30% sucrose for 3–12 h. The tissue was then embedded in OCT, frozen on dry ice, and 10 μm sections were stained with Oil Red O and counterstained with haematoxylin.
DNA and RNA extraction, Q-RT–PCR, and microarray analysis
Genomic tail DNA was used for preliminary genotyping. Liver DNA was purified using the Qiagen DNeasy Tissue Kit. Liver RNA was extracted using the Versagene RNA Tissue Kit (Gentra Systems). cDNA was prepared from 2 to 3 μg RNA using random hexanucleotide primers (Applied Biosystems) and M-MLV Reverse Transcriptase (Promega). For Q-RT–PCR, cDNA was analysed using iQ SYBR Green Supermix (Bio-Rad). Primer sequences are available on request.
For the microarray analysis, RNA was prepared from individual whole livers, tested for RNA quality, and high-quality RNA from 10 control and 10 Hdac3-null livers was pooled to normalize for biological variability between animals before being hybridized to Applied Biosystems 1700 Mouse Expression Array System chips for analysis in the Vanderbilt Microarray Shared Resource. Biological replicates of pools were performed to further normalize for biological variability and a total of at least three arrays were performed for each genotype and each time point. The data were analysed using GeneSpring (Agilent Technologies) and genes whose expression changed at least two-fold using a t-test were included in the data sets. Ingenuity Pathway Analysis software (Ingenuity Systems, Mountain View, CA) and Panther Classification System software were used to group the regulated genes into ontology groups (Thomas et al, 2003; Mi et al, 2007).
Chromatin immunoprecipitation
The ChIP assay was performed following the standard protocol of the Chromatin Immunoprecipitation Assay Kit from Upstate Cell Signaling, with slight modifications using minced tissue for formaldehyde crosslinking. DNA was purified using the Qiagen PCR Purification Kit. Primers designed to the designated promoter sequences were used for quantification of histone acetylation using Q-PCR, with histone H3 and input sonicated DNA as reference controls.
Metabolic analysis
Serum levels of ALT were measured using the ALT Reagent Colorimetric Endpoint Method (Teco Diagnostics) following the standard protocol with slight modifications. Glucose levels were measured in whole blood using the Free Style Flash glucose monitor (courtesy of Dr William Russell). Serum levels of insulin and glucagon were measured using a double-antibody radioimmunoassay, with all primary reagents supplied by Linco Research Inc. (St Charles, MO). Hormone levels were determined by the Vanderbilt Diabetes Center Hormone Assay Core. To perform the glucose tolerance test, 10-week-old mice were fasted for 6 h and injected i.p. with glucose at 1.5 mg/g body weight. Blood glucose levels were measured through the tail tip using the Free Style Flash glucose monitor during the indicated time course. Total plasma cholesterol and triglycerides were measured by standard enzymatic assays. Liver tissue extraction and analysis of lipids and cholesterol were performed by the Vanderbilt Mouse Metabolic Phenotyping Center (MMPC) Analytical Resources Core, as previously described (Zhu et al, 2005).
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Acknowledgments
We thank the members of the Hiebert lab for helpful discussions and encouragement, and the Vanderbilt-Ingram Cancer Center (CA68485) and the Vanderbilt Digestive Diseases Research Center (5P30DK58404) for support and the use of shared resources including flow cytometry, microarray analysis, DNA sequencing, transgenic/embryonic stem cell, immunohistochemistry, and histological analysis. This work was supported by the TJ Martell Foundation, the National Institutes of Health (NIH) grants RO1-CA64140 and RO1-CA77274 (SWH), and Leukemia and Lymphoma Society postdoctoral fellowship #5074-03 (BJC) and by T32 CA009385 (SKK).
References
- Alcalay M, Zangrilli D, Pandolfi PP, Longo L, Mencarelli A, Giacomucci A, Rocchi M, Biondi A, Rambaldi A, Lo Coco F, E, F, PG (1991) Translocation breakpoint of acute promyelocytic leukemia lies within the retinoic acid receptor alpha locus. Proc Natl Acad Sci USA 88: 1977–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann JM, Nip J, Strom DK, Lutterbach B, Harada H, Lenny N, Downing JR, Meyers S, Hiebert SW (2001) ETO, a target of t(8;21) in acute leukemia, makes distinct contacts with multiple histone deacetylases and binds mSin3A through its oligomerization domain. Mol Cell Biol 21: 6470–6483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner BP, Westerheide SD, Baldwin AS Jr (2001) The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol Cell Biol 21: 7065–7077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskara S, Chyla BJ, Amann JM, Knutson SK, Hiebert SW (2008) Deletion of Histone Deacetylase 3 reveals critical roles in S-phase progression and DNA damage control. Molecular Cell (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NF, Stefanovic-Racic M, Sipula IJ, Perdomo G (2007) The mammalian target of rapamycin regulates lipid metabolism in primary cultures of rat hepatocytes. Metabolism 56: 1500–1507 [DOI] [PubMed] [Google Scholar]
- Chakrabarti SR, Nucifora G (1999) The leukemia-associated gene TEL encodes a transcription repressor which associates with SMRT and mSin3A. Biochem Biophys Res Commun 264: 871–877 [DOI] [PubMed] [Google Scholar]
- Clapham JC, Arch JR (2007) Thermogenic and metabolic antiobesity drugs: rationale and opportunities. Diabetes Obes Metab 9: 259–275 [DOI] [PubMed] [Google Scholar]
- de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB (2003) Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 370: 737–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeljak N, Fink M, Rozman D (2003) Many facets of mammalian lanosterol 14alpha-demethylase from the evolutionarily conserved cytochrome P450 family CYP51. Arch Biochem Biophys 409: 159–171 [DOI] [PubMed] [Google Scholar]
- Fajas L, Egler V, Reiter R, Hansen J, Kristiansen K, Debril MB, Miard S, Auwerx J (2002) The retinoblastoma-histone deacetylase 3 complex inhibits PPARgamma and adipocyte differentiation. Dev Cell 3: 903–910 [DOI] [PubMed] [Google Scholar]
- Fenrick R, Amann JM, Lutterbach B, Wang L, Westendorf JJ, Downing JR, Hiebert SW (1999) Both TEL and AML-1 contribute repression domains to the t(12;21) fusion protein. Mol Cell Biol 19: 6566–6574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenrick R, Wang L, Nip J, Amann JM, Rooney RJ, Walker-Daniels J, Crawford HC, Hulboy DL, Kinch MS, Matrisian LM, Hiebert SW (2000) TEL, a putative tumor suppressor, modulates cell growth and cell morphology of ras-transformed cells while repressing the transcription of stromelysin-1. Mol Cell Biol 20: 5828–5839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingar DC, Blenis J (2004) Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23: 3151–3171 [DOI] [PubMed] [Google Scholar]
- Fischle W, Dequiedt F, Hendzel MJ, Guenther MG, Lazar MA, Voelter W, Verdin E (2002) Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell 9: 45–57 [DOI] [PubMed] [Google Scholar]
- Gallinari P, Di Marco S, Jones P, Pallaoro M, Steinkuhler C (2007) HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Res 17: 195–211 [DOI] [PubMed] [Google Scholar]
- Gartenberg MR (2000) The Sir proteins of Saccharomyces cerevisiae: mediators of transcriptional silencing and much more. Curr Opin Microbiol 3: 132–137 [DOI] [PubMed] [Google Scholar]
- Glaser KB, Li J, Staver MJ, Wei RQ, Albert DH, Davidsen SK (2003) Role of class I and class II histone deacetylases in carcinoma cells using siRNA. Biochem Biophys Res Commun 310: 529–536 [DOI] [PubMed] [Google Scholar]
- Gregoire S, Xiao L, Nie J, Zhang X, Xu M, Li J, Wong J, Seto E, Yang XJ (2007) Histone deacetylase 3 interacts with and deacetylates myocyte enhancer factor 2. Mol Cell Biol 27: 1280–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignani F, De Matteis S, Nervi C, Tomassoni L, Gelmetti V, Cioce M, Fanelli M, Ruthardt M, Ferrara FF, Zamir I, Seiser C, Grignani F, Lazar MA, Minucci S, Pelicci PG (1998) Fusion proteins of the retinoic acid receptor-alpha recruit histone deacetylase in promyelocytic leukaemia. Nature 391: 815–818 [DOI] [PubMed] [Google Scholar]
- Guan HP, Ishizuka T, Chui PC, Lehrke M, Lazar MA (2005) Corepressors selectively control the transcriptional activity of PPARgamma in adipocytes. Genes Dev 19: 453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther MG, Barak O, Lazar MA (2001) The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol Cell Biol 21: 6091–6101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidez F, Ivins S, Zhu J, Soderstrom M, Waxman S, Zelent A (1998) Reduced retinoic acid-sensitivities of nuclear receptor corepressor binding to PML- and PLZF-RARalpha underlie molecular pathogenesis and treatment of acute promyelocytic leukemia. Blood 91: 2634–2642 [PubMed] [Google Scholar]
- Guidez F, Petrie K, Ford AM, Lu H, Bennett CA, MacGregor A, Hannemann J, Ito Y, Ghysdael J, Greaves M, Wiedemann LM, Zelent A (2000) Recruitment of the nuclear receptor corepressor N-CoR by the TEL moiety of the childhood leukemia-associated TEL-AML1 oncoprotein. Blood 96: 2557–2561 [PubMed] [Google Scholar]
- Haga S, Ogawa W, Inoue H, Terui K, Ogino T, Igarashi R, Takeda K, Akira S, Enosawa S, Furukawa H, Todo S, Ozaki M (2005) Compensatory recovery of liver mass by Akt-mediated hepatocellular hypertrophy in liver-specific STAT3-deficient mice. J Hepatol 43: 799–807 [DOI] [PubMed] [Google Scholar]
- Hartman HB, Yu J, Alenghat T, Ishizuka T, Lazar MA (2005) The histone-binding code of nuclear receptor co-repressors matches the substrate specificity of histone deacetylase 3. EMBO Rep 6: 445–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18: 1926–1945 [DOI] [PubMed] [Google Scholar]
- He LZ, Guidez F, Tribioli C, Peruzzi D, Ruthardt M, Zelent A, Pandolfi PP (1998) Distinct interactions of PML-RARalpha and PLZF-RARalpha with co-repressors determine differential responses to RA in APL. Nat Genet 18: 126–135 [DOI] [PubMed] [Google Scholar]
- Hernandez R, Teruel T, Lorenzo M (2001) Akt mediates insulin induction of glucose uptake and up-regulation of GLUT4 gene expression in brown adipocytes. FEBS Lett 494: 225–231 [DOI] [PubMed] [Google Scholar]
- Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP (2002) HDAC6 is a microtubule-associated deacetylase. Nature 417: 455–458 [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403: 795–800 [DOI] [PubMed] [Google Scholar]
- Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN (2004) Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 6: 1122–1128 [DOI] [PubMed] [Google Scholar]
- Juan LJ, Shia WJ, Chen MH, Yang WM, Seto E, Lin YS, Wu CW (2000) Histone deacetylases specifically down-regulate p53-dependent gene activation. J Biol Chem 275: 20436–20443 [DOI] [PubMed] [Google Scholar]
- Kuhn R, Schwenk F, Aguet M, Rajewsky K (1995) Inducible gene targeting in mice. Science 269: 1427–1429 [DOI] [PubMed] [Google Scholar]
- Lagger G, O'Carroll D, Rembold M, Khier H, Tischler J, Weitzer G, Schuettengruber B, Hauser C, Brunmeir R, Jenuwein T, Seiser C. (2002) Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J 21: 2672–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahm A, Paolini C, Pallaoro M, Nardi MC, Jones P, Neddermann P, Sambucini S, Bottomley MJ, Lo Surdo P, Carfi A, Koch U, De Francesco R, Steinkuhler C, Gallinari P (2007) Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc Natl Acad Sci USA 104: 17335–17340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA (1995) An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem 270: 12953–12956 [DOI] [PubMed] [Google Scholar]
- Li J, Wang J, Wang J, Nawaz Z, Liu JM, Qin J, Wong J (2000) Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J 19: 4342–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kao GD, Garcia BA, Shabanowitz J, Hunt DF, Qin J, Phelan C, Lazar MA (2006) A novel histone deacetylase pathway regulates mitosis by modulating Aurora B kinase activity. Genes Dev 20: 2566–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RJ, Nagy L, Inoue S, Shao W, Miller WH Jr, Evans RM (1998) Role of the histone deacetylase complex in acute promyelocytic leukaemia. Nature 391: 811–814 [DOI] [PubMed] [Google Scholar]
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN (2002) Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 10: 457–468 [DOI] [PubMed] [Google Scholar]
- Luo J, Su F, Chen D, Shiloh A, Gu W (2000) Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature 408: 377–381 [DOI] [PubMed] [Google Scholar]
- Marks PA, Richon VM, Breslow R, Rifkind RA (2001) Histone deacetylase inhibitors as new cancer drugs. Curr Opin Oncol 13: 477–483 [DOI] [PubMed] [Google Scholar]
- Mi H, Guo N, Kejariwal A, Thomas PD (2007) PANTHER version 6: protein sequence and function evolution data with expanded representation of biological pathways. Nucleic Acids Res 35: D247–D252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordier S, Iynedjian PB (2007) Activation of mammalian target of rapamycin complex 1 and insulin resistance induced by palmitate in hepatocytes. Biochem Biophys Res Commun 362: 206–211 [DOI] [PubMed] [Google Scholar]
- Ono H, Shimano H, Katagiri H, Yahagi N, Sakoda H, Onishi Y, Anai M, Ogihara T, Fujishiro M, Viana AY, Fukushima Y, Abe M, Shojima N, Kikuchi M, Yamada N, Oka Y, Asano T (2003) Hepatic Akt activation induces marked hypoglycemia, hepatomegaly, and hypertriglyceridemia with sterol regulatory element binding protein involvement. Diabetes 52: 2905–2913 [DOI] [PubMed] [Google Scholar]
- Papeleu P, Loyer P, Vanhaecke T, Elaut G, Geerts A, Guguen-Guillouzo C, Rogiers V (2003) Trichostatin A induces differential cell cycle arrests but does not induce apoptosis in primary cultures of mitogen-stimulated rat hepatocytes. J Hepatol 39: 374–382 [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L (2004) Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429: 771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postic C, Magnuson MA (2000) DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis 26: 149–150 [DOI] [PubMed] [Google Scholar]
- Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA (1999) Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem 274: 305–315 [DOI] [PubMed] [Google Scholar]
- Ren D, Collingwood TN, Rebar EJ, Wolffe AP, Camp HS (2002) PPARgamma knockdown by engineered transcription factors: exogenous PPARgamma2 but not PPARgamma1 reactivates adipogenesis. Genes Dev 16: 27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini V, Gozzini A, Ferrari G (2007) Histone deacetylase inhibitors: molecular and biological activity as a premise to clinical application. Curr Drug Metab 8: 383–393 [DOI] [PubMed] [Google Scholar]
- Sanyal AJ (2005) Mechanisms of disease: pathogenesis of nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol 2: 46–53 [DOI] [PubMed] [Google Scholar]
- Shimazu T, Komatsu Y, Nakayama KI, Fukazawa H, Horinouchi S, Yoshida M (2006) Regulation of SV40 large T-antigen stability by reversible acetylation. Oncogene 25: 7391–7400 [DOI] [PubMed] [Google Scholar]
- Takami Y, Nakayama T (2000) N-terminal region, C-terminal region, nuclear export signal, and deacetylation activity of histone deacetylase-3 are essential for the viability of the DT40 chicken B cell line. J Biol Chem 275: 16191–16201 [DOI] [PubMed] [Google Scholar]
- Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, Diemer K, Muruganujan A, Narechania A (2003) PANTHER: a library of protein families and subfamilies indexed by function. Genome Res 13: 2129–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM (1994) Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 79: 1147–1156 [DOI] [PubMed] [Google Scholar]
- Trivedi CM, Luo Y, Yin Z, Zhang M, Zhu W, Wang T, Floss T, Goettlicher M, Noppinger PR, Wurst W, Ferrari VA, Abrams CS, Gruber PJ, Epstein JA (2007) Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3 beta activity. Nat Med 13: 324–331 [DOI] [PubMed] [Google Scholar]
- Wang L, Hiebert SW (2001) TEL contacts multiple co-repressors and specifically associates with histone deacetylase-3. Oncogene 20: 3716–3725 [DOI] [PubMed] [Google Scholar]
- Werman A, Hollenberg A, Solanes G, Bjorbaek C, Vidal-Puig AJ, Flier JS (1997) Ligand-independent activation domain in the N terminus of peroxisome proliferator-activated receptor gamma (PPARgamma). Differential activity of PPARgamma1 and -2 isoforms and influence of insulin. J Biol Chem 272: 20230–20235 [DOI] [PubMed] [Google Scholar]
- Widmer J, Fassihi KS, Schlichter SC, Wheeler KS, Crute BE, King N, Nutile-McMenemy N, Noll WW, Daniel S, Ha J, Kim KH, Witters LA (1996) Identification of a second human acetyl-CoA carboxylase gene. Biochem J 316 (Part 3): 915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Rychnovsky SD, Belani JD, Hobbs HH, Cohen JC, Rawson RB (2005) Dual roles for cholesterol in mammalian cells. Proc Natl Acad Sci USA 102: 14551–14556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HG, Chan DW, Huang ZQ, Li J, Fondell JD, Qin J, Wong J (2003) Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1. EMBO J 22: 1336–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Matsusue K, Kashireddy P, Cao WQ, Yeldandi V, Yeldandi AV, Rao MS, Gonzalez FJ, Reddy JK (2003) Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J Biol Chem 278: 498–505 [DOI] [PubMed] [Google Scholar]
- Zhang YL, Hernandez-Ono A, Siri P, Weisberg S, Conlon D, Graham MJ, Crooke RM, Huang LS, Ginsberg HN (2006) Aberrant hepatic expression of PPARgamma2 stimulates hepatic lipogenesis in a mouse model of obesity, insulin resistance, dyslipidemia, and hepatic steatosis. J Biol Chem 281: 37603–37615 [DOI] [PubMed] [Google Scholar]
- Zhu MY, Hasty AH, Harris C, Linton MF, Fazio S, Swift LL (2005) Physiological relevance of apolipoprotein E recycling: studies in primary mouse hepatocytes. Metabolism 54: 1309–1315 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Alvares K, Huang Q, Rao MS, Reddy JK (1993) Cloning of a new member of the peroxisome proliferator-activated receptor gene family from mouse liver. J Biol Chem 268: 26817–26820 [PubMed] [Google Scholar]
- Zupkovitz G, Tischler J, Posch M, Sadzak I, Ramsauer K, Egger G, Grausenburger R, Schweifer N, Chiocca S, Decker T, Seiser C (2006) Negative and positive regulation of gene expression by mouse histone deacetylase 1. Mol Cell Biol 26: 7913–7928 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4