There are about 869 species of ticks described so far [1], most of which are responsible for the transmission of a huge diversity of microorganisms belonging to almost all the trees of life (viruses, bacteria, protozoa, fungi, and nematodes) [2–4]. Despite the strong opportunity for interaction between these pathogenic species, little is known about the competitive interactions between tick-borne diseases within the vector and the vertebrate host (see [3] for review).
Severity of symptoms is often found associated with co-infection by different pathogens (e.g., [3,5–7]). Moreover, dual infections may affect the therapeutic strategies [5]. Exploring the incidence of co-infections and testing for possible interactions (positive or negative) thus represent important objectives.
Theileria annulata, Babesia bovis, and Anaplasma marginale are among the most economically important haemoparasitic tick-borne diseases of ruminants worldwide [8] and represent a serious economic challenge, particularly in developing countries [9–11]. In this paper we analyse the co-occurrence of these pathogens within individual bovine hosts in northeastern Algeria (North Africa) from one original dataset and one published dataset from the same region [12] that we have reanalysed, and test for the existence of positive or negative associations between these pathogens. We then discuss the implications of our findings in terms of therapeutic strategies and further studies.
All samples were collected around Boutheldja, a small town located on the northeast border of Algeria (close to Tunisia) and east of Annaba in Algeria (GPS coordinates 36° 45′ 7.0 N; 8° 10′ 0 E) corresponding to an area of 113.53 km2 (Figure S1). The sampling period extended from July to December 2004. Ill cattle were diagnosed by private veterinarians, who were contacted directly by their owners. The main criteria used for diagnosis were the presence of the ticks, a hyperthermic condition, presence of icterus, ganglionic hypertrophy, feebleness, lack of appetite, anorexia, anaemia, and dehydration. In case of positive diagnostic a blood smear was carried out, as described in Table 1. Parasites were then identified according to morphology, localisation in the erythrocytes, and using the key proposed by Morel in 1981 [13]. We also reanalysed the data from a published descriptive work [12] undertaken in 2002 in the same part of Algeria on 54 cattle with an identical approach as described above.
Table 1.
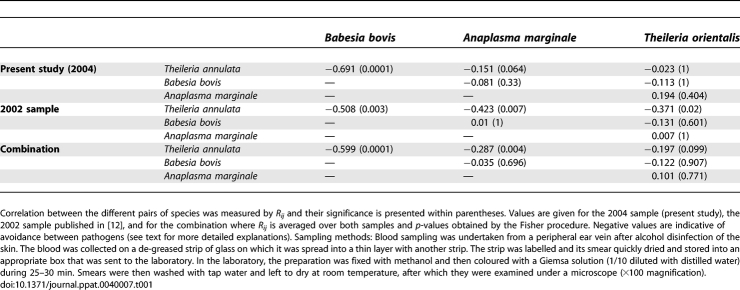
Within-Cattle Occurrence Correlation between Pathogens
Four species were identified in the samples: T. annulata, B. bovis, A. marginale, and Theileria orientalis. Note that Anaplasma are rickettsial bacteriae while the others are apicomplexan sporozoan (piroplasms), and that transovarial transmission is only known for Babesia sp. and not for the three other species. In the area investigated, Theileria sp. are transmitted by different Hyaloma ticks, B. bovis is transmitted by the tick Boophilus annulatus, and A. marginale by B. annulatus and Rhipicephalus bursa [4]. All are intracellular parasites [14].
In 2004, the most frequently encountered pathogenic agent was T. annulata (47.6% of infected cattle), followed by A. marginale (40.5%), B. bovis (33.3%), and T. orientalis (2.4%). In 2002, as presented by [12], some differences were observed with 74%, 24%, 8%, and 16% for T. annulata, A. marginale, B. bovis, and T. orientalis, respectively. The comparison of microbe prevalences between 2002 and 2004 samples was undertaken with an approximation of the Fisher exact test with 1,000,000 randomisations using the module Struc of Genepop version 3.1.c [15]. Randomisation tests gave significant differences (p-values ≤ 0.036) for all species but A. marginale (p-value = 0.117). In 2004 (original sample), 12 hosts appeared negative, 15 were infected by T. annulata only, ten by B. bovis only, seven by A. marginale, and four were infected both by B. bovis and A. marginale, five by T. annulata and A. marginale, and one by T. orientalis and A. marginale. In 2002 [12], four hosts appeared negative, 29 were infected by T. annulata only, three by B. bovis only, four by A. marginale, three by T. orientalis, and one was infected both by B. bovis and A. marginale, five by T. annulata and A. marginale, two by T. orientalis and A. marginale, and three by T. annulata and T. orientalis. Presence or absence of a particular micropathogen was treated as a phenotypic character. Thus there were four such phenotypic characters each taking the value 1 (absence) or 2 (presence). To study the association between these four characters a correlation coefficient, initially designed for genetic data, was adapted to our data. This coefficient of correlation is noted Rij, where i and j stands for the pair of loci (or as here, phenotypic characters), the association of which is under study. It was computed by Genetix 4.02 [16]. This parameter is described in a series of articles cited in the Genetix help menu (i.e., [17–19]). If n 11 is the number of cattle without pathogens i or j, n 12 the number of cattle infected by j but not by i, n 21 those infected by i but not j, n 22 those infected by both species, and n the total sample size, then for a given pathogen pair ij:
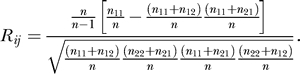 |
The significance of Rij was tested by a permutation procedure. Phenotypes (1 or 2) of the two characters studied (e.g., T. annulata and B. bovis) are associated at random a number of times (10,000) and a statistic R is recalculated on the randomised data set. The exact p-value is estimated as the proportion of statistics from randomised data sets that are larger or equal to the one observed in the real data set. This test was undertaken under Genetix 4.02, which uses Weir's R [20] as the statistic. However, given how we coded data, this statistic simply corresponds to Rij absolute value (bi-lateral testing). The 12 ill cows that were negative according to the cytological tests in 2004 were not taken into account for this study, considering that microbes might have been missed (false negative). The four negative cattle from [12] were also ignored. However, undertaking the same tests and including these data as true negatives (no microbe) did not change the results described below. To unite both data sets (2004 and 2002), we simply averaged Rij (unweighted mean) and combined p-values with the Fisher procedure [21] where the quantity 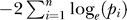 can be compared to a chi-square distribution with 2n degrees of freedom, with n the number of tests (here n = 2) and pi the p-value of the ith test. The results of the correlation study between pairs of pathogenic agents provided mainly negative correlations (five out of six for combined data, Table 1). For the original sample (2004), only one of the correlations, between T. annulata and B. bovis, was highly significant. In fact, no cattle individual was found co-infected by both pathogens despite an expected co-occurrence of 0.476*0.333*42 = 7 co-infected individuals. The second interesting result, though marginally non-significant, was observed between T. annulata and A. marginale, for which only five individuals were found co-infected for an expected frequency of height. All other pairs were not significant, but we can notice that with only one case reported for T. orientalis, the rarity of this species in 2004 makes any conclusion impossible for this pathogen. Nevertheless, the analysis combining 2002 [12] and 2004 samples confirms all the tendencies observed (Table 1). An absolute exclusion exists between T. annulata and B. bovis (no co-infection observed), a strong avoidance characterises the relationship between T. annulata and A. marginale, and a moderate one (if any) between this species and T. orientalis.
can be compared to a chi-square distribution with 2n degrees of freedom, with n the number of tests (here n = 2) and pi the p-value of the ith test. The results of the correlation study between pairs of pathogenic agents provided mainly negative correlations (five out of six for combined data, Table 1). For the original sample (2004), only one of the correlations, between T. annulata and B. bovis, was highly significant. In fact, no cattle individual was found co-infected by both pathogens despite an expected co-occurrence of 0.476*0.333*42 = 7 co-infected individuals. The second interesting result, though marginally non-significant, was observed between T. annulata and A. marginale, for which only five individuals were found co-infected for an expected frequency of height. All other pairs were not significant, but we can notice that with only one case reported for T. orientalis, the rarity of this species in 2004 makes any conclusion impossible for this pathogen. Nevertheless, the analysis combining 2002 [12] and 2004 samples confirms all the tendencies observed (Table 1). An absolute exclusion exists between T. annulata and B. bovis (no co-infection observed), a strong avoidance characterises the relationship between T. annulata and A. marginale, and a moderate one (if any) between this species and T. orientalis.
Because Rij is oriented and because we coded absence as 1 and presence as 2 for each phenotype, a positive value would bear witness to a positive association between the two pathogenic agents within the same individual hosts, while a negative value gives evidence for avoidance between the pairs of microbes studied. In the first case, different interpretations can be formulated as cooperation between pathogens (immunocompromising by one agent, opening the gate to infection by the other) or identical ecological needs (e.g., same vector). Immunosuppressive effects are what seem to be most of the time observed for various pathogenic agents, in particular in tick-borne diseases, as babesioses with other parasites in mice, or anaplasmosis with louping ill virus in sheep and goats (reviewed in [22]). In the case of a negative association, a hypothesis for competitive exclusion or at least a negative interference (cross-immunisation) can be advanced. This is the case for T. annulata and B. bovis, which appear to strictly avoid co-infection. This is also the case for T. annulata and A. marginale, though to a lower extent and probably also for T. annulata and T. orientalis, though the rarity of the latter species (low statistical power) forbids a definitive conclusion. This may seem to contradict the conclusions found in other studies on similar systems [23–25], though prevalence differences, lack of detailed data, and absence of specific analysis may be the main causes of this apparent discrepancy. Significant negative correlations always involve T. annulata against the other pathogen species. In the area studied, Theileria sp. and the other pathogens (B. bovis, A. marginale) are transmitted by different tick species [4]. Thus, competition must occur within the vertebrate host. Within-host competition between microbes (mostly intra-specific) was reviewed in Read and Taylor [26], with the best documented examples apparently found in Plasmodium sp. Competition was poorly studied in tick-borne diseases and only suspected once (to our knowledge) between Anaplasma phagocytophilum, Ehrlichia muris, and Babesia microti in unfed adults of Ixodes persulcatus in northwestern Russia [27]. This might confirm a generalised weak compatibility between these three kinds of intracellular pathogens within the vector [27] as well as within the vertebrate host (present study). Weak compatibility between these pathogens was never evidenced in vertebrate hosts for which specific studies are scarce or difficult to interpret in that perspective. Immunological surveys are not conclusive enough, as they may better reflect the history of sequential infections, not necessarily all successful, experienced by one host. Many studies report the co-occurrence of tick-borne micro-pathogens (e.g., [3,5,12,23,24] and references therein), and specific analyses seemed to conclude with positive associations or even suggested cooperation in multi-infected mice: between Borrelia burgdorferi (Lyme disease agent) and B. microti (transmission to tick is enhanced) [6] or between B. burgdorferi and A. phagocytophilum (Borrelia number is increased in co-infected mice) [28] (see also [29] for review and references therein). Synchronous infections with Babesia divergens and A. phagocytophila seem very common, but their interaction remains poorly understood and a suppression of Babesia by Anaplasma even seems possible (reviewed in [25]). As suggested above, such exclusion may be mediated by host immune system (concomitant immunity) or by direct interference (one pathogen tends to eliminate the other). Within the vector, most studies only report the co-occurrence of different microbes [10,29–37]. Associations between different pathogenic species were rarely tested, and only positive associations [6,28,38,39] or no effect [40] between borrelia spirochaetes and co-infecting microbes were detected.
Exclusion between pathogens may appear beneficial to the host, as severity of symptoms is often found associated with co-infection by different pathogens (e.g., [3,5–7]). Nevertheless, depending on the mechanisms involved and which, if any, of the pathogens is competitively dominant, this phenomenon may severely affect the outcome of prophylactic or vaccination campaigns. If one pathogenic species is less sensitive to treatment but at a competitive disadvantage against the most sensitive one, this may lead to the opening of the widest gate to the most pathogenic microbe. It is thus essential to better understand the origin and mechanisms of such competitive interactions, not only for babesioses, theilerioses, and anaplasmoses that represent a real threat to livestock on a global scale, particularly in developing countries where they constrain economic improvement [8–10], but also for other vector-borne or other diseases affecting animals or humans. This parameter is thus worth investigating further, because it may open new perspectives in the design of therapeutic strategies of economically and medically important pathogenic agents, particularly in tick-borne and vector-borne diseases, where it has attracted little attention so far.
Supporting Information
Several local breed cattle, as those studied in the present study, can be seen. All cattle belong to the so-called Atlas brown breed. The Cheurfa and Guelmoise sub-breeds harbor various clear coat colors, almost white for some. The Sétifienne displays a uniform black coat and the Chelefienne a fawn coat. Photo credit: Loubna Dib.
(2.8 MB TIF)
Acknowledgments
We thank Franck Prugnolle for very useful and stimulating discussions and one anonymous referee for helpful suggestions.
Footnotes
Loubna Dib is with the Institut Vétérinaire, Centre Universitaire El Tarf, Route de Matroha, El Tarf, Algeria. Idir Bitam is with the Laboratoire d'Entomologie Médicale, Annexe Sidi Fredj, Institut Pasteur d'Alger, Algeria . Maja Tahri and Mourad Bensouilah are with the Laboratoire d'Ecobiologie des Milieux Marin et Littoraux, Université Badji Mokthar, Annaba, Algeria. Thierry De Meeûs is with the Génétique et Evolution des Maladies Infectieuses (GEMI), UMR 2724 IRD/CNRS, Centre IRD, Montpellier, France.
Author contributions. MAB conceived and designed the experiments. LD, IB, and MT performed the experiments. IB and TDM analyzed the data. IB contributed reagents/materials/analysis tools. TDM wrote the paper.
Funding. Funding of this work was supported by the Centre Universitaire El Tarf, the CNRS, and the IRD. Thierry De Meeûs is supported by the CNRS and IRD.
Competing interests. The authors have declared that no competing interests exist.
References
- Camicas JL, Hervy JP, Adam F, Morel PC. The ticks of the world (Acarida, Ixodida) Paris: ORSTOM; 1998. 233 [Google Scholar]
- Aeschlimann A, Burgdorfer W, Matile H, Peter O, Wyler R. Aspects nouveaux du rôle de vecteur joué par Ixodes ricinus L. en Suisse. Acta Trop. 1979;36:181–191. [PubMed] [Google Scholar]
- Belongia EA. Epidemiology and impact of coinfections acquired from Ixodes ticks. Vector Borne Zoonot Dis. 2002;2:265–273. doi: 10.1089/153036602321653851. [DOI] [PubMed] [Google Scholar]
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129:S3–S14. doi: 10.1017/s0031182004005967. [DOI] [PubMed] [Google Scholar]
- Nadelman RB, Horowitz HW, Hsieh TC, Wu JM, Aguero-Rosenfeld ME, et al. Simultaneous human granulocytic ehrlichiosis and Lyme borreliosis. N Engl J Med. 1997;337:27–30. doi: 10.1056/NEJM199707033370105. [DOI] [PubMed] [Google Scholar]
- Thompson C, Spielman A, Krause PJ. Coinfecting deer-associated zoonoses: Lyme disease, babesiosis, and ehrlichiosis. Clin Infect Dis. 2001;33:676–685. doi: 10.1086/322681. [DOI] [PubMed] [Google Scholar]
- Le Hesran JY, Akiana J, El Ndiaye HM, Dia M, Senghor P, et al. Severe malaria attack is associated with high prevalence of Ascaris lumbricoides infection among children in rural Senegal. Trans R Soc Trop Med Hyg. 2004;98:397–399. doi: 10.1016/j.trstmh.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Uilenberg G. International collaborative research: significance of tick-borne hemoparasitic diseases to world animal health. Vet Parasitol. 1995;57:19–41. doi: 10.1016/0304-4017(94)03107-8. [DOI] [PubMed] [Google Scholar]
- D'oliveira C, Vanderweide M, Habela MA, Jacquiet P, Jongejan F. Detection of Theileria annulata in blood-samples of carrier cattle by PCR. J Clin Microbiol. 1995;33:2665–2669. doi: 10.1128/jcm.33.10.2665-2669.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cringoli G, Otranto D, Testini G, Buono V, Di Giulio G, et al. Epidemiology of bovine tick-borne diseases in southern Italy. Vet Res. 2002;33:421–428. doi: 10.1051/vetres:2002028. [DOI] [PubMed] [Google Scholar]
- Barros SL, Madruga CR, Araujo FR, Menk CF, de Almeida MAO, et al. Serological survey of Babesia bovis, Babesia bigemina, and Anaplasma marginale antibodies in cattle from the semi-arid region of the state of Bahia, Brazil, by enzyme-linked immunosorbent assays. Mem Inst Oswaldo Cruz. 2005;100:613–617. doi: 10.1590/s0074-02762005000600003. [DOI] [PubMed] [Google Scholar]
- Ziam H, Benaouf H. Prevalence of blood parasites in cattle from wilayates of Annaba and El Tarf east Algeria. Arch Inst Pasteur Tunis. 2004;81:27–30. [PubMed] [Google Scholar]
- Morel PC. Maladies à tiques du bétail en Afrique. In: Troncy PM, Itard J, Morel PC, editors. Précis de parasitologie vétérinaire tropicale. Maisons-Alfort Ministere de la Cooperation et du Developpement; 1981. p. 473. [Google Scholar]
- Uilenberg G. Tick-borne livestock diseases and their vectors. 2. Epizootiology of tick-borne diseases. World Animal Review. 1976;17:8–15. [Google Scholar]
- Raymond M, Rousset F. Genepop (version 1.2): population genetics software for exact tests and ecumenicism. J Hered. 1995;86:248–249. [Google Scholar]
- Belkhir k, Borsa P, Chikhi L, Raufaste N, Bonhomme F. GENETIX 4.05, logiciel sous Windows TM pour la génétique des populations. Montpellier: Laboratoire Génome, Populations, Interactions CNRS UMR 5000, Université de Montpellier II; 2004. [Google Scholar]
- Cockerham CC, Weir BS. Digenic descent measures for finite populations. Genet Res. 1979;30:121–147. [Google Scholar]
- Black IVWC, Krafsur ES. A FORTRAN program for the calculation and analysis of two-locus linkage disequilibrium coefficients. Theor Appl Genet. 1985;70:491–496. doi: 10.1007/BF00305981. [DOI] [PubMed] [Google Scholar]
- Garnier-Géré P, Dillmann C. A computer program for testing pairwise linkage disequilibria in subdivided populations. J Hered. 1992;83:239. doi: 10.1093/oxfordjournals.jhered.a111204. [DOI] [PubMed] [Google Scholar]
- Weir BS. Inferences about linkage disequilibrium. Biometrics. 1979;35:235–254. [PubMed] [Google Scholar]
- Fisher RA. Statistical methods for research workers, 14th edition. Edinburgh: Oliver and Boyd; 1970. 362 [Google Scholar]
- Persing DH. The cold zone: a curious convergence of tick-transmitted diseases. Clin Infect Dis. 1997;25:S35–S42. doi: 10.1086/516170. [DOI] [PubMed] [Google Scholar]
- Georges K, Loria GR, Riili S, Greco A, Caracappa S, et al. Detection of haemoparasites in cattle by reverse line blot hybridisation with a note on the distribution of ticks in Sicily. Vet Parasitol. 2001;99:273–286. doi: 10.1016/s0304-4017(01)00488-5. [DOI] [PubMed] [Google Scholar]
- Almeria S, Castella J, Ferrer D, Gutierrez JF, Estrada-Pena A, et al. Reverse line blot hybridization used to identify hemoprotozoa in Minorcan cattle. Ann N Y Acad Sci. 2002;969:78–82. doi: 10.1111/j.1749-6632.2002.tb04354.x. [DOI] [PubMed] [Google Scholar]
- Zintl A, Mulcahy G, Skerrett HE, Taylor SM, Gray JS. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin Microbiol Rev. 2003;16:622–636. doi: 10.1128/CMR.16.4.622-636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read AF, Taylor LH. The ecology of genetically diverse infections. Science. 2001;292:1099–1102. doi: 10.1126/science.1059410. [DOI] [PubMed] [Google Scholar]
- Alekseev AN, Dubinina HV, Jushkova OV. Firs report on the coexistence and compatibility of seven tick-borne pathogens in unfed adult Ixodes persulcatus Schulze (Acarina: Ixodidae) Int J Med Microbiol. 2004;293:104–108. doi: 10.1016/s1433-1128(04)80015-9. [DOI] [PubMed] [Google Scholar]
- Holden K, Hodzic E, Feng S, Freet KJ, Lefebvre RB, et al. Coinfection with Anaplasma phagocytophilum alters Borrelia burgdorferi population distribution in C3H/HeN mice. Infect Immun. 2005;73:3440–3444. doi: 10.1128/IAI.73.6.3440-3444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenberg EI. Problems in the study and prophylaxis of mixed infections transmitted by ixodid ticks. Int J Med Microbiol. 2004;293:80–85. doi: 10.1016/s1433-1128(04)80012-3. [DOI] [PubMed] [Google Scholar]
- Schwartz I, Fish D, Daniels TJ. Prevalence of the rickettsial agent of human granulocytic ehrlichiosis in ticks from a hyperendemic focus of Lyme disease. N Engl J Med. 1997;337:49–50. doi: 10.1056/NEJM199707033370111. [DOI] [PubMed] [Google Scholar]
- Leutenegger CM, Pusterla N, Mislin CN, Weber R, Lutz H. Molecular evidence of coinfection of ticks with Borrelia burgdorferi sensu lato and the human granulocytic ehrlichiosis agent in Switzerland. J Clin Microbiol. 1999;37:3390–3391. doi: 10.1128/jcm.37.10.3390-3391.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ML, des Vignes F, Fish D. Disparity in the natural cycles of Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis. Emerg Infect Dis. 1999;5:204–208. doi: 10.3201/eid0502.990203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouls LM, Van de Pol I, Rijpkema SGT, Schot CS. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J Clin Microbiol. 1999;37:2215–2222. doi: 10.1128/jcm.37.7.2215-2222.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelson ME, Rao RVS, Tilton RC, Cabets K, Eskow E, et al. Prevalence of Borrelia burgdorferi, Bartonella spp., Babesia microti, and Anaplasma phagocytophila in Ixodes scapularis ticks collected in northern New Jersey. J Clin Microbiol. 2004;42:2799–2801. doi: 10.1128/JCM.42.6.2799-2801.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halos L, Jamal T, Maillard R, Beugnet F, Le Menach A, et al. Evidence of Bartonella sp in questing adult and nymphal Ixodes ricinus ticks from France and co-infection with Borrelia burgdorferi sensu lato and Babesia sp. Vet Res. 2005;36:79–87. doi: 10.1051/vetres:2004052. [DOI] [PubMed] [Google Scholar]
- Halos L, Vourc'h G, Cotte V, Gasqui P, Barnouin J, et al. Prevalence of Anaplasma phagocytophilum, Rickettsia sp and Borrelia burgdorferi sensu lato DNA in questing Ixodes ricinus ticks from France. Ann N Y Acad Sci. 2006;1078:316–319. doi: 10.1196/annals.1374.059. [DOI] [PubMed] [Google Scholar]
- Molad T, Mazuz ML, Fleiderovitz L, Fish L, Savitsky I, et al. Molecular and serological detection of A. centrale- and A. marginale- infected cattle grazing within an endemic area. Vet Microbiol. 2006;113:55–62. doi: 10.1016/j.vetmic.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Jenkins A, Kristiansen BE, Allum AG, Aakre RK, Strand L, et al. Borrelia burgdorferi sensu lato and Ehrlichia spp. in Ixodes ticks from southern Norway. J Clin Microbiol. 2001;39:3666–3671. doi: 10.1128/JCM.39.10.3666-3671.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meeûs T, Lorimier Y, Renaud F. Lyme borreliosis agents and the genetics and sex of their vector, Ixodes ricinus . Microbes Infect. 2004;6:299–304. doi: 10.1016/j.micinf.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Levin ML, Fish D. Acquisition of coinfection and simultaneous transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis ticks. Infect Immun. 2000;68:2183–2186. doi: 10.1128/iai.68.4.2183-2186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Several local breed cattle, as those studied in the present study, can be seen. All cattle belong to the so-called Atlas brown breed. The Cheurfa and Guelmoise sub-breeds harbor various clear coat colors, almost white for some. The Sétifienne displays a uniform black coat and the Chelefienne a fawn coat. Photo credit: Loubna Dib.
(2.8 MB TIF)