Abstract
Pyruvate ferredoxin oxidoreductase (POR) has been previously purified from the hyperthermophilic archaeon, Pyrococcus furiosus, an organism that grows optimally at 100°C by fermenting carbohydrates and peptides. The enzyme contains thiamine pyrophosphate and catalyzes the oxidative decarboxylation of pyruvate to acetyl-CoA and CO2 and reduces P. furiosus ferredoxin. Here we show that this enzyme also catalyzes the formation of acetaldehyde from pyruvate in a CoA-dependent reaction. Desulfocoenzyme A substituted for CoA showing that the cofactor plays a structural rather than a catalytic role. Ferredoxin was not necessary for the pyruvate decarboxylase activity of POR, nor did it inhibit acetaldehyde production. The apparent Km values for CoA and pyruvate were 0.11 mM and 1.1 mM, respectively, and the optimal temperature for acetaldehyde formation was above 90°C. These data are comparable to those previously determined for the pyruvate oxidation reaction of POR. At 80°C (pH 8.0), the apparent Vm value for pyruvate decarboxylation was about 40% of the apparent Vm value for pyruvate oxidation rate (using P. furiosus ferredoxin as the electron acceptor). Tentative catalytic mechanisms for these two reactions are presented. In addition to POR, three other 2-keto acid ferredoxin oxidoreductases are involved in peptide fermentation by hyperthermophilic archaea. It is proposed that the various aldehydes produced by these oxidoreductases in vivo are used by two aldehyde-utilizing enzymes, alcohol dehydrogenase and aldehyde ferredoxin oxidoreductase, the physiological roles of which were previously unknown.
Keywords: archaea, aldehyde, decarboxylation/2-keto acid/thiamine pyrophosphate
Pyrococcus furiosus is one of the best studied of an unusual group of microorganisms, the so-called hyperthermophilic archaea (formerly archaebacteria), which thrive at extreme temperatures and inhabit shallow and deep sea volcanic environments (1, 2). P. furiosus grows optimally at 100°C and ferments either peptides or carbohydrates with the production of organic acids, H2 and CO2. It also reduces elemental sulfur (S°) to H2S, although the organism grows well in the absence of S° (3). Carbohydrates are converted to pyruvate predominantly via an unusual Embden–Meyerhof pathway (4, 5), and pyruvate is oxidatively decarboxylated to acetyl-CoA and CO2 via pyruvate ferredoxin oxidoreductase (POR) (6). Acetyl-CoA can be used directly for energy conservation via acetyl-CoA synthetase (7). The oxidation of the reduced ferredoxin (Fd) that is generated by the POR reaction is coupled to S° reduction and H2 production via sulfide dehydrogenase (8) and hydrogenase (9, 10). In addition to POR, three other types of 2-keto acid Fd oxidoreductase are uniquely present in the hyperthermophilic archaea and these are involved in peptide fermentation. They use 2-ketoglutarate (KGOR) (11), indolepyruvate (IOR) (12), and 2-ketoisovalerate (VOR) (13) as substrates, and function to oxidatively decarboxylate the 2-keto acids generated by the transamination of glutamate, aromatic amino acids, and branched chain amino acids, respectively, to the corresponding CoA derivative (13).
The growth of hyperthermophilic archaea such as P. furiosus is also unusual in that it is dependent upon tungsten (14), a metal seldom used in biological systems (15). Three different tungsten-containing- enzymes have been purified from these organisms: aldehyde Fd oxidoreductase (AOR) (16), formaldehyde Fd oxidoreductase (17), and glyceraldehyde-3-phosphate Fd oxidoreductase (5). Glyceraldehyde-3-phosphate Fd oxidoreductase is thought to be involved in glycolysis (5), but the functions of AOR and formaldehyde Fd oxidoreductase are not clear. For example, formaldehyde Fd oxidoreductase only oxidizes C1–C3 aldehydes, whereas AOR has a broad substrate specificity and is able to use both aliphatic and aromatic aldehydes (18). The latter correspond to the aldehyde derivatives of transaminated amino acids, but it is not known how such aldehydes are generated.
An NADP(H)-dependent alcohol dehydrogenase (ADH) is present in various species of Pyrococcus and Thermococcus, organisms that grow well in the absence of S°, although its specific activity in cell-free extracts is quite low (19). Moreover, in Thermococcus strain ES-1, a peptide-utilizing archaeon whose growth is obligately dependent upon S°, the cellular concentration of ADH increased 20-fold when ES-1 was grown under S°-limited conditions (20). Kinetic analysis of pure ES-1 ADH showed that the enzyme preferentially used aldehydes rather than alcohols as substrates, and it was postulated that such aldehydes were generated during amino acid oxidation, although the mechanism was unclear (20).
Here we have identified a source of the aldehydes that are proposed to serve as substrates for AOR and ADH. It is shown that during pyruvate oxidation by POR from P. furiosus, a significant fraction of the substrate is converted to acetaldehyde in a CoA-dependent reaction. We suggest that this may be a general property of all 2-ketoacid oxidoreductases.
MATERIALS AND METHODS
Purification of POR.
P. furiosus (DSM 3638) was grown in a 500-liter fermentor (21) and POR (6) and Fd (22) were purified under anaerobic conditions from the harvested cells. The pyruvate oxidation activity of POR was routinely determined by the pyruvate- and CoA- dependent reduction of methyl viologen under anaerobic conditions at 80°C (6). The enzyme preparation used had a specific activity of 20 μmol pyruvate oxidized per min/mg under these conditions.
Determination of Acetaldehyde and Acetyl-CoA.
The pyruvate decarboxylation activity of P. furiosus POR was measured by acetaldehyde production. For the routine assay, the 2 ml mixture was prepared in a vial (8 ml) sealed with a stopper under argon and contained the following: 50 mM CAPS (3-[cyclohexylamino]-1-propanesulfonic acid) (pH 10.2), 10 mM pyruvate, 0.1 mM thiamine pyrophosphate (TPP), 1.0 mM CoA, and 5 μM Fd. The vial was shaken (150 rpm) in a water bath at 80°C for 1 min and the reaction was initiated by the addition of 92 μg POR. After 20 min, the reaction was stopped by transferring the vials to an ice bath and adding 2 ml of saturated 2,4-dinitrophenylhydrazine (DNPH) in 2 M HCl. The vial was then incubated at 35°C with shaking (150 rpm) for 48 hr. The acetaldehyde–DNPH derivative that was formed was extracted by adding 1 ml of methylene dichloride and shaking (350 rpm) at 35°C for 15 min. After centrifugation (1,000 × g for 5 min), the organic phase was removed and the extraction process was repeated. The combined organic phases were evaporated by incubation for 16 hr in a vacuum desiccator. The dry yellowish-red powder (containing both acetaldehyde–DNPH and excess DNPH) was resuspended in acetonitrile (8 ml) and after 16 hr at 4°C the solution was filtered (0.2 μm, Gelman). An aliquot (0.7 ml) was analyzed using a high-performance liquid chromatograph (Dionex) fitted with an Ultracarb 5 μ ODS column (250 × 4.6 mm, Phenomenex, Torrance, CA). The mobile phase was acetonitrile/water (80:20), and the flow-rate was 1 ml/min. Known concentrations of acetaldehyde were analyzed under the same assay conditions to obtain a calibration curve. Acetyl-CoA formation from pyruvate oxidation catalyzed by POR was measured by a coupled malate dehydrogenase/citrate synthase assay (23). Both enzymes were obtained from Boehringer Mannheim.
RESULTS AND DISCUSSION
Acetaldehyde Formation from Pyruvate.
It had been previously shown that pure P. furiosus POR, in the presence of CoA, catalyzes the anaerobic oxidation of pyruvate to acetyl-CoA and CO2 at 80°C. P. furiosus Fd or artificial electron acceptors such as methyl viologen serve as the electron acceptor (6). We have now found that under the same conditions, acetaldehyde is also a catalytic product of the POR reaction. P. furiosus POR contains four different subunits and the enzyme preparation used here was homogeneous as judged by SDS/PAGE (Fig. 1), indicating that acetaldehyde production is not catalyzed by a contaminating enzyme. As shown in Table 1, acetaldehyde production, as measured by its DNPH derivative, was dependent upon pyruvate and CoA, but this activity did not require the addition of Fd. Moreover, desulfocoenzyme A was as effective as CoA in the decarboxylation reaction, showing that this cofactor does not have a catalytic role in acetaldehyde production. In contrast to some PORs from mesophilic organisms, e.g., ref. 24, P. furiosus POR contains tightly bound TPP (1.0 ± 0.1 mol TPP/tetramer; ref. 6), and additional TPP had no effect on either its pyruvate oxidation or pyruvate decarboxylation activities (Table 1). No acetaldehyde was produced if the reaction was carried out aerobically, or if POR was omitted (Table 1). Therefore, in addition to pyruvate oxidation, POR catalyzes a CoA-dependent, anaerobic, nonoxidative decarboxylation of pyruvate to acetaldehyde and CO2.
Figure 1.
SDS/PAGE of purified POR from P. furiosus. Lanes: 1 and 4, molecular weight standard marker; 2, 2.5 μg of purified POR; 3, 5 μg of purified POR.
Table 1.
Acetaldehyde production from pyruvate catalyzed by P. furiosus POR
| Conditions | Acetaldehyde produced, μmol | Relative activity, % |
|---|---|---|
| Complete assay* | 7.72 | 100 |
| −pyruvate | 0.028 | 0.4 |
| −TPP | 7.62 | 99 |
| −CoA | 0.056 | 0.7 |
| −Fd | 7.40 | 96 |
| −POR | 0.066 | 0.9 |
| +air† | 0.070 | 0.9 |
| +desulfocoenzyme A‡ | 8.03 | 104 |
Acetaldehyde production was measured at 80°C under standard assay conditions as described.
The 2 ml assay mixture contained 10 mM pyruvate, 0.1 mM TPP, 1.0 mM CoA, 5 μM P. furiosus Fd, and 92 μg P. furiosus POR in 50 mM CAPS (3-[cyclohexylamino]-l-propanesulfonic acid), pH 10.2.
The reaction was carried out under aerobic conditions.
Complete assay without CoA and with desulfocoenzyme A (1.0 mM).
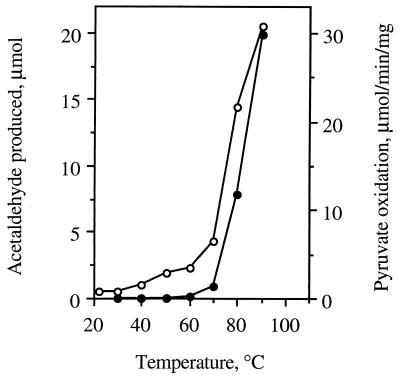
The temperature dependence of acetaldehyde production by POR was consistent with this being an enzyme-catalyzed reaction rather than a direct chemical process. As shown in Fig. 2, acetaldehyde production was significant only at temperatures above 60°C, with an optimum above 90°C. These data mirror the temperature dependence of the pyruvate oxidation activity of POR (as measured by methyl viologen reduction; Fig. 2), as well as that of several other P. furiosus enzymes (for example, see refs. 12, 14, and 16). Notably, the rate of acetaldehyde production was directly proportional to the amount of POR added to the reaction mixture (data not shown). The pH dependence of pyruvate decarboxylation was also consistent with an enzyme-catalyzed reaction (Fig. 3). Maximal activity was observed near pH 10.0, with about 60% of this activity at pH 8. In contrast, pyruvate oxidation activity of POR is maximal at pH 8, with about 10% of this activity at pH 10 (6). These data obviously suggest that POR is a bifunctional enzyme that catalyzes both the oxidative and nonoxidative decarboxylation of pyruvate.
Figure 2.
Temperature dependence of acetaldehyde formation from pyruvate and of the pyruvate oxidation activity of P. furiosus POR. •, Acetaldehyde production; ○, pyruvate oxidation (data were taken from ref. 6).
Figure 3.
pH dependence of acetaldehyde formation from pyruvate and of the pyruvate oxidation activity of P. furiosus POR. •, Acetaldehyde production measured as described in Materials and Methods, except that the buffers used 50 mM phosphate (pH 6.4), 50 mM EPPS [N-(2-hydroxyethyl)piperazine-N′-3-propanesulfonic acid] (pH 7.2–8.6) or 50 mM CAPS (pH 9.6–11.5), and the incubation time was 5 rather than 20 min; ○, pyruvate oxidation activity catalyzed by POR (data were taken from ref. 6).
Kinetics of Pyruvate Decarboxylation.
The rate of pyruvate decarboxylation reaction of POR was fairly constant over a 40 min period after a short but reproducible lag phase (data not shown). Within 40 min, approximately 80% of the pyruvate (10 mM) initially present in the reaction mixture had been converted to acetaldehyde. Under standard assay conditions at 80°C, kinetics parameters for the reaction were estimated by varying the concentrations of pyruvate (0.5–10 mM, using 1.0 mM CoA) and CoA (0.05–1.0 mM, using 10 mM pyruvate). In both cases, linear Lineweaver–Burk plots were obtained, confirming that the reaction is enzyme catalyzed. The apparent Km values for pyruvate and CoA were 1.1 mM and 0.11 mM, respectively. These values are very similar to those previously determined for the pyruvate oxidation reaction of POR (0.46 mM and 0.11 mM for pyruvate and CoA, respectively; ref. 6). Because CoA does not play a catalytic role in acetaldehyde production, its Km value may be appropriately interpreted as its binding affinity.
These kinetic data also indicate that in the standard assay for the pyruvate decarboxylation reaction, which used 10 mM pyruvate and 1.0 mM CoA, both substrates were approaching saturating concentrations. In support of this, the measured rate of aldehyde production and the calculated apparent Vm value were very similar (4.3 ± 0.3 μmol acetaldehyde produced per min/mg). For the pyruvate oxidation activity of POR using Fd as the electron acceptor (apparent Km, 7 μM; ref. 6), the apparent Vm value at 80°C was reported to be 7.4 μmol pyruvate oxidized per min/mg at 80°C and pH 8.0 (6). Hence, at 80°C, the rates of pyruvate decarboxylation by POR were about 60% (at pH 10.2) and 40% (at pH 8.0) of the rate of pyruvate oxidation activity of the enzyme (at pH 8.0).
Pyruvate Oxidation Versus Decarboxylation.
POR is irreversibly inactivated by oxygen (6) and in the presence of O2 (air) does not catalyze either the oxidation or decarboxylation of pyruvate (Table 1). Pyruvate oxidation obviously requires the presence of an electron acceptor such as Fd (6), but the pyruvate decarboxylation reaction was not dependent upon Fd, and Fd did not inhibit the reaction (using a concentration equivalent to its apparent Km value in the oxidation reaction; ref. 6). Similarly, desulfocoenzyme, which supports the decarboxylation reaction, inhibited pyruvate oxidation by POR (data not shown). These data suggest that pyruvate decarboxylation by POR is not an alternative reaction to pyruvate oxidation, rather, the two reactions must occur simultaneously, providing a suitable electron acceptor is present. We therefore analyzed for the production of both acetaldehyde and acetyl-CoA by POR in the same reaction mixture (using 10 mM methyl viologen as the electron acceptor). As shown in Fig. 4, POR did indeed generate both products with the initial rate of acetaldehyde production being ≈10% of that of acetyl-CoA. However, the rate of acetyl-CoA formation decreased with time, presumably as the concentration of reduced electron acceptor (methyl viologen) increased, whereas the rate of acetaldehyde production appeared to increase under the same conditions (Fig. 4).
Figure 4.
Simultaneous production of acetyl–CoA and acetaldehyde catalyzed by P. furiosus POR. The reaction was carried out as described in the legend to Table 1, except that methyl viologen (10 mM) replaced P. furiosus Fd and the POR concentration was 11 μg/ml. •, Acetaldehyde production; ○, acetyl-CoA production.
Catalytic Mechanisms of Decarboxylation.
In addition to the P. furiosus enzyme, PORs have been purified from various microorganisms, most of which are strict anaerobes (24–32). In contrast, most aerobic organisms carry out pyruvate oxidation using the pyruvate dehydrogenase complex (33). The reactions catalyzed by pyruvate dehydrogenase and POR both involve the formation of a hydroxyethyl–TPP complex and the transfer of the acyl moiety to CoA. Pyruvate oxidation by pyruvate dehydrogenase involves acyl transfer by lipoic acid and the overall mechanism has been firmly established (33). In contrast, PORs lack lipoic acid and mechanisms based on a TPP–radical species have been proposed (25, 31), including for the P. furiosus enzyme (34). PORs do contain multiple iron–sulfur clusters, and these participate in electron transfer to the external electron acceptor, typically Fd (23, 35). Although the mechanism by which PORs catalyze acyl transfer to CoA in the absence lipoic acid is not clear (36), the newly discovered acetaldehyde production activity of POR reported herein can be evaluated in mechanistic terms.
Although acetaldehyde production by P. furiosus POR is most easily explained by its direct conversion from the hydroxyethyl–TPP intermediate, this cannot be the case, because the reaction is dependent upon CoA. The decarboxylation reaction of POR must therefore differ from that of pyruvate decarboxylases. Pyruvate decarboxylases have been purified from a variety of organisms (37–41) and the crystal structure of one is known (42, 43). That they are also TPP-containing enzymes and the mechanism of pyruvate decarboxylation, which does involve direct conversion of the hydroxyethyl–TPP complex to the aldehyde, is well understood (43–45). In contrast to POR, the pyruvate decarboxylation reaction of pyruvate decarboxylases is unaffected by O2, nor does it require CoA. Because acetaldehyde production by POR was dependent upon CoA (Table 1), and the affinity of the enzyme for CoA (as determined by the apparent Km value) was the same as in the pyruvate oxidation reaction, it seems likely that CoA binds to the same site on the enzyme for both the decarboxylation and oxidation reactions. The ability of the desulfocoenzyme to support acetaldehyde production shows that CoA must have a structural role in that its binding to the enzyme is a prerequisite for further catalysis.
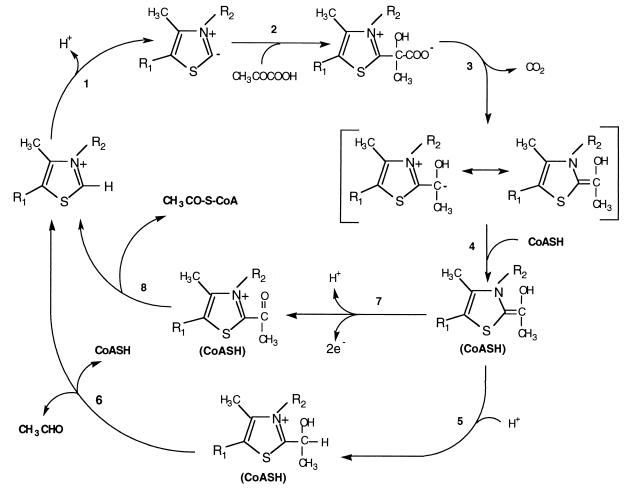
Based on all of the kinetic data for P. furiosus POR and established TPP chemistry, we propose a “switch” mechanism for the bifunctional activity of this enzyme. As shown in Fig. 5, the enzyme-bound TPP cofactor loses a proton to generate the ylid form (Step 1) (43). Based on the pyruvate decarboxylase reaction, the ylid form of TPP attacks the carbonyl carbon of pyruvate (Step 2), and after the release of CO2, a resonance-stabilized carbanion will be generated (Step 3). The conversion of the hydroxyacyl–TPP intermediate to either acetyl-CoA or acetaldehyde depends upon the binding of CoA (Step 4). This must cause a conformational change in the enzyme, which allows catalysis to continue. Thus, the carbanion is protonated (Step 5) to generate hydroxyethyl–TPP, which eliminates acetaldehyde to regenerate TPP and presumably CoA is released (Step 6). At this point it is not clear whether CoA bind to the enzyme before or after the binding of substrate. However, the catalytic cycle of the decarboxylation (Fig. 5) remains unchanged.
Figure 5.
Proposed catalytic mechanism of pyruvate decarboxylation under different conditions. See text for details.
This proposed mechanism for pyruvate decarboxylation is independent of the redox state of POR, but this is not the case for the oxidation of pyruvate. For acetyl-CoA to be produced, the hydroxyacyl–TPP intermediate must first be oxidized in a two electron step to generate acetyl–TPP (Step 7). This must occur by two separate one electron transfer reactions, since the iron–sulfur clusters of POR are one electron carriers. Consequently, a hydroxyacyl TPP radical intermediate can be observed under certain conditions in vitro (25, 31, 34). The iron–sulfur clusters of POR ultimately donate their electrons to Fd, and acetyl-CoA is released (Step 8). In the presence of excess oxidized Fd, which accepts the electrons released from Step 7, this catalytic cycle can be repeated. However, acetyl–TPP cannot be generated (Step 7) if the iron–sulfur clusters of POR are reduced, which would eventually occur after enzyme turnover in the absence of Fd. Hence, continued acetyl–CoA production is dependent upon Step 7, which depends upon the ability of the enzyme to dispose of the reductant generated from pyruvate oxidation. The redox state of the enzyme in vivo will be determined by the ability of the cell to oxidize reduced Fd and the overall redox states of the cytoplasm, which in turn will depend on the growth conditions.
Physiological Significance of Pyruvate Decarboxylation by POR.
From the results presented here we conclude that acetaldehyde production by P. furiosus POR is an intrinsic property of the enzyme, and presumably, this reaction must also occur in vivo. POR is present in significant amounts in the cytoplasm of this organism (6), and the enzyme plays an essential role in the primary pathway of carbohydrate fermentation. Moreover, P. furiosus and related organisms contain three other Fd-dependent, 2-keto acid oxidoreductases (IOR, VOR, and KGOR), which are highly similar to POR in all of their properties except substrate specificity (13, 35, 36). For example, POR and VOR of P. furiosus have one of their four subunits in common and overall show approximately 45% sequence identity (35). Thus, it is further assumed that, like POR, these other enzymes also produce aldehydes in vivo. Our preliminary experiments with IOR (12), analogous to those reported here for POR, show that phenyl acetaldehyde is produced during the oxidation of phenyl pyruvate (data not shown). Therefore, the question arises as to the metabolic fate of the aldehydes that these enzymes generate.
Two different types of aldehyde-utilizing enzyme have been purified and characterized from hyperthermophilic heterotrophic archaea such as P. furiosus, AOR (16, 18) and ADH (19, 20). Both types exhibit high catalytic efficiencies with the aldehydes that would be produced by the four 2-keto acid oxidoreductases present in these organisms. For example, the best substrates for AOR are acetaldehyde, phenylacetaldehyde, and isovaleraldehyde (Km values < 100 μM; ref. 18), which would be the primary aldehydes produced by POR, IOR and VOR, respectively. Similarly, ADH reduces acetaldehyde and phenylacetaldehyde with high efficiency (Km values < 250 μM; ref. 20). AOR is present in significant concentrations in the cytoplasm of hyperthermophilic archaea that do not require S° for growth, such as P. furiosus (5), as well as in those that do, such as Thermococcus strain ES-1, which grows obligately, dependent upon the presence of S° (20). Both types of organisms produce organic acids as end products of fermentation (3, 20). Hence, as shown in Fig. 6, AOR is proposed to be the primary enzyme responsible for oxidizing the aldehydes that are produced by the 2-keto acid oxidoreductases.
Figure 6.
Proposed pathway for the metabolism of aldehydes produced during glucose and amino acid fermentation in heterotrophic hyperthermophilic archaea. POR, IOR, VOR, and KGOR represent oxidoreductases that use pyruvate, indolepyruvate, 2-keto isovalerate, and 2-ketoglutarate, respectively. Fdox and Fdred, oxidized and reduced Fd, respectively; ACS, acyl-CoA synthetase.
The situation is different with ADH, since only low activities of this enzyme are present in species of Pyrococcus and Thermococcus under the usual conditions used to grow these organisms (19, 20). However, under S° limitation, the ADH activity of Thermococcus strain ES-1 increases dramatically and alcohols are excreted into the medium. Presumably, in the absence of sufficient amounts of the terminal electron acceptor, S°, reductant is disposed of using the ADH reaction, wherein aldehydes are reduced to alcohols. Thus, in this case, AOR and ADH would “compete” for aldehydes produced by peptide fermentation. From the perspective of maintaining the cellular redox balance, the ADH reaction, which generates an oxidized electron carrier (NADP), would be preferred to the AOR reaction, as this generates a reduced one (reduced Fd). Although ADH expression is regulated by S° availability, this appears not to be the case with AOR, since its activity in extracts of ES-1 were unaffected by S° limitation (20). Assigning a physiological role to P. furiosus AOR is significant, because this enzyme is one of the best characterized of all hyperthermophilic, as well as tungsten-containing, enzymes (15, 45).
Another issue is whether aldehyde production is a general property of all 2-keto acid oxidoreductases, not just those from the hyperthermophilic archaea. Interestingly, some thermophilic and mesophilic acetogens exhibit tungsten-dependent growth and they contain an enzyme termed carboxylic acid reductase, whose molecular and catalytic properties resemble those of P. furiosus AOR (15, 46). Carboxylic acid reductase represents about 4% of the cytoplasmic protein but its function is unknown. Because these acetogenic organisms also contain significant amounts of pyruvate oxidoreductase, a role for carboxylic acid reductase in oxidizing the acetaldehyde produced by the clostridial POR is possible. On the other hand, the hyperthermophilic archaea are considered the most slowly evolving of all known organisms (47), and sequence analyses indicate that the POR of P. furiosus represents an ancestral-type compared with mesophilic PORs (35). Thus, the latter enzymes may have evolved to prevent or minimize the anaerobic, CoA-dependent aldehyde production seen in the hyperthermophilic oxidoreductases. It will obviously be of great interest to determine if all POR-type enzymes decarboxylate 2-keto acids.
Acknowledgments
We thank Dr. Tadhg Begley for suggesting the desulfocoenzyme experiments and for helpful discussions. This research was supported by grants from the Department of Energy (FG09–88ER13901) and the National Science Foundation (BC5-9632657).
ABBREVIATIONS
- POR
pyruvate ferredoxin oxidoreductase
- Fd
ferredoxin
- IOR
indolepyruvate Fd oxidoreductase
- VOR
2-ketoisovalerate Fd oxidoreductase
- KGOR
2-ketoglutarate Fd oxidoreductase
- ADH
alcohol dehydrogenase
References
- 1.Stetter K O, Fiala G, Huber G, Huber R, Segerer G. FEMS Microbiol Rev. 1990;75:117–124. [Google Scholar]
- 2.Blöchl E, Burggraf S, Fiala F, Lauerer G, Huber G, Huber R, Rachel R, Segerer A, Stetter K O, Völkl P. World J Microbiol Biotechnol. 1995;11:9–16. doi: 10.1007/BF00339133. [DOI] [PubMed] [Google Scholar]
- 3.Fiala G, Stetter K O. Arch Microbiol. 1986;145:56–61. [Google Scholar]
- 4.Kengen S W M, Debok F A M, Vanloo N D, Dijkema C, Stams A J M, Devos W M. J Biol Chem. 1994;269:17537–17541. [PubMed] [Google Scholar]
- 5.Mukund S, Adams M W W. J Biol Chem. 1995;270:8389–8392. doi: 10.1074/jbc.270.15.8389. [DOI] [PubMed] [Google Scholar]
- 6.Blamey J M, Adams M W W. Biochim Biophys Acta. 1993;1161:19–27. doi: 10.1016/0167-4838(93)90190-3. [DOI] [PubMed] [Google Scholar]
- 7.Schäfer T, Selig M, Schönheit P. Arch Microbiol. 1993;159:72–83. [Google Scholar]
- 8.Ma K, Adams M W W. J Bacteriol. 1994;176:6509–6517. doi: 10.1128/jb.176.21.6509-6517.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma K, Schicho R N, Kelly R M, Adams M W W. Proc Natl Acad Sci USA. 1993;90:5341–5344. doi: 10.1073/pnas.90.11.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma K, Zhou Z H, Adams M W W. FEMS Microbiol Lett. 1994;122:263–266. [Google Scholar]
- 11.Mai X, Adams M W W. J Inorg Chem. 1993;51:459. [Google Scholar]
- 12.Mai X, Adams M W W. J Biol Chem. 1994;269:16726–16732. [PubMed] [Google Scholar]
- 13.Heider J, Mai X, Adams M W W. J Bacteriol. 1996;178:780–787. doi: 10.1128/jb.178.3.780-787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly R M, Adams M W W. Antonie van Leeuwenhoek. 1994;66:247–270. doi: 10.1007/BF00871643. [DOI] [PubMed] [Google Scholar]
- 15.Kletzin A, Adams M W W. FEMS Microbiol Rev. 1996;18:5–64. doi: 10.1016/0168-6445(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 16.Mukund S, Adams M W W. J Biol Chem. 1991;266:14208–14216. [PubMed] [Google Scholar]
- 17.Mukund S, Adams M W W. J Biol Chem. 1993;268:13592–13600. [PubMed] [Google Scholar]
- 18.Heider J, Ma K, Adams M W W. J Bacteriol. 1995;177:4757–4764. doi: 10.1128/jb.177.16.4757-4764.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma K, Robb F T, Adams M W W. Appl Environ Microbiol. 1994;60:562–568. doi: 10.1128/aem.60.2.562-568.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma K, Loessner H, Heider J, Johnson M K, Adams M W W. J Bacteriol. 1995;177:4748–4756. doi: 10.1128/jb.177.16.4748-4756.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryant F O, Adams M W W. J Biol Chem. 1989;264:5070–5079. [PubMed] [Google Scholar]
- 22.Aono S, Bryant F O, Adams M W W. J Bacteriol. 1989;171:3433–3439. doi: 10.1128/jb.171.6.3433-3439.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Decker K. Methods Enzym Anal. 1985;7:187–192. [Google Scholar]
- 24.Wahl R C, Orme-Johnson W H. J Biol Chem. 1987;262:10489–10496. [PubMed] [Google Scholar]
- 25.Kerscher L, Oesterhelt D. Eur J Biochem. 1981;116:595–600. doi: 10.1111/j.1432-1033.1981.tb05377.x. [DOI] [PubMed] [Google Scholar]
- 26.Uyeda K, Rabinowitz J C. J Biol Chem. 1971;246:3111–3119. [PubMed] [Google Scholar]
- 27.Meinicke B, Bertram J, Gottschalk G. Arch Microbiol. 1987;152:244–250. doi: 10.1007/BF00409658. [DOI] [PubMed] [Google Scholar]
- 28.Williams K, Lowe P N, Leadlay P F. Biochem J. 1987;246:529–536. doi: 10.1042/bj2460529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brostedt E, Nordlund S. Biochem J. 1991;279:155–158. doi: 10.1042/bj2790155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blamey J M, Adams M W W. Biochemistry. 1994;33:1000–1007. doi: 10.1021/bi00170a019. [DOI] [PubMed] [Google Scholar]
- 31.Pieulle L, Guigliarelli B, Asso M, Dole F, Bernadac A, Hatchikian E C. Biochim Biophys Acta. 1995;1250:49–59. doi: 10.1016/0167-4838(95)00029-t. [DOI] [PubMed] [Google Scholar]
- 32.Hughes N L, Chalk P A, Clayton C L, Kelly D J. J Bacteriol. 1995;177:3953–3959. doi: 10.1128/jb.177.14.3953-3959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wieland O H. Rev Biochem Physiol Pharmacol. 1983;96:123–170. doi: 10.1007/BFb0031008. [DOI] [PubMed] [Google Scholar]
- 34.Smith E T, Blamey J M, Adams M W W. Biochemistry. 1994;33:1008–1016. doi: 10.1021/bi00170a020. [DOI] [PubMed] [Google Scholar]
- 35.Kletzin A, Adams M W W. J Bacteriol. 1996;178:248–257. doi: 10.1128/jb.178.1.248-257.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams M W W, Kletzin A. Adv Protein Chem. 1996;48:101–180. doi: 10.1016/s0065-3233(08)60362-9. [DOI] [PubMed] [Google Scholar]
- 37.Sieber M, König S, Hübner G, Schellenberger A. Biomed Biochim Acta. 1983;42:343–349. [PubMed] [Google Scholar]
- 38.Bringer-Meyer S, Schimz K L, Sahm H. Arch Microbiol. 1986;146:105–110. [Google Scholar]
- 39.Rivoal J, Ricard B, Pradet A. Eur J Biochem. 1990;194:791–797. doi: 10.1111/j.1432-1033.1990.tb19471.x. [DOI] [PubMed] [Google Scholar]
- 40.Zehender H, Trescher D, Ullrich J. Eur J Biochem. 1987;167:149–154. doi: 10.1111/j.1432-1033.1987.tb13316.x. [DOI] [PubMed] [Google Scholar]
- 41.Mücke U, König S, Hübner G. Biol Chem Hoppe-Seyler. 1995;376:111–117. doi: 10.1515/bchm3.1995.376.2.111. [DOI] [PubMed] [Google Scholar]
- 42.Dyda F, Furey W, Swaminathan S, Sax M, Farrenkopf B, Jordan F. Biochemistry. 1993;32:6165–6170. doi: 10.1021/bi00075a008. [DOI] [PubMed] [Google Scholar]
- 43.Arjunan P, Umland T, Dyda F, Swaminathan S, Furey W, Sax M, Farrenkopf B, Gao Y, Zhang D, Jordan F. J Mol Biol. 1996;256:590–600. doi: 10.1006/jmbi.1996.0111. [DOI] [PubMed] [Google Scholar]
- 44.Kluger R. Chem Rev. 1987;87:863–876. [Google Scholar]
- 45.Koga J C. Biochim Biophys Acta. 1995;1249:1–13. doi: 10.1016/0167-4838(95)00011-i. [DOI] [PubMed] [Google Scholar]
- 46.White H, Feicht R, Huber C, Lottspeich F, Simon H. Biol Chem Hoppe-Seyler. 1991;372:999–1005. doi: 10.1515/bchm3.1991.372.2.999. [DOI] [PubMed] [Google Scholar]
- 47.Woese C R, Kandler O, Wheelis M L. Proc Natl Acad Sci USA. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]