Abstract
Leukocytes contain both nicotinic and muscarinic receptors, and while activation of nicotinic receptors suppresses immune/inflammatory responses, the role of muscarinic receptors in immunity is unclear. We examined the effects of a muscarinic receptor antagonist (atropine) and agonist (oxotremorine), administered chronically through miniosmotic pumps, on immune/inflammatory responses in the rat. Results show that while oxotremorine stimulated, atropine inhibited the antibody and T-cell proliferative responses. Moreover, atropine also suppressed the turpentine-induced leukocytic infiltration and tissue injury, and inhibited chemotaxis of leukocytes toward neutrophil and monocyte/lymphocyte chemoattractants. Thus, activation of nicotinic and muscarinic receptors has opposite effects on the immune/inflammatory responses.
Keywords: muscarinic receptors; nicotinic receptors; atropine; oxotremorine; immunity, inflammation; chemotaxis
1. Introduction
The nervous system and the immune system communicate bidirectionally, and lymphoid tissues are innervated by the autonomic nervous system (Blalock, 1994). However, increasing evidence suggests the presence of a non-neuronal cholinergic system on many cell types, in particular, the immunocompetent cells at the site of inflammation (Kurzen et al., 2007). Cells of the immune system contain most components of the cholinergic system, including choline acetyltransferase, choline transporters, and acetylcholinesterase, and they produce acetylcholine (Kawashima and Fujii, 2004). In addition, lymphocytes contain both muscarinic receptors (mAChRs) and nicotinic receptors (nAChRs) (Bering et al., 1987; Kawashima and Fujii, 2004; Razani-Boroujerdi et al., 2007). Therefore, it is likely that the cholinergic system on immune cells plays a role in regulating the immune responses. Indeed, activation of nAChRs, either through direct interaction with nicotinic agonists or indirectly through activation of the autonomic and vagal systems, inhibits adaptive and innate immune responses (Sopori, 2002; Czura et al., 2003; Ulloa, 2005). Moreover, chronic nicotine administration increases the lung burden of influenza virus (Razani-Boroujerdi et al., 2004) and Cryptococcus neoformans (Razani-Boroujerdi et al., unpublished). Thus, activation of nicotinic receptors suppresses the immune system and increases the susceptibility to pathogenic infections.
The role of muscarinic receptors in modulating the immune system is not clear. Vagotomy and atropinization moderate lethal anaphylaxis and histamine shock (Levy et al., 1976), and carbacholine increases the number of leukocytes in the splenic venous blood that is blocked by atropine treatment (Sandberg, 1994). Direct addition of acetylcholine to spleen cell cultures enhances the Con A-induced T-cell proliferation (Qiu et al., 1996). Therefore, activation of mAChRs may activate the immune system. However, because acetylcholine can react with both nicotinic and muscarinic receptors, it is difficult to ascertain whether the effects reflect its interaction with muscarinic and/or nicotinic receptors. In this study, we used selective muscarinic agonists/antagonists to show that while mAChR agonists stimulate, mAChR antagonists inhibit immune and inflammatory responses. These data suggest that nAChRs and mAChRs might affect the immune/inflammatory responses in an opposite manner, and may represent the yin and yang of the immune system’s homeostasis.
2. Materials and Methods
2.1 Animals
Male pathogen-free Lewis rats were purchased from Charles River (Wilmington, MA). Animals were housed individually in filter-top plastic cages and maintained in a 12-h light/dark cycle at 30 ± 1°C. Food and water were provided ad libitum. Lovelace Respiratory Research Institute is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC; accreditation number 000200).
2.2 Reagents
Formyl-methionyl-leucyl-phenylalanine (fMLP) and recombinant rat monocyte chemoattractant protein-1 (MCP-1) were purchased from BD Pharmingen (San Diego, CA). All other reagents, unless otherwise mentioned, were obtained from Sigma (St. Louis, MO).
2.3 Drug treatment and immunization
Rats were anesthetized with isoflurane-oxygen, and 28-day constant release miniosmotic pumps (Alzet Corp., Palo Alto, CA) containing either atropine, oxotremorine, nicotine, nicotine plus mecamylamine, or saline were implanted subcutaneously in the back of the neck. The pumps delivered about 2 mg/kg body weight of atropine, nicotine, nicotine plus mecamylamine, or 0.1 mg/kg of oxotremorine per day. The blood cotinine concentration of nicotine-treated animals was less than that of two-pack per day human smokers (Geng et al., 1995). At 3 weeks after the treatment, rats were immunized with sheep red blood cells (SRBC) purchased from Colorado Serum Company (Denver, CO). Animals were injected intravenously (using the tail vein) with 2 × 109 SRBC in 200 µl saline 4 days prior to sacrifice.
2.4 Turpentine administration and histopathology
Sterile abscess was induced by subcutaneous injection of 100 µl turpentine in the hind leg, a method widely used to induce localized inflammation in animals (Kozak et al., 1997; Razani-Boroujerdi et al., 2004). Histopathology of tissue sections from control and turpentine-treated legs was carried out as described previously (Razani-Boroujerdi et al., 2004). Briefly, legs were fixed and decalcified, and serial cuts were made every 4 mm through the muscles near the site of turpentine injection. The tissue sections were stained with H&E and examined microscopically. Tissue injury/inflammation was scored on a scale from 0 (no detectable effect) to 4 (extensive inflammation and tissue necrosis).
2.5 Isolation of peripheral blood mononuclear cells (PBMC)
To obtain PBMC, 5 to 10 ml of rat heparinized blood was diluted with RPMI to get a total volume of 30 ml. The diluted blood was slowly added on top of 20 ml Histopaque-1083 in a 50-ml centrifuge tube and centrifuged over the gradient at 400 g for 30 min at room temperature. Leukocytes were collected, and washed twice with PBS containing 2% bovine serum albumin (BSA). Cells were resuspended in the same medium to be used for migration assays described previously (Razani-Boroujerdi et al., 2004).
2.6 Preparation of splenocytes
Spleen cells were prepared as described elsewhere (Geng et al., 1996). Briefly, spleens were pressed through stainless steel mesh and the red blood cells in the cell suspension were lysed by NH4Cl treatment. Cells were washed three times with PBS and resuspended in complete medium (RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 50 µM 2-mercaptoethanol, and 1% pen/strep).
2.7 Measurement of cell migration
A standard micropore filter assay using blind-well chambers was performed essentially as described (Razani-Boroujerdi et al., 2004). To obtain the chemoattractant concentration for optimal migration of PBMC, we determined the dose response for MCP-1 and fMLP. Briefly, the lower well of the chamber was filled with PBS-BSA in the presence or absence of various concentrations of MCP-1 or fMLP. The well was then overlaid with an 8-µm pore size, 150-µm-thick nitrocellulose filter prewetted in PBS-BSA. The upper well was screwed down onto the filter and 105 PBMC in 200 µl were added to the upper well. The chambers were then incubated at 37°C for 1–4 h and, at the end of incubation; the filters were harvested, fixed in 70% ethanol, stained with hematoxylin, dehydrated, cleared, and mounted for microscopy. All samples were tested in duplicate with three independent cell counts per replicate filter at the depth of 30-µm from the top of the filter at 400 X magnification. A total of six readings were taken from each sample. These experiments indicated that, under these conditions, rat PBMC required a 3-h incubation period for a reproducible migratory response, and that the optimal chemoattractant concentrations were 5 nM and 500 nM for MCP-1 and fMLP, respectively. These conditions were subsequently used for migration experiments.
2.8 Antibody-forming cell (AFC) assay
The anti-SRBC AFC assay was performed as described (Geng et al., 1996). Briefly, spleen cells (2 × 105 in 100 µl complete medium) were mixed with 20 µl of SRBC solution containing 25 × 106 SRBC and 20 µl of guinea pig complement (Cederlane, Hornby, ON, Canada) pre-absorbed on SRBC. Aliquots were distributed in duplicate on Cunningham slides and incubated for 45 min at 37°C. The AFC plaques were counted and normalized to 1 × 106 cells.
2.9 T-cell proliferation assay
Spleen cells (2 × 105 cells) were cultured in 0.2 ml of complete medium in the presence of 1, 3, or 5 µg/ml concentrations of Con A or 2 µg/ml anti-T-cell antigen-receptor (TCR) + CD28 in a 96-well plate. The cultures were incubated at 37°C in the presence of 5% CO2, and cells were harvested after 3 days by a Skatron cell harvester (Skatron Inc., Sterling, VA). Cell proliferation was assayed by pulsing the culture wells with 0.5 µCi of [3H]-thymidine (3H-Tdr; ICN, Irvine, CA) at 18 h before harvest.
2.10 Statistical analysis
A Student’s t-test or a two-way analysis of variance by Prism Software 3.0 (GraphPad Inc., San Diego, CA) was used for comparison of values in all experiments. A p ≤ 0.05 was considered statistically significant.
3. Results
3.1 Unlike nicotine, the mAChR agonist oxotremorine enhances T-cell responses
To test the hypothesis that activation of mAChRs activates the immune system, rats were treated for 3 weeks with the nonselective mAChR agonist oxotremorine that does not interact with the nAChRs. Saline- and oxotremorine-treated rats were immunized with the T-cell-dependent antigen SRBC, and splenocytes from these animals were tested for the antigen-specific antibody cell (AFC) response. As seen in Fig. 1A, oxotremorine treatment significantly enhanced the AFC response of spleen cells to SRBC. To determine whether oxotremorine also enhanced the T cell antigen-receptor (TCR)-mediated T-cell proliferation, spleen cells from control and oxotremorine-treated animals were cultured in the presence of anti-TCR + anti-CD28 antibodies and assayed for T-cell proliferation. Fig. 1B shows that oxotremorine treatment significantly elevated the TCR-mediated spleen cell proliferation. These results suggest that oxotremorine treatment enhances both the antigen-induced antibody response and the antigen-mediated T-cell proliferation.
Fig. 1. Oxotremorine treatment enhances anti-SRBC AFC and proliferation of spleen cells after ligation of the TCR.
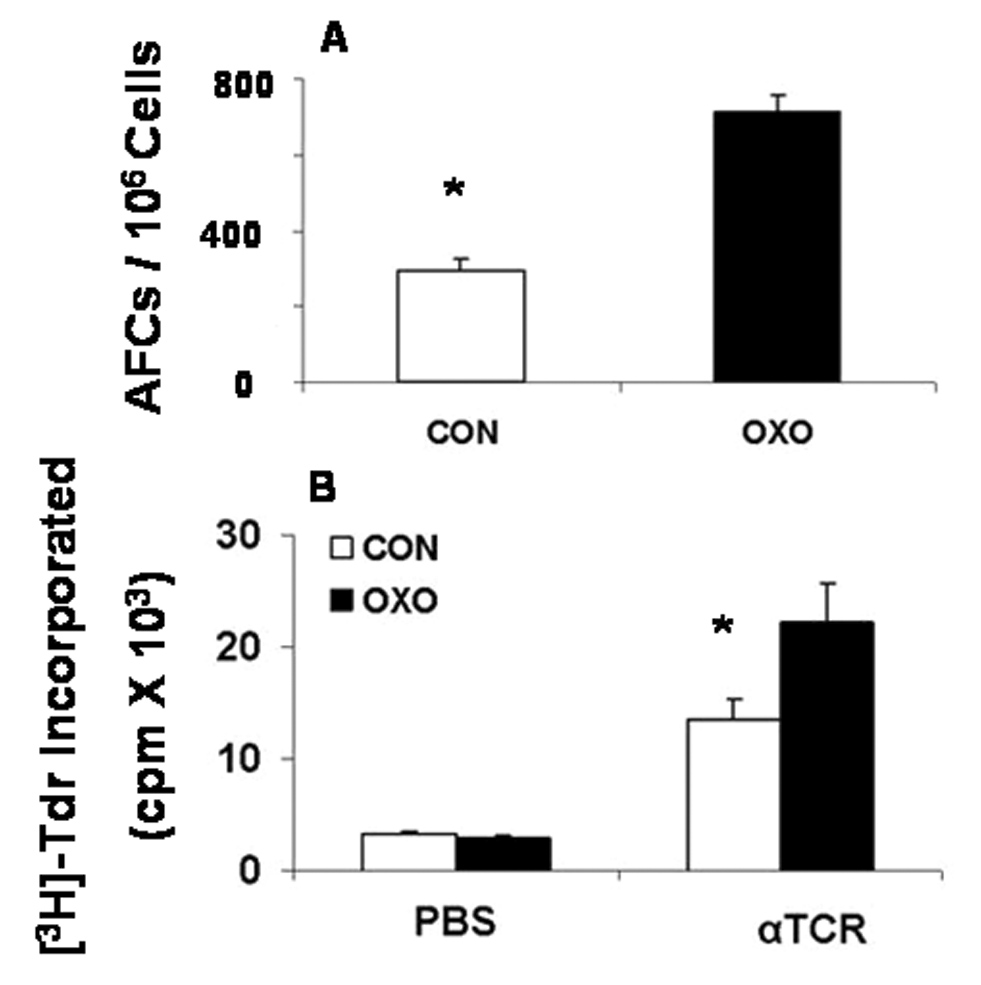
Lewis rats (6 animals/group) were exposed to saline (CON) or oxotremorine (OXO) for 3 weeks and immunized with SRBC intravenously as described in Material and Methods. (A) The AFC response is expressed as AFC/106 spleen cells. (B) Spleen cells from CON and OXO animals were cultured with previously determined optimal levels of anti-TCR + anti-CD28 (αTCR). The cultures were labeled with 3H-Tdr as described in Materials and Methods, and the results are presented as mean cpm ± SEM. * p < 0.05.
Although we and others have shown that nicotine treatment inhibits both immune and inflammatory responses (Sopori, 2002; Razani-Boroujerdi et al., 2004), to ascertain whether under the conditions of oxotremorine treatment nicotine suppresses T-cell responses, rats were treated in parallel with a nicotine concentration that produces blood nicotine/cotinine of less than a two-pack per day smoker (Geng et al., 1995), mecamylamine, or mecamylamine + nicotine. Results presented in Fig. 2 show that nicotine significantly suppressed the anti-SRBC AFC response (Fig. 2A) as well as the anti-TCR + CD28-mediated T-cell proliferation (Fig. 2B). Both responses were blocked by the nAChR antagonist mecamylamine (Fig. 2). Thus, unlike mAChR activation, activation of nAChRs suppresses the immune system and nicotinic receptor antagonists block the nicotine-induced immunosuppression.
Fig. 2. Nicotine inhibits T-cell-dependent immune responses.
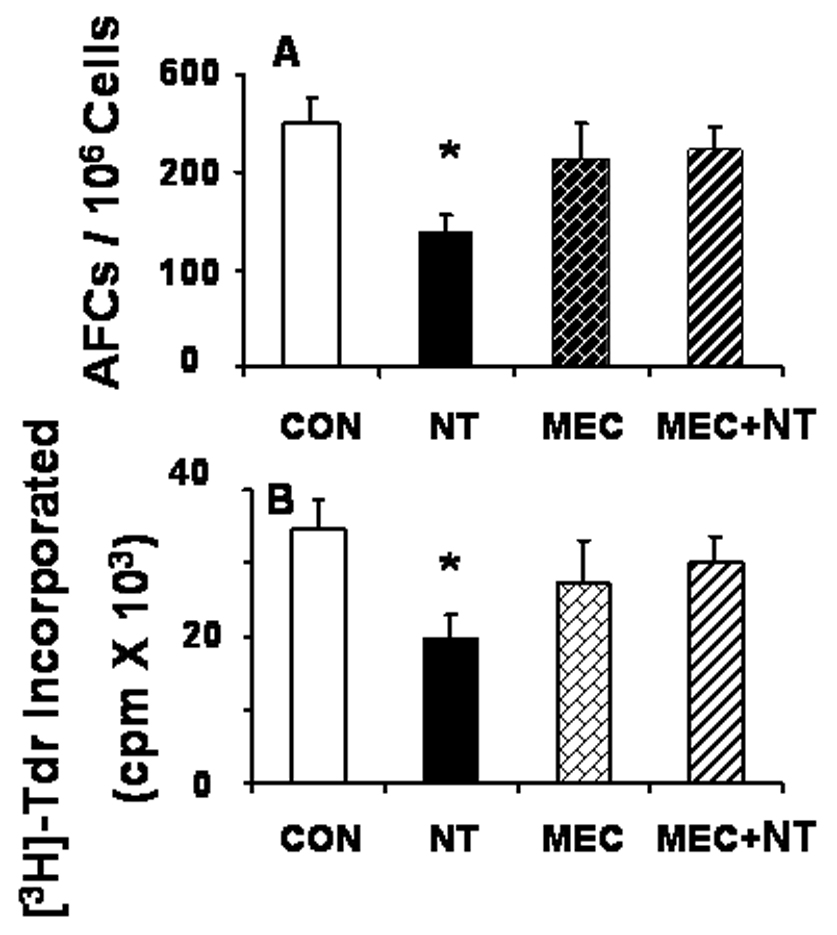
Rats (6 animals/group) were treated with saline (CON), nicotine (NT), mecamylamine (MEC), or MEC +NT for 3 weeks and then immunized with SRBC intravenously as described in Fig. 1. Animals were sacrificed on day 4 after immunization. Spleen cells were tested for the anti-SRBC AFC (A) and the anti-TCR + CD28 (αTCR, 2 µg/ml of each antibody)-mediated proliferative (B) responses as described in Materials and Methods. Bar graphs represent mean ± SEM. *Significant (p ≤ 0.05) change from CON and MEC + NT.
3.2 The mAChR antagonist atropine suppresses T-cell responses
To ascertain whether mAChR antagonists will affect the immune system opposite to that of the receptor agonists (i.e., suppress T-cell responses), rats were exposed to saline or the non-selective mAChR antagonist atropine for 3 weeks and then immunized with SRBC. As seen in Fig. 3A, atropine treatment strongly suppressed the anti-SRBC AFC response of spleen cells. Similarly, compared to saline-treated control spleen cells, atropine-treated spleen cells exhibited significantly lower proliferation in response to anti-TCR + CD28 antibodies (2 µg/ml) or the T-cell mitogen Con A (1µg/ml) (Fig. 3B). Similar results were obtained when cells were cultured with 3 and 5 µg/ml of Con A (not shown). These results suggest that while mAChR agonists stimulate, antagonists of mAChR inhibit the antigen-stimulated as well as mitogen-stimulated T-cell responses.
Fig. 3. Atropine treatment suppresses anti-SRBC AFC and T-cell mitogenic responses of splenocytes.

Lewis rats (6 animals/group) were exposed to atropine for 3 weeks then immunized with SRBC intravenously. The AFC response of the spleen cells was determined as described in Material and Methods and expressed as AFC/106 spleen cells (A). The mitogenic response to saline (CON), anti-TCR + CD28 antibodies (αTCR, 2 µg/ml), and Con A (1 µg/ml) (B) are expressed as mean cpm ± SEM. * p < 0.05.
3.3 Atropine attenuates inflammatory responses
To examine the possibility that, in addition to T-cell responses, mAChR antagonists also suppress innate immune responses, rats were treated for 3 weeks with saline or atropine, and then injected with turpentine in the leg to cause a sterile abscess. Animals were sacrificed at 48 h after turpentine administration. Microscopic examination of histology sections of the leg showed that while a saline injection did not cause any significant histological changes in the leg (i.e., histopathology score 0; not shown), tissue sections from control animals treated with turpentine exhibited a large accumulation of leukocytes near the site of turpentine injection in the leg (Fig. 4A). However, animals treated with atropine prior to turpentine injection had dramatically reduced inflammatory changes at the site of injection (Fig. 4B). Quantitation of various tissue injury indices (cellulitis, tissue necrosis, and inflammation) suggests that atropine treatment significantly reduced tissue injury in response to sterile abscess (Fig. 4C). These results suggest that atropine is an anti-inflammatory that suppresses both inflammation (leukocyte accumulation) and tissue injury in response to a sterile abscess.
Fig. 4. Chronic atropine treatment attenuates tissue inflammatory responses.
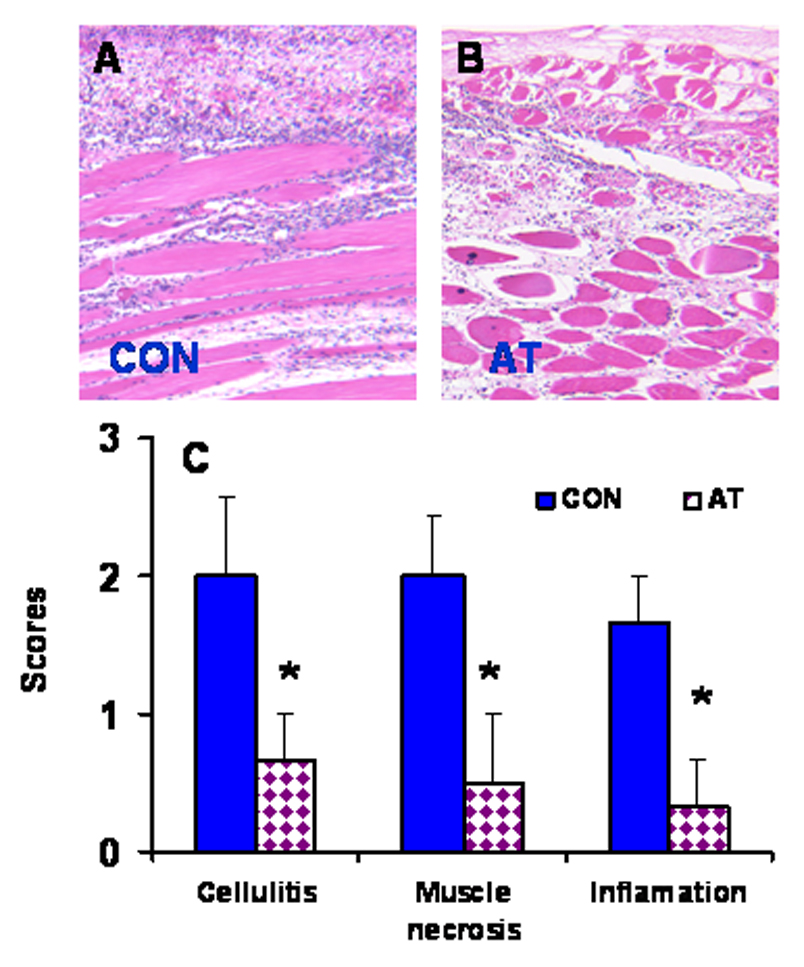
Animals were treated with atropine for 3 weeks and then injected with turpentine as described in Material and Methods. A representative of leg histopathology around the turpentine-injected (CON) site from six CON (A) and six atropine-treated (AT; panel B) rats. (C) Bar graphs representing mean ± SEM of various tissue injury responses to sterile abscess. * p < 0.05.
3.4 Atropine decreases chemokinesis and chemotaxis of PBMC
To ascertain if attenuation of inflammation by atropine resulted from the inability of leukocytes to respond to chemoattractants, we investigated the effects of atropine on the migratory behavior of PBMC in response to chemoattractants. PBMC cells were obtained from turpentine-treated control and atropine-treated animals. The migratory response of these cells was determined by the blind-well migration assay in the absence of chemoattractants (random), and in the presence of an optimal concentration of a chemoattractant in both the upper and the lower wells (chemokinesis) and in lower wells only (chemotaxis). While in the absence of chemoattractants (random), neither control- nor atropine-treated PBMC exhibited significant migration (Fig. 5), the presence of chemoattractant induced cell migration. However, atropine treatment reduced both chemokinesis and chemotaxis of PBMC in response to the potent neutrophil/macrophage chemoattractant fMLP (Fig. 5A). It also reduced the cell mobility in response to the lymphocyte/monocyte chemoattractant MCP-1 (Fig. 5B). Thus, atropine inhibits both chemokinesis and chemotaxis of leukocytes.
Fig. 5. Atropine inhibits leukocyte migration in response to fMLP, and MCP-1.
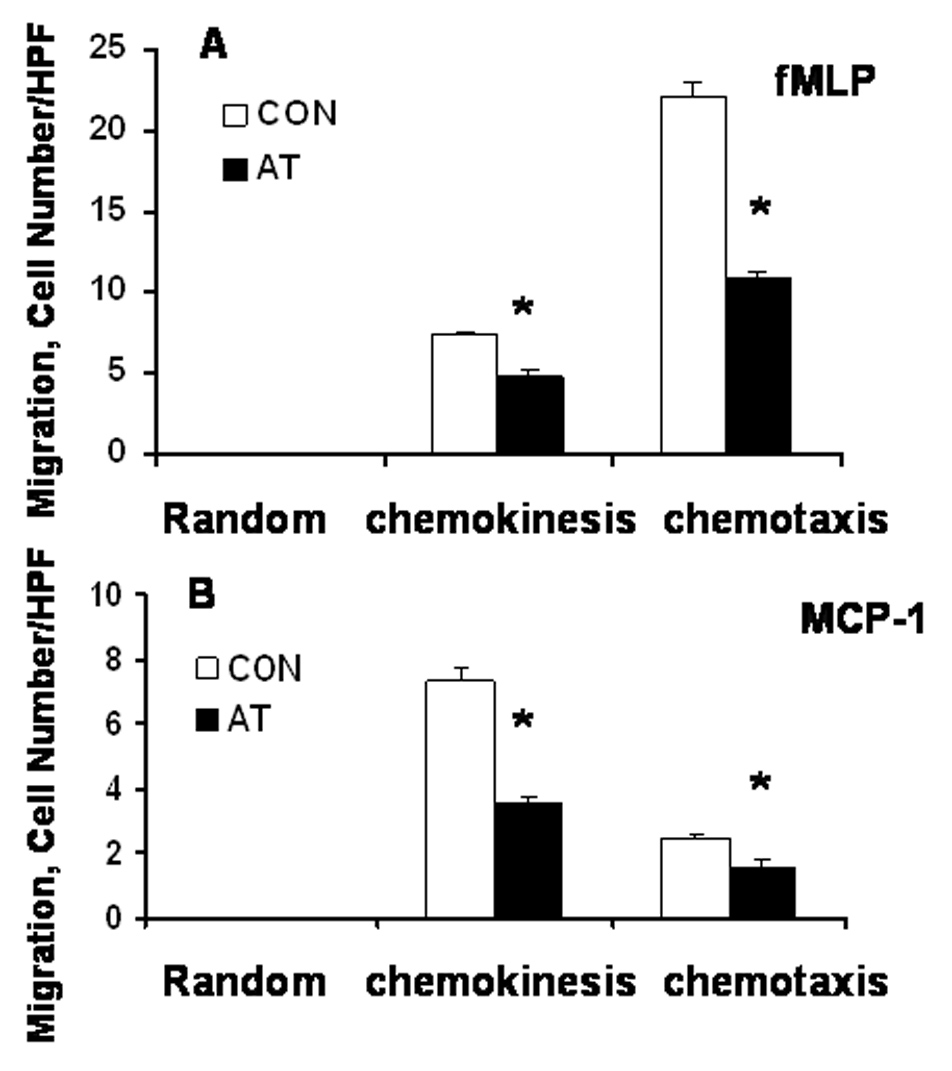
(A) Random migration (no chemoattractant) and fMLP-induced chemokinesis and chemotaxis of PBMC from (A) turpentine-treated (CON) and atropine-treated (AT) animals. (B) Random and MCP-1-induced migration of PBMC from CON and AT-treated animals. Bar graphs represent mean ± SEM (n = 6). *Significant change from CON (i.e., p ≤ 0.05).
4. Discussion
The occurrence of a non-neuronal cholinergic system has been shown in a number of cell types, including the cells of the immune system (Kurzen et al., 2007). T cells and monocytes have high levels of acetylcholine (Neumann et al., 2007), and T cells express nAChRs (Sato et al., 1999; Razani-Boroujerdi et al., 2007) as well as mAChRs (Okuma and Nomura, 2001; Kawashima et al., 2007). However, the relationship between nAChRs and mAChRs in the regulation of T-cell function is not clear. Because the only known biological ligand for both muscarinic and nicotinic receptors is acetylcholine, it would be difficult to tease out the contribution of nicotinic and muscarinic receptors on immune cell function by using acetylcholine-like molecules to activate these receptors. Previous studies have clearly shown that activation of nAChRs by nicotine suppresses both the adaptive and innate immune responses (Sopori, 2002; Czura et al., 2003; Razani-Boroujerdi et al., 2004); however, very little is known about the role of mAChRs in the regulation of immune and inflammatory responses. Muscarinic receptors might play an important function in some autoimmune diseases: for example, the density of muscarinic receptors is significantly increased on CD4+ T cells in patients with chronic progressive multiple sclerosis (Anlar et al., 1992), and muscarinic receptor agonist-like auto-antibodies may play a role in autoimmune idiopathic dilated cardiomyopathy (Fu et al., 1993). Preincubation of T cells with the muscarinic receptor agonist physostigmine enhances the mitogen-induced IL-2 production (Nomura et al., 2003; Okuma and Nomura, 2001). Similarly, acetylcholine enhances the Con A-induced T-cell proliferation in spleen cell cultures (Qiu et al., 1996). Moreover, development of cytotoxic T cells was defective in M1-muscarinic receptor knockout mice (Zimring et al., 2005; Vezys et al., 2007). On the other hand, Wen et al. (2006) observed that arecoline inhibited T-cell responses through the activation of mAChRs. Thus, immunostimulatory effects of mAChRs activation are debatable.
Belladonna extracts derived from Datura stramonium have been used over centuries in homeopathic medicine as a topical anti-inflammatory and in the treatment of Parkinsonism and asthma (Kapp, 1992; Sourkes, 1999). Belladonna contains atropine, and atropine was found to markedly reduce immune complex deposition in microvasculature and alleviate anaphylaxis (Levy et al., 1976). Results presented herein clearly show that chronic treatment with the mAChR agonist oxotremorine stimulates the T-cell-dependent responses, including the response of T cells to antigens (simulated by the ligation of TCR with anti-TCR antibodies), Con A-stimulated T-cell mitogenesis, and the AFC response to the T-cell-dependent antigen SRBC. Under the same conditions, however, nicotine inhibited the antibody response and T-cell proliferation, and the nicotine-induced suppression was blocked by the non-selective nAChR antagonist, mecamylamine. On the other hand, the mAChR antagonist atropine significantly inhibited the AFC response and T-cell proliferation in response to TCR ligation and Con A. In addition, atropine inhibited the migration of leukocytes toward the site of inflammation caused by turpentine-induced sterile abscess, and significantly decreased various indices of inflammation and tissue injury. This inference was further supported by the results that PBMC isolated from atropine-treated animals failed to exhibit chemokinesis in response to turpentine treatment and chemotaxis in response to neutrophil and monocyte/lymphocyte chemoattractants. Thus, while mAChR agonists stimulate immune/inflammatory responses, mAChR antagonists inhibit these responses. These results are opposite to those seen with nicotinic receptor agonists, which inhibit these responses, and while nicotinic antagonists do not per se stimulate the immune/inflammatory response, they block the immunosuppressive effects of nicotine. Therefore, it is likely that the activation of mAChRs and nAChRs stimulate and inhibit the immune and inflammatory responses, respectively. Hence the differential interaction of acetylcholine with nicotinic and muscarinic receptors may represent the yin and yang of immune regulation with cholinergic compounds.
Acknowledgements
This work was supported in part by grants from NIH (RO1 DA017003, R01 DA04208-15, and RO1 DA04208-7S) and from the Lovelace Respiratory Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anlar B, Karaszewski JW, Reder AT, Arnason BG. Increased muscarinic cholinergic receptor density on CD4+ lymphocytes in progressive multiple sclerosis. J. Neuroimmunol. 1992;36:171–177. doi: 10.1016/0165-5728(92)90048-p. [DOI] [PubMed] [Google Scholar]
- Bering B, Moises HW, Müller WE. Muscarinic cholinergic receptors on intact human lymphocytes. Properties and subclass characterization. Biol. Psychiatry. 1987;22:1451–1458. doi: 10.1016/0006-3223(87)90103-x. [DOI] [PubMed] [Google Scholar]
- Blalock JE. The syntax of immune-neuroendocrine communication. Immunol. Today. 1994;15:504–511. doi: 10.1016/0167-5699(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Czura CJ, Friedman SG, Tracey KJ. Neural inhibition of inflammation: the cholinergic anti-inflammatory pathway. J. Endotoxin Res. 2003;9:409–413. doi: 10.1179/096805103225002755. [DOI] [PubMed] [Google Scholar]
- Fu LX, Magnusson Y, Bergh CH, Liljeqvist JA, Waagstein F, Hjalmarson A, Hoebeke J. Localization of a functional autoimmune epitope on the muscarinic acetylcholine receptor-2 in patients with idiopathic dilated cardiomyopathy. J. Clin. Invest. 1993;91:1964–1968. doi: 10.1172/JCI116416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Savage SM, Johnson LJ, Seagrave J, Sopori ML. Effects of nicotine on the immune response. I. Chronic exposure to nicotine impairs antigen receptor-mediated signal transduction in lymphocytes. Toxicol. Appl. Pharmacol. 1995;135:268–278. doi: 10.1006/taap.1995.1233. [DOI] [PubMed] [Google Scholar]
- Geng Y, Savage SM, Razani-Boroujerdi S, Sopori ML. Effects of nicotine on the immune response. II. Chronic nicotine treatment induces T cell anergy. J. Immunol. 1996;156:2384–2390. [PubMed] [Google Scholar]
- Kapp W. The history of drugs for the treatment of Parkinson’s disease. J. Neural. Transm. Suppl. 1992;38:1–6. [PubMed] [Google Scholar]
- Kawashima K, Fujii T. Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function. Front Biosci. 2004;9:2063–2085. doi: 10.2741/1390. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007;80:2314–2319. doi: 10.1016/j.lfs.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Kozak W, Poli V, Soszynski D, Conn CA, Leon LR, Kluger MJ. Sickness behavior in mice deficient in interleukin-6 during turpentine abscess and influenza pneumonitis. Am. J. Physiol. 1997;272:R621–R630. doi: 10.1152/ajpregu.1997.272.2.R621. [DOI] [PubMed] [Google Scholar]
- Kurzen H, Wessler I, Kirkpatrick CJ, Kawashima K, Grando SA. The nonneuronal cholinergic system of human skin. Horm. Metab. Res. 2007;39:125–135. doi: 10.1055/s-2007-961816. [DOI] [PubMed] [Google Scholar]
- Levy RM, Rose JE, Johnson JS. Effect of vagotomy on anaphylaxis in the rat. Clin. Exp. Immunol. 1976;24:96–101. [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Razen M, Habermehl P, Meyer CU, Zepp F, Kirkpatrick CJ, Wessler I. The non-neuronal cholinergic system in peripheral blood cells: effects of nicotinic and muscarinic receptor antagonists on phagocytosis, respiratory burst and migration. Life Sci. 2007;80:2361–2364. doi: 10.1016/j.lfs.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Nomura J, Hosoi T, Okuma Y, Nomura Y. The presence and functions of muscarinic receptors in human T cells: the involvement in IL-2 and IL-2 receptor system. Life Sci. 2003;72:2121–2126. doi: 10.1016/s0024-3205(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Okuma Y, Nomura Y. Roles of muscarinic acetylcholine receptors in interleukin-2 synthesis in lymphocytes. Jpn. J. Pharmacol. 2001;85:16–19. doi: 10.1254/jjp.85.16. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Peng Y, Wang J. Immunoregulatory role of neurotransmitters. Adv. Neuroimmunol. 1996;6:223–231. doi: 10.1016/s0960-5428(96)00018-6. [DOI] [PubMed] [Google Scholar]
- Razani-Boroujerdi S, Singh SP, Knall C, Hahn FF, Pena-Philippides JC, Kalra R, Langley RJ, Sopori ML. Chronic nicotine inhibits inflammation and promotes influenza infection. Cell Immunol. 2004;230:1–9. doi: 10.1016/j.cellimm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Razani-Boroujerdi S, Boyd RT, Davila-Garcia MI, Nandi JS, Mishra NC, Singh SP, Pena-Philippides JC, Langley R, Sopori ML. T cells express {alpha}7-nicotinic acetylcholine receptor subunits that require a functional TCR and leukocyte-specific protein tyrosine kinase for nicotine-induced Ca2+ response. J. Immunol. 2007;179:2889–2898. doi: 10.4049/jimmunol.179.5.2889. [DOI] [PubMed] [Google Scholar]
- Sandberg G. Leukocyte mobilization from the guinea pig spleen by muscarinic cholinergic stimulation. Experientia. 1994;50:40–43. doi: 10.1007/BF01992047. [DOI] [PubMed] [Google Scholar]
- Sato KZ, Fujii T, Watanabe Y, Yamada S, Ando T, Kazuko F, Kawashima K. Diversity of mRNA expression for muscarinic acetylcholine receptor subtypes and neuronal nicotinic acetylcholine receptor subunits in human mononuclear leukocytes and leukemic cell lines. Neurosci. Lett. 1999;266:17–20. doi: 10.1016/s0304-3940(99)00259-1. [DOI] [PubMed] [Google Scholar]
- Sopori M. Effects of cigarette smoke on the immune system. Nat. Rev. Immunol. 2002;2:372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- Sourkes TL. “Rational hope” in the early treatment of Parkinson’s disease. Can. J. Physiol. Pharmacol. 1999;77:375–382. [PubMed] [Google Scholar]
- Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat. Rev. Drug Discov. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- Vezys V, Masopust D, Desmarets M, Wess J, Zimring JC. Analysis of CD8+ T cell-mediated anti-viral responses in mice with targeted deletions of the M1 or M5 muscarinic cholinergic receptors. Life Sci. 2007;80:2330–2333. doi: 10.1016/j.lfs.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen XM, Zhang YL, Liu XM, Guo SX, Wang H. Immune responses in mice to arecoline mediated by lymphocyte muscarinic acetylcholine receptor. Cell Biol. Int. 2006;30:1048–1053. doi: 10.1016/j.cellbi.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Zimring JC, Kapp LM, Yamada M, Wess J, Kapp JA. Regulation of CD8+ cytolytic T lymphocyte differentiation by a cholinergic pathway. J. Neuroimmunol. 2005;164:66–75. doi: 10.1016/j.jneuroim.2005.03.018. [DOI] [PubMed] [Google Scholar]


