Abstract
Evidence suggests that compensatory behaviors operate in infants and preschool children, such that the high variance characteristic of single eating occasions is much reduced over the day. However, the concept has not been fully explored in adults. The present with-in subject, observational study investigated short-term dietary compensation patterns in fifty, weight-stable, normal weight (n = 27), overweight (n = 14), and obese (n = 9) free-living adults (11M, 39F; age 30 ± 11 y; BMI 26.3 ± 5.9). Twenty four-hour diet recalls were obtained for 7 consecutive days, by the multi-pass technique. Each 24-h period was divided into 7 eating occasions. The coefficient of variation for energy intake was calculated for each adult, for each eating occasion, and over each 24-h period. Sub-group variability was assessed by BMI and frequency of consumption of sweetened energy-yielding beverages. The mean coefficient of variation for energy intake for the 7 eating occasions was 110.5%, compared to 28.9% for the day as a whole. Correlations between energy intakes at successive eating events were uniformly negative. No significant differences were noted in the sub-group analyses. Significantly greater variation in energy intake was noted for snacks compared to meals (P < 0.0001). These data suggest that adults regulate energy intake over a 24-h period more closely than they do at individual eating occasions, similar to the pattern previously observed in children. Further studies of compensatory responses by larger sub-groups of individuals at risk for weight gain are warranted.
Keywords: Short-term energy regulation, dietary compensation, food intake, snacking, energy-yielding beverages
Introduction
Maintenance of energy balance requires ongoing adjustments of energy intake to match energy expenditure [1,2,3,4]. Whether the increasing incidence of overweight/obesity [5,6] is due to increased energy intake [7–9] or declining energy expenditure [10,11] has not been established, but it is clear that energy consumption has been exceeding energy expenditure. This fact raises questions about the accuracy and precision of compensatory dietary behaviors in humans. Ultimately, accuracy is of primary importance for energy balance while knowledge of precision is vital for accurate measurement of intake. Evidence suggests that compensatory behaviors operate in infants and preschool children, with the precision of intake regulation improving over longer periods of time (i.e., the day versus a meal). However, the concept has not been fully explored in adults.
The concept of energy intake regulation in children emerged from observations in newly weaned infants [12,13]. In the absence of adult influences, the infants (aged 6–11 mo) self-selected foods over a period of months that supported growth and health, despite an erratic meal-to-meal food intake pattern. Later, infants adjusted their formula intake in response to modifications of energy density (ED) to maintain a constant energy intake [14].
Within the constraints of a fixed mealtime protocol, children (aged 3.5–5 y) revealed stronger regulation of reported energy intake over a 24-h period, despite high variation in energy intake at individual eating occasions [15]. The mean coefficient of variation (CV) for energy intake at individual eating occasions was 33.6%. In contrast, the mean CV for total daily energy intake was only 10.4%. Later, similar patterns of short-term energy intake regulation were observed in free-living pre-school children [16]. The mean CV for energy intake for the six specified eating occasions over the day was 95.4% (46.5–165.8%), while the mean CV for energy intake for the full 24-h period was only 30.3%. Thus, similar to the previous study [15], the meal-to-day proportion of variability was approximately 3 to 1.
Further research demonstrated that children aged 2–3 years maintained constant energy intakes regardless of portion size while children aged 4–6 years consumed more energy as the portion size increased [17]. This led to the suggestion that the mechanisms involved in energy intake regulation become disordered after pre-school years, and that by adulthood, such mechanisms are weak and imprecise [18–21]. Failure of adults to adjust food intake in response to variations in the ED of various preloads [22–26] has been cited as providing further support for the concept of imprecise regulation among adults.
Given the fundamental pre-requisite for weight gain is excess energy intake relative to energy expenditure, overweight and obese states result from inaccurate intake regulation, which may be exacerbated by poor precision [27]. While some studies have reported that obese adults exhibit a poorer ability to compensate for covert manipulation of a food’s energy content than lean counterparts [28,29], the differential finding has not been consistent [25,30,31].
The lack of overall consensus regarding the compensatory ability of adults may merely be reflective of sampling over a short timeframe [32]. Studies using the preload paradigm generally assess energy intake within meals or at the next meal only, thus failing to assess energy compensation that may occur later. The short timeframe of preload studies may also limit associative learning influences on energy intake regulation [33], thus, possibly leading to erroneous conclusions from an intervention. Although lack of compensation, or imprecise compensation, following a preload of energy is indicative of dysregulation of energy intake within a meal, it cannot be assumed to be indicative of longer term dysregulation. Regulation may act on a day-to-day basis (short-term regulation), by adjusting the total ingested over the course of the entire day to the state of overall energy balance, or it may act over periods of weeks or months (long-term regulation), by subtly altering a bias that operates persistently in the face of powerful, but short-lived influences [34].
The present study aimed to examine patterns of energy intake regulation in free-living adults over a consecutive 7-d period, to contrast responses to published data from children. We aimed to explore this precision across BMI categories and level of sweetened energy-yielding beverage (SEB) consumption, to identify possible differential patterns of regulation. We chose to explore the relationship between SEB consumption and energy intake regulation due to the concern that SEB consumption leads to weight gain [35]. It was hypothesized that normal weight adults would regulate energy intake over a short-term period in a pattern similar to that observed in children, but the degree of regulatory error would increase with BMI. Further, it was expected that consumers of SEBs would have weaker compensation than non-consumers.
Methods
Data were collected from 128 participants recruited through public advertisements. Eligibility criteria included weight stable, male or female, 18–60 years of age, and in good health. Exclusion criteria included smoker, pregnant, lactating, or use of medications reported to affect appetite or body weight. The Institutional Review Board of Purdue University, IN approved the study protocol, and informed consent was obtained from each participant. Participants were informed that the aim of this activity was to better understand customary dietary behavior to aid development and assessment of therapeutic diets. They were compensated monetarily for their participation.
Anthropometrics
Height was measured (± 0.1 cm) on a wall-mounted stadiometer. Body weight was measured (± 0.1 kg) using calibrated scales and body composition by bioelectrical impedance (model TBF-305; Tanita, Arlington Heights, IL), with participants wearing no shoes and a light gown on Day 1. Participants were categorized as normal weight (BMI ≤ 24.9 kg/m2), overweight (BMI 25–29.9 kg/m2), or obese (BMI ≥ 30.0 kg/m2) [36].
Eating Behavior
Each participant completed the Three-Factor Eating Questionnaire (TFEQ) on Day 1 [37]. This scale measured cognitive dietary restraint (conscious intent to control food intake in an effort to manage body weight), disinhibition (over consumption of food in response to cognitive or emotional cues), and susceptibility to hunger (food intake in response to feelings and perceptions of hunger).
Assessment of Hydration Status
Hydration status was assessed by measurement of urinary osmolality on two spot urine samples collected on Day 1 and Day 7. Osmolality (mOsm/kg) was determined by freezing point depression (Advanced™ OSMOMETER, Model 3D3, Advanced Instruments, Inc., Norwood, MA). Participants classified as dehydrated (n = 6) (mean urinary osmolality > 1000 mOsm/kg) were eliminated from the study sample, to exclude dehydration as a possible confounding variable.
Habitual Dietary Intake
Data on habitual food and beverage intake (time, type, quantity, brand, etc.) were collected daily for 7 consecutive days, starting on Tuesdays, by telephone-administered 24-h dietary recalls, using multi-pass software (NDS-R; version 2005; Nutrition Coordinating Center, University of Minnesota, Minneapolis). To aid recall and improve accuracy participants were instructed to keep a food diary, recording intake at the time of ingestion, and were educated on portion size estimation. The importance of reporting habitual dietary intake was emphasized. Complete 7-d records of food and beverage intake was necessary for meaningful analyses. Thus, participants with incomplete 7-d records were removed from the analytic sample (n = 40). However, these participants did not different significantly from the final sample with respect to age, gender, or BMI.
To reduce the bias introduced by under-reporting [38], reported intakes were compared with the estimated basal metabolic rate (BMRest) for each participant. BMR was estimated from the participant’s weight after adjusting for age and sex [39]. Reported energy intake (EI) data were treated as 7-d records, with a pooled within-subject variation in EI (CVwEI) of 30% [40]. The coefficient of variation in BMR predictions (CVwB) was taken as 8.5%, and mean physical activity level (PAL) value as 1.55 [48]. The cutoff value for identifying low energy reporters (LER) was lower than 1.03 for a ratio of EI: BMRest. Thirty two participants (39%) were identified as LERs and subsequently removed from the analysis. This level is comparable to levels reported in the literature [41]. The LER group had a mean BMI 2 units higher than the accurate energy reporters, but no differences were found with respect to age or gender.
Each 24-h dietary recall was divided into seven eating occasions (with the following labels assigned only to facilitate discussion of the data): breakfast (06:00–09:29h), morning snack (09:30–11:29h), lunch (11:30–14:59h), afternoon snack (15:00–16:59h), dinner (17:00–20:29h), evening snack (20:30 – 23:59h), overnight (00:00 – 05:59h), as reported previously [13]. When food was consumed more than once within a given eating occasion, energy values for all food consumed within that occasion were summed. When no food was consumed, reported energy intake for that eating occasion was marked as zero. Total daily energy intake reported for each eating occasion and for each 24-h period was calculated for each of the 7 days.
Assessment of Variability in Energy Intake
The CV (standard deviation divided by the mean) was used as the index of intra-individual variability in reported energy intake, as cited previously [15,16]. For each participant, the mean CVs across the 7 days for reported energy intake at each individual eating occasion (e.g., across the 7 breakfast energy intakes) and for total daily energy intake reported across the 7-d period were calculated. The mean CV of the 7 individual eating occasions (i.e., mean of breakfast, morning snack, lunch, afternoon snack, dinner, evening snack, and overnight) was compared with the observed mean CV over the 24-h period, using a paired sample t-test. Additionally, evidence of self-regulation was determined by comparing the expected CV for each participant with their observed CV for the 24-h period [16]. The expected CV was calculated as the square root of the expected variance divided by the observed mean whole-day energy intake. Lack of compensation is reflected as an expected variance equal to the sum of the observed variances i.e. the expected variance of the total daily energy intake is equal to the sum of the observed variances of the energy intakes at each individual eating occasion over that same period. In contrast, compensation is reflected as a smaller observed variance i.e. the expected variance of the total daily energy intake is greater than the sum of the observed variances of the energy intakes at each individual eating occasion over that same period. Thus, the expected CV was used as a benchmark for comparative purposes. A paired sample t-test was used to compare these two values.
Furthermore, to examine the impact of eating occasions on each other, reported energy intakes at successive eating occasions were correlated, between participants, across the 7-d period. To minimize concerns of possible confounding of the meal-to-meal correlations [42] energy intakes were converted to standardized Z scores before correlation analysis. The binomial probability distribution was used to determine random nature of the pattern of correlations [43]. A consistent pattern of negative correlations were assumed to reflect energy compensation [34], as negative correlations between successive eating events reflects the rank ordering of participant intake (i.e., high rank at one eating event followed by low rank at the next), rather than simply reflecting a pattern of high energy eating events followed by smaller ones.
Assessment of Beverage Consumption and Variability in Energy Intake
Participants were classified according to frequency of days in the week that SEBs were consumed: non-consumers (consume 0 SEB d/wk), infrequent consumers (consume SEB 1–5d/wk), or frequent consumers (consume SEB 6–7d/wk). SEBs were defined as sweetened soft drinks, including sweet carbonated drinks and sweetened non-alcoholic wine, and sweetened fruit drinks [35]. To investigate the hypothesis that energy from sweetened beverages are not regulated, each participant’s reported mean daily energy intake was compared between days when such beverages were and were not consumed, using paired sample t-tests.
Statistical Analysis
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, version 14.0, SPSS Inc., Chicago, IL). Descriptive statistics were calculated for group characteristics and reported as mean ± standard deviation (SD). Pearson cross-correlation coefficients were computed to examine bivariate relationships between variables of interest. Chi-square and analysis of variances tests were used to investigate differences between all computed categories. Multiple comparisons were performed at the significance level of α = 0.05, with Bonferroni adjustment when the main effects were significant. A two-tailed level P < 0.05 was set as the criterion for significance.
RESULTS
Anthropometry
Table 1 summarizes the anthropometric characteristics of the final sample of 50 adults included for analysis. There was a gender bias towards women (n = 39), but there was no significant difference between men and women with respect to age or BMI. Twenty-seven of the participants were normal weight, 14 were overweight, and nine were obese. Mean (± SD) BMI within these categories was 22.0 (± 1.9) kg/m2, 28.4 (± 1.4) kg/m2, and 36.0 (± 4.8) kg/m2, respectively. The sample bias towards normal weight was introduced following the elimination of LERs. Overweight and obese individuals were significantly older than normal weight individuals (P < 0.05). The mean ages were 26 (± 10) years in the normal weight group, but 34 (± 12) years in the overweight and obese group.
Table 1.
Mean (range) and standard deviations (SD) of BMR, weight, height, BMI, age and hunger, disinhibition and restraint scores for total group and according to gender.
| Total
n=50 |
Males
n = 11 |
Females
n = 39 |
||||
|---|---|---|---|---|---|---|
| Mean (range) | SD | Mean (range) | SD | Mean (range) | SD | |
| BMR | 1547 (1169–2305) | 253 | 1906 (1515–2305)* | 206 | 1446 (1169–1940) | 155 |
| Weight (kg) | 73 (46–134) | 17 | 84 (55–107) | 15 | 69 (46–134) | 17 |
| Height (cm) | 166 (154–182) | 7 | 172 (162–182) | 7 | 164 (154–178) | 6 |
| BMI (kg/m2) | 26 (18–44) | 6 | 28 (21–40) | 6 | 26 (18–44) | 6 |
| Age (y) | 30 (19–60) | 12 | 28 (20–60) | 12 | 31 (19–57) | 12 |
| Hunger (0–14) | 6 (1–14) | 3 | 5 (1–14) | 4 | 6 (1–14) | 3 |
| Disinhibition (0–16) | 10 (1–16) | 5 | 8 (2–16) | 6 | 11 (1–16) | 5 |
| Restraint (0–21) | 7 (2–14) | 3 | 6 (3–12) | 3 | 8 (2–14) | 3 |
Significantly greater than females, (P < 0.05, t-test).
Variability in Energy Intake
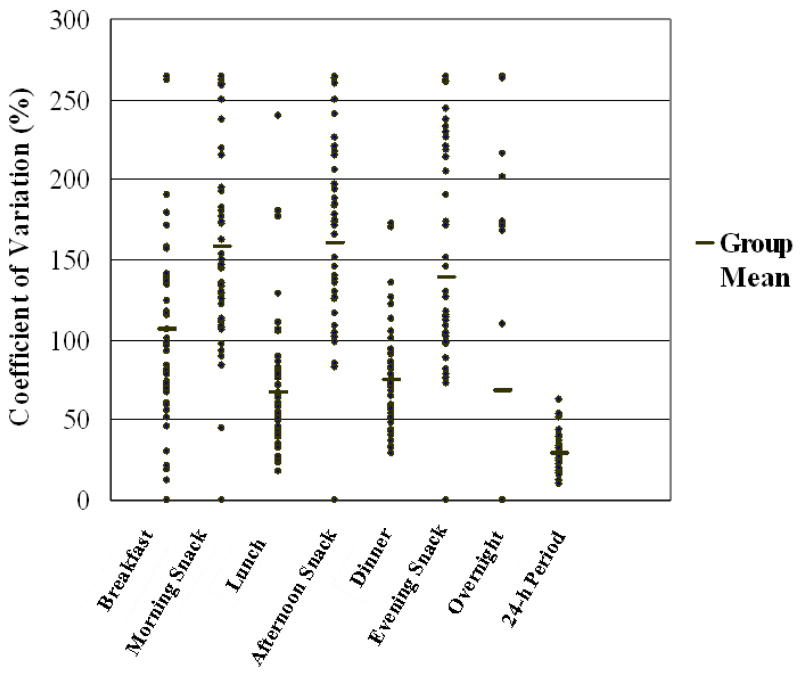
Table 2 shows the CV for reported energy intake at each of the seven eating occasions, and for reported total daily energy intake for the total group (within-participant variability). Mean CV for energy intake for each of the seven eating occasions ranged from 67.6 (± 69.7) % to 158.1 (± 67.4) %. The mean CV for energy intake at each eating occasion was 110.5 (± 24.5) %, while the CV for energy intake for the full 24-h period was 28.9 (± 10.9) %. Thus, the ratio of meal to day variability was 3.8 to 1. Figure 1 illustrates wide variation in reported energy intake across eating occasions, but smaller variation across the whole day. Greater variability was noted in energy intake reported during snack time periods than during main meal time periods (P < 0.001). For main meals, the mean CV for energy intake was 82.9%; however, the mean CV for snacks was 152.0%, excluding overnight intake.
Table 2.
Mean and standard deviation (SD) of coefficients of variation in energy intake for seven eating occasions and for the 24-h period for the total group and according to gender, BMI, age, and sweetened energy-yielding beverage (SEB) frequency.
| n | Eating Occasion
Mean ± SD |
24-h Period
Mean ± SD |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Breakfast | Morning Snack | Lunch | Afternoon Snack | Dinner | Supper Snack | Overnight | ||||
| Total | 50 | 106.1 ± 69.6 | 158.1 ± 67.4 | 67.6 ± 41.9 | 159.7 ± 79.3 | 75.0 ± 31.5 | 138.3 ± 88.6 | 68.3 ± 108.8 | 28.9 ± 10.9 | |
| Gender | Males | 11 | 81.5 ± 34.7 | 185.9 ± 64.9 | 76.1 ± 51.9 | 168.3 ± 76.2 | 66.0 ± 24.1 | 127.0 ± 100.9 | 66.8 ± 106.6 | 28.7 ± 11.0 |
| Females | 39 | 98.3 ± 64.5 | 158.1 ± 64.5 | 63.5 ± 37.9 | 165.1 ± 72.8 | 75.4 ± 32.9 | 134.5 ± 90.9 | 67.9 ± 111.7 | 28.3 ± 9.9 | |
| BMI (kg/m2) | ≤24.9 | 27 | 112.3 ± 62.5 | 146.1 ± 63.4 | 62.7 ± 36.2 | 160.8 ± 74.6 | 81.8 ± 35.6 | 142.4 ± 85.9 | 71.4 ± 114.2 | 28.8 ± 9.7 |
| 25 – 29.9 | 14 | 97.4 ± 79.2 | 188.0 ± 65.4 | 72.5 ± 52.6 | 161.7 ± 100.6 | 66.2 ± 28.0 | 98.9 ± 101.4 | 46.2 ± 93.8 | 29.0 ± 13.9 | |
| ≥30 | 9 | 101.1 ± 80.5 | 147.6 ± 75.0 | 75.0 ± 42.6 | 153.2 ± 62.4 | 68.1 ± 18.1 | 187.1 ± 45.4 | 93.1 ± 118.9 | 28.8 ± 10.3 | |
| ≤30 | 31 | 121.1 ± 81.1 | 141.1 ± 64.1 | 62.4 ± 34.4 | 154.4 ± 81.9 | 80.5 ± 34.5 | 145.2 ± 81.4 | 69.1 ± 111.8 | 28.9 ± 11.1 | |
| Age (y) | > 30 | 19 | 81.6 ± 34.7 | 185.9 ± 64.9 | 76.2 ± 51.9 | 168.4 ± 76.2 | 66.0 ± 24.1 | 127.1 ± 100.9 | 66.8 ± 106.6 | 28.7 ± 11.1 |
| 0 | 8 | 88.5 ± 58.2 | 177.0 ± 98.0 | 65.8 ± 47.9 | 158.2 ± 91.5 | 79.2 ± 23.5 | 145.3 ± 75.1 | 46.6 ± 95.5 | 24.6 ± 5.9 | |
| SEB (d/week) | 1 – 5 | 28 | 109.5 ± 75.4 | 154.4 ± 55.7 | 77.7 ± 45.4 | 146.9 ± 75.6 | 77.1 ± 32.5 | 131.1 ± 99.0 | 92.9 ± 120.1 | 31.4 ± 13.1 |
| 6 – 7 | 11 | 101.5 ± 72.8 | 166.2 ± 77.5 | 53.5 ± 21.7 | 179.6 ± 85.8 | 68.0 ± 37.6 | 159.1 ± 85.2 | 39.7 ± 90.7 | 27.0 ± 6.3 | |
Figure 1.
Coefficients of variation for total daily energy intake (24-h period) and for energy intake at seven eating periods (n = 50). Each point represents the mean value for a single participant for seven days, except where the values for two or more participants coincide and one data point is shown.
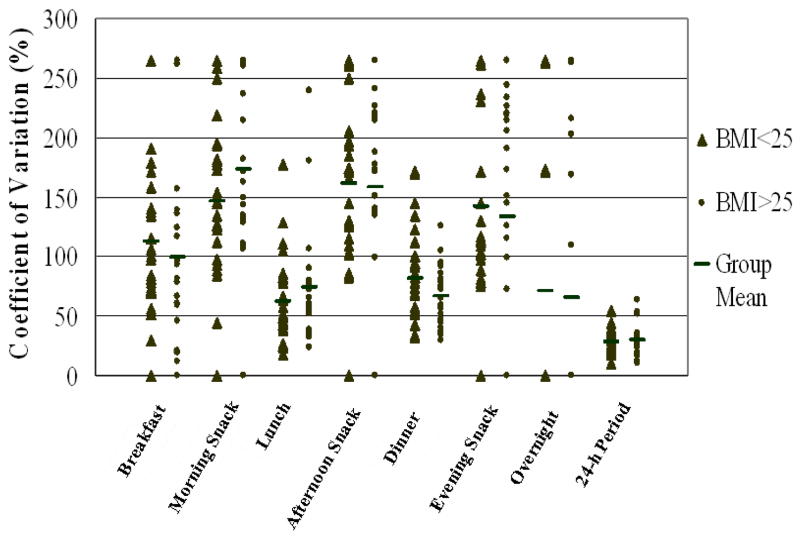
A similar pattern of greater variation in reported energy intake between eating occasions than in energy intake reported for the whole day was demonstrated across all classification categories (Table 2). The CV for energy intake for the full 24-h period was similar in the overweight/obese category to the normal weight category (Figure 2). Mean cognitive dietary restraint was comparable among normal weight and overweight individuals, with a mean value of 7 (± 3) in both groups, but was significantly higher among obese individuals (10 ± 4) (P < 0.05). However, this did not result in statistically significant differences between the groups with respect to variation in energy intake.
Figure 2.
Coefficients of variation for total daily energy intake (24-h period) and for energy intake at seven eating periods for normal weight (n = 27) and overweight and obese (n = 23) participants. Each point represents the mean value for a single participant for seven days, except where the values for two or more participants coincide and one data point is shown.
The CV for reported total daily energy intake was larger in consumers of SEBs than non-consumers. The greatest variation occurred in participants who consumed the beverages infrequently. Frequent consumers had greater CVs in reported total daily energy intake than non-consumers, but less variation than infrequent consumers (Table 2). However, the differences did not reach statistical significance.
The expected CV was calculated at 42.6%, which was significantly greater than the observed CV of 28.9% (t = 15.756, df = 49, P < 0.001). Furthermore, negative correlations were found between each successive eating occasion, with the correlation reaching significance 11 of 35 times (Table 3). Based on the binomial probability distribution this is a non-random pattern of correlations (P < 0.001).
Table 3.
Group correlation coefficients (r) between successive eating occasions, following standardization to Z scores, for 7 consecutive days for total sample (n = 50).
| Eating | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | Sunday |
|---|---|---|---|---|---|---|---|
| Occasion | r | r | r | r | r | r | r |
| Breakfast Mid Morning | −0.07 | −0.22 | −0.41** | −0.22 | −0.19 | −0.30* | −0.20 |
| Mid Morning Lunch | −0.35* | −0.18 | −0.08 | −0.16 | −0.05 | −0.37** | −0.22 |
| Lunch Afternoon | −0.12 | −0.05 | −0.07 | −0.37** | −0.21 | −0.15 | −0.16 |
| Afternoon Dinner | −0.48* | −0.03 | −0.29* | −0.08 | −0.31** | −0.03 | −0.18 |
| Dinner Supper | −0.32* | −0.03 | −0.18 | −0.29* | −0.40** | −0.27 | −0.27 |
Significantly correlated at the 0.05 level (2-tailed).
Significantly correlated at the 0.01 level (2-tailed).
Daily Food and Beverage Intake
Reported mean daily energy intake was 8443 ± 1576 kJ (Table 4). Energy intake was not significantly higher on the weekend days. While energy intake was higher in men than women, the difference was not significant. Multiple comparisons, with Bonferroni adjustments, revealed reported mean daily energy intake was significantly higher in the obese than normal weight participants (P < 0.05). A significant positive association was observed between reported daily energy intake and BMI (r = 0.331, P < 0.05). No significant differences in energy intakes were noted with respect to the TFEQ variables. Mean daily intake of energy and macronutrients did not differ between age groups. Overall, protein, fat, carbohydrate, and alcohol contributed 15%, 33%, 49%, and 3% of total daily energy intake, respectively.
Table 4.
Mean, standard deviation and range of total daily energy, protein, fat, carbohydrate (CHO), and alcohol intake for the total group and according to gender, BMI, age, and sweetened energy-yielding beverage (SEB) frequency.
| n | Daily Energy Intake (kJ) (range) | Protein (g) (range) | Fat (g) (range) | CHO (g) (range) | Alcohol (g) (range) | ||
|---|---|---|---|---|---|---|---|
| Total | 50 | 8443 ± 1576 (6489–13120) | 79.0 ± 21.3 (39–171) | 76.8 ± 17.3 (41–121) | 253.1 ± 57.5 (119–412) | 8.8 ± 18.5 (0–110) | |
| Gender | Males | 11 | 9420 ± 1418 | 96.3 ± 28.2 | 80.9 ± 15.5 | 259.3 ± 53.4 | 21.6 ± 34.4 |
| Females | 39 | 8167 ± 1523 | 74.1 ± 16.2 | 75.6 ± 17.8 | 251.3 ± 59.2 | 5.1 ± 8.3 | |
| BMI (kg/m2) | ≤24.9 | 27 | 8141 ± 1224 | 76.0 ± 14.3 | 70.5 ± 15.0 | 255.8 ± 57.1 | 7.6 ± 13.3 |
| 25 – 29.9 | 14 | 8250 ± 1569 | 75.8 ± 19.1 | 78.2 ± 15.2* | 235.3 ± 33.1 | 11.6 ± 29.3 | |
| ≥30 | 9 | 9645 ± 2088* | 92.8 ± 35.4 | 93.3 ± 17.0* | 272.5 ± 83.0 | 8.0 ± 10.7 | |
| ≤30 | 31 | 8525 ± 1470 | 80.4 ± 21.1 | 73.8 ± 15.3 | 256.9 ± 53.6 | 12.9 ± 22.4 | |
| Age (y) | > 30 | 19 | 8308 ± 1769 | 76.7 ± 21.9 | 81.7 ± 19.6 | 246.8 ± 64.4 | 2.1 ± 4.7 |
| 0 | 8 | 8085 ± 1307 | 89.5 ± 37.1 | 76.1 ± 19.0 | 221.6 ± 29.3 | 8.8 ± 12.0 | |
| SEB (d/wk) | 1 – 5 | 28 | 8245 ± 1550 | 75.7 ± 18.9 | 76.1 ± 17.4 | 241.3 ± 55.7 | 11.1 ± 23.2 |
| 6 – 7 | 11 | 9413 ± 1728 | 80.5 ± 12.5 | 82.0 ± 17.8 | 303.4 ± 54.8 | 5.0 ± 8.9 |
Significantly higher than BMI ≤ 24.9 kg/m2 (Independent sample t -test): p < 0.05.
A paired sample t-test revealed that reported energy intake was significantly higher on days when SEBs were consumed, compared to days when they were not (t = 3.69, df = 36, P < 0.001). Reported mean energy intake was 7913 ± 1077 kJ/d on days when not consumed and 9296 ± 767 kJ/d on days when they were consumed. The mean difference in energy intake was 1437 ± 2365 kJ/d. Multiple comparisons of mean daily energy intake at different levels of daily beverage consumption revealed a significantly higher energy intake in participants who consumed SEBs frequently (6 – 7d/wk) than those who consumed them infrequently (1 – 5d/wk) (P < 0.05). Multiple comparisons, with Bonferroni adjustments, revealed women who did not consume SEBs and those who consumed sweetened beverages infrequently consumed significantly less energy than women who consumed sweetened beverages 6 – 7 d/wk (P < 0.05).
Discussion
Variability in Energy Intake
The present findings indicate marked dietary energy compensation occurs within days in adults. Intra-individual variability in intake is much smaller over the day relative to individual eating occasions. The inverse association between intake at successive eating events and a significantly greater expected CV than the observed CV provide further support for meal-to-meal compensation. This pattern of reduced variance over the day relative to individual eating events replicates that reported previously in children [15,16], and indicates the contributing regulatory mechanisms do not necessarily decline markedly with age as speculated [18–21]. The proportion of variability in reported energy intake at individual eating occasions to variability in energy intake reported over the 24-h period was 3.8:1. This is comparable to the 3:1 ratio noted in children [15,16]. While strong compensation was observed, it still is not precise. The daily CV was 28.9%. This may be further reduced by longer-term dietary adjustments and thermogenic regulatory mechanisms, such as non-exercise activity thermogenesis [44,45].
This analysis did not reveal significantly different patterns of regulation between BMI categories. This refutes our hypothesis that the precision would be less in overweight and obese individuals, as overweight and obese status represents a previous or current state of positive energy balance [46]. There may be several explanations. First, it may be that there is no difference between these groups. Variations in food intake may not be the primary determinant of weight gain in humans. Second, overweight/obese individuals may have only episodic periods of positive marked energy imbalance that remain incompletely corrected [47,48]. As participants in this study reported they were weight stable prior to recruitment, this may have been missed. Differential patterns in energy intake regulation may only be captured during the dynamic phase of weight gain [49]. Altered physiological signals have been noted in weight-gaining obese individuals, but not in a similar weight-stable obese group [50]. During periods of dynamic weight gain, physiological signals, possibly hormonal or metabolic, that are involved in the short-term (cues to terminate meals; meal size) and long-term (cues to initiate eating occasions; meal frequency) regulation may be altered, and lead to a heightened food intake and positive energy balance [49]. Third, it must be re-emphasized that the variability evaluated here reflects the precision of daily energy intake rather than accuracy. Obese individuals may have similar daily variability, but a small systematic positive error in energy intake relative to energy expenditure. If a lack of precision leads to even a small, sustained positive energy balance, the error may result in substantive weight gain [51]. Fourth, the multi-factorial nature of obesity may also account for the failure to find a significant difference between BMI categories. Identification of a contribution of differential compensation may require additional knowledge of individual attributes such as birth weight, age of onset, body fat distribution, body weight fluctuations, binge-eating behavior, or family history of obesity. Regulatory influences in individuals who are obese from childhood likely differ from those who have adult onset obesity [52]. Thus, there may be sub-groups of the overweight where poor dietary compensation is a greater source of error. It is also possible that the difference between lean and overweight/obese individuals is small and this study lacked the power to detect such a difference. Only 9 individuals were obese. This bias limits the confidence with which these findings can be applied to obese individuals, but warrants further investigation in larger cohorts of individuals. Future studies may be best directed to comparisons between the extremes of the body fat distribution. An additional consideration when interpreting these findings and planning future studies is the possibility that the magnitude of the positive energy balance needed to produce even a small weight gain over a year may be smaller than the random error associated with the measurement of energy intake over a consecutive 7-d period [53].
There are data indicating incomplete dietary compensation for a variety of foods [34], however, the substantive contribution SBE’s make to daily energy intake [54] and growing evidence for their especially weak satiety property [55], prompted additional consideration of the compensatory responses to their ingestion. SEB consumption was associated with greater daily energy intake among participants in this study. Additionally, the CV of reported energy intake for participants who consumed SEBs was greater than that for participants who did not. Together these findings extend prior observations that beverages fail to trigger complete compensation for the energy they provide [56–59] and lead to weight gain [35]. The mechanisms by which SEBs escape appetitive controls and regulation is not known, but may include cognitive, sensory, osmotic, gastrointestinal transit, and endocrine processes [55]. As SEBs are an important source of energy in Western diets [62] these findings are of concern. The observational nature of the present study precludes inference of causality, but such evidence is available [e.g. 58].
Significantly greater variation in energy intake was observed at “snack” (morning, afternoon, evening) compared to “meal” times (breakfast, lunch, dinner). The similarity of this pattern with those observed previously in children [15,16] suggests that ‘snacking’ may be an important mechanism whereby energy compensation acts to regulate, or dysregulate, energy intake. It is important to note that the term ‘snacking’ in this case refers to an eating pattern characterized by short inter-meal ingestive events, rather than referring to the consumption of commercially available ‘snack foods’. A greater level of dietary compensation has been noted in frequent snack consumers compared to consumers of fewer, larger meals [60,61]. Furthermore, compensation has been observed under controlled conditions in normal weight men following the introduction of 3 mandatory snacks across the day [62]. Similarly, improved compensation has been observed in obese individuals with increased meal frequency [63]. However, there are reports of an indirect, no, or direct relationship between eating frequency and BMI or obesity [64–67]. Snack-related sensory, palatability, energy density, and macronutrient characteristics may play a role in these differential findings [68]. In particular, high-fat snack foods and high-fat diets may disrupt energy compensation and lead to dysregulation of energy intake and weight gain [68]. Thus, the patterns of regulation observed in the present study, where fat intake contributed moderately to reported total daily energy intake (33%), may become disrupted on a diet composed of higher fat snack foods. Given commercially available snack foods tend be high in fat content [62] the concept of snacks fine-tuning meal time energy intake to match energy requirements demands further study amongst all ages and weight categories, in the context of free-living conditions. Snack-dominated and meal-dominated eating patterns must be compared, at various macronutrient levels, to ascertain any differential patterns.
Although the 7-d food intake diary used in this study, in conjunction with 24-h dietary recalls, has been shown to be a reliable method for estimating habitual energy intakes in free-living humans [69,70], it is not without error. This technique has been shown to underestimate intakes, especially in obese [71,72]. However, additional steps were taken to improve the internal validity of the data. First, data were collected by using multi-pass software, the currently viewed best approach. Second, participants were educated on portion size estimation. Third, participants with incomplete 7-d records were removed from the analytical sample. Fourth, LERs were identified by a validated method (Goldberg cut-off [40]) and subsequently removed from the analytical sample, minimizing the confounding effects of implausible reports.
In summary, the present study documents clear evidence of short-term dietary compensation in free-living adults that is comparable to levels noted previously in children [15,16]. While compensation occurred, it was not precise in any group over the interval studied. The implications of such imprecise regulation for energy balance remains to be clarified. However, as greater compensation was observed over the day relative to individual meals, the present study raises questions about the use of single meal feeding trials for advancing the study of compensatory responses and longer-term energy balance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edholm OG, Fletcher JG, Widdowson EM, McCance RA. The energy expenditure and food intake of individual men. Br J Nutr. 1955;9(3):286–300. doi: 10.1079/bjn19550040. [DOI] [PubMed] [Google Scholar]
- 2.Verboeket-van de Venne WP, Westerterp KR, Kester AD. Effect of the pattern of food intake on human energy metabolism. Br J Nutr. 1993;70(1):103–15. doi: 10.1079/bjn19930108. [DOI] [PubMed] [Google Scholar]
- 3.Pannemans DL, Westerterp KR. Energy expenditure, physical activity and basal metabolic rate of elderly subjects. Br J Nutr. 1995;73(4):571–81. doi: 10.1079/bjn19950059. [DOI] [PubMed] [Google Scholar]
- 4.Wynne K, Stanley S, McGowan B, Bloom S. Appetite Control. J Endocrinol. 2005;184:291–318. doi: 10.1677/joe.1.05866. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 6.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–50. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen SJ, Siega-riz AM, Popkin BM. Trends in energy intake in U.S. between 1977 and 1996:similar shifts seen across age groups. Obes Res. 2002;10:370–8. doi: 10.1038/oby.2002.51. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen SJ, Popkin BM. Patterns and trends in food portion sizes, 1977–1998. JAMA. 2003;289:450–3. doi: 10.1001/jama.289.4.450. [DOI] [PubMed] [Google Scholar]
- 9.Kant AK, Graubard BI. Secular trends in patterns of self-reported food consumption of adult Americans:NHANES 1971–1975 to NHANES 1999–2002. Am J Clin Nutr. 2006;84:1215–23. doi: 10.1093/ajcn/84.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindstrom M, Isacsson SO, Merlo J. Increasing prevalence of overweight, obesity and physical inactivity:Two population based studies 1986 and 1994. Eur J Public Health. 2003;13:306–12. doi: 10.1093/eurpub/13.4.306. [DOI] [PubMed] [Google Scholar]
- 11.Adams J. Trends in physical activity and inactivity amongst US 14–18 year olds by gender, school grade and race, 1993–2003:evidence from the youth risk behavior survey. BMC Public Health. 2006;6:57–63. doi: 10.1186/1471-2458-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis CM. Self-selection of diet by newly weaned infants:an experimental study. Am J Dis Child. 1928;36:651–75. [Google Scholar]
- 13.Davis CM. Results of the self-selection of diets by young children. Can Med Assoc J. 1939;41:257–61. [PMC free article] [PubMed] [Google Scholar]
- 14.Fomon S, Filer L, Thomas L, Anderson T, Nelson S. Influence of formula concentration on caloric intake and growth of normal infants. Acta Paediatr Scand. 1975;64:172–81. doi: 10.1111/j.1651-2227.1975.tb03818.x. [DOI] [PubMed] [Google Scholar]
- 15.Birch LL, Johnson SL, Andresen G, Peters JC, Schulte MC. The variability of young children’s energy intake. N Engl J Med. 1991;324:232–5. doi: 10.1056/NEJM199101243240405. [DOI] [PubMed] [Google Scholar]
- 16.Shea S, Stein AD, Basch CE, Contento IR, Zybert P. Variability and self-regulation of energy intake in young children in their everyday environment. Pediatrics. 1992;90:542–6. [PubMed] [Google Scholar]
- 17.Rolls BJ, Engell D, Birch LL. Serving portion size influences 5-year-old but not 3-year-old children’s food intakes. J Am Diet Assoc. 2000;100:232–4. doi: 10.1016/S0002-8223(00)00070-5. [DOI] [PubMed] [Google Scholar]
- 18.Birch LL, Deysher M. Caloric compensation and sensory specific satiety: Evidence for self-regulation of food intake by young children. Appetite. 1986;7:323–31. doi: 10.1016/s0195-6663(86)80001-0. [DOI] [PubMed] [Google Scholar]
- 19.Fisher JO, Rolls BJ, Birch LL. Children’s bite size and intake of an entree are greater with large portions than with age-appropriate or self-selected portions. Am J Clin Nutr. 2003;77:1164–70. doi: 10.1093/ajcn/77.5.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox MK, Devaney B, Reidy K, Razafindrakoto C, Ziegler P. Average portions of foods commonly eaten by infants and toddlers in the United States. J Am Diet Assoc. 2006;106:S66–76. doi: 10.1016/j.jada.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 21.Johnson SL, Taylor-Holloway LA. Non-Hispanic white and Hispanic elementary school children’s self-regulation of energy intake. Am J Clin Nutr. 2006;83:1276–82. doi: 10.1093/ajcn/83.6.1276. [DOI] [PubMed] [Google Scholar]
- 22.Stubbs RJ, Ritz P, Coward WA, Prentice AM. Covert manipulation of the ratio of dietary fat to carbohydrate and energy density:effect on food intake and energy balance in free-living men eating ad libitum. Am J Clin Nutr. 1995;62:330–7. doi: 10.1093/ajcn/62.2.330. [DOI] [PubMed] [Google Scholar]
- 23.Stubbs RJ, Johnstone AM, O’Reilly LM, Barton K, Reid C. The effect of covertly manipulating the energy density of mixed diets on ad libitum food intake in ‘pseudo free-living’ humans. Int J Obes Relat Metab Disord. 1998;22:980–7. doi: 10.1038/sj.ijo.0800715. [DOI] [PubMed] [Google Scholar]
- 24.Bell EA, Castellanos VH, Pelkman CL, Thorwart ML, Rolls BJ. Energy density of foods affects energy intake in normal-weight women. Am J Clin Nutr. 1998;67:412–20. doi: 10.1093/ajcn/67.3.412. [DOI] [PubMed] [Google Scholar]
- 25.Bell EA, Rolls JR. Energy density of foods affects energy intake across multiple levels of fat content in lean and obese women. Am J Clin Nutr. 2001;73:1010–18. doi: 10.1093/ajcn/73.6.1010. [DOI] [PubMed] [Google Scholar]
- 26.Rolls BJ, Roe LS, Kral TVE, Meengs JS, Wall DE. Increasing the portion size of a packaged snack increases energy intake in men and women. Appetite. 2004;42:63–9. doi: 10.1016/S0195-6663(03)00117-X. [DOI] [PubMed] [Google Scholar]
- 27.Woods SC, Schwartz MW, Baskin DG, Seeley RJ. Food intake and the regulation of body weight. Annu Rev Psychol. 2000;51:255–77. doi: 10.1146/annurev.psych.51.1.255. [DOI] [PubMed] [Google Scholar]
- 28.Speechly DP, Buffenstein R. Appetite dysfunction in obese males:evidence for role of hyperinsulinaemia in passive overconsumption with a high fat diet. Eur J Clin Nutr. 2000;53:225–33. doi: 10.1038/sj.ejcn.1600924. [DOI] [PubMed] [Google Scholar]
- 29.Fricker J, Chapelot D, Pasquet P, Rozen R, Apfelbaum M. Effect of a covert fat dilution on the spontaneous food intake by lean and obese subjects. Appetite. 1995;24:121–37. doi: 10.1016/s0195-6663(95)99343-6. [DOI] [PubMed] [Google Scholar]
- 30.Ebbeling CB, Sinclair KB, Pereira MA, Garcia-Lago E, Feldman HA, Ludwig DS. Compensation for energy intake from fast food among overweight and lean adolescents. JAMA. 2004;291:2828–33. doi: 10.1001/jama.291.23.2828. [DOI] [PubMed] [Google Scholar]
- 31.Rolls BJ, Morris EL, Roe LS. Portion size of food affects energy intake in normal-weight and overweight men and women. Am J Clin Nutr. 2002;76:1207–13. doi: 10.1093/ajcn/76.6.1207. [DOI] [PubMed] [Google Scholar]
- 32.Almiron-Roig E, Chen Y, Drewnowski A. Liquid calories and the failure of satiety:how good is the evidence? Obes Rev. 2003;4:201–12. doi: 10.1046/j.1467-789x.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 33.Brunstrom JM. Associative learning and the control of human dietary behavior. Appetite. 2007;49(1):268–71. doi: 10.1016/j.appet.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 34.de Castro JM. The effect of the spontaneous ingestion of particular foods or beverages on the meal pattern and overall nutrient intake of humans. Physiol Behav. 1993;53:1133–44. doi: 10.1016/0031-9384(93)90370-u. [DOI] [PubMed] [Google Scholar]
- 35.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain:a systematic review. Am J Clin Nutr. 2006;84:274–88. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Institutes of Health [NIH] Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults – the evidence report. Obes Res. 1998;51(suppl):51S–209S. [PubMed] [Google Scholar]
- 37.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 38.Bailey RL, Mitchell DC, Miller C, Smiciklas-Wright H. Assessing the effect of underreporting energy intake on dietary patterns and weight status. J Am Diet Assoc. 2007;107:64–71. doi: 10.1016/j.jada.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Schofield WN. Predicting basal metabolic rate – review and prediction, together with an annotated bibliography of source material. Hum Nutr Clin Nutr. 1984;39:1–96. [PubMed] [Google Scholar]
- 40.Black AE. Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int J Obes Relat Metab Disord. 2000;24:1119–30. doi: 10.1038/sj.ijo.0801376. [DOI] [PubMed] [Google Scholar]
- 41.Huang TK, Roberts SB, Howarth NC, McCrort MA. Effect of screening out implausible energy intake reports on relationships between diet and BMI. Obesity Res. 2005;13:1205–17. doi: 10.1038/oby.2005.143. [DOI] [PubMed] [Google Scholar]
- 42.Mrdjenovic G, Levitsky DA. Children eat what they are served: the imprecise regulation of energy intake. Appetite. 2005;44(3):273–82. doi: 10.1016/j.appet.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Darlington RB. Radicals and Squares: Statistical methods for the behavioral sciences. 1974 [Google Scholar]
- 44.Levine JA. Non-exercise activity thermogenesis [NEAT] Nutr Rev. 2004;62:S82–97. doi: 10.1111/j.1753-4887.2004.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 45.Kotz CM, Levine JA. Role of nonexercise activity thermogenesis [NEAT] in obesity. Minn Med. 2005;88:54–7. [PubMed] [Google Scholar]
- 46.Schutz Y, Garrow JS. Energy and substrate balance, and weight regulation. In: Garrow JS, James WPT, Ralph A, editors. Human Nutrition and Dietetics. 10. London: Harcourt Publishers Limited; 2000. pp. 137–47. [Google Scholar]
- 47.Yanovski JA, Yanovski SZ, Sovik KN, Nguyen TT, O’Neil PM, Sebring NG. A prospective study of holiday weight gain. New Engl J Med. 2000;342:861–67. doi: 10.1056/NEJM200003233421206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hull HR, Radley D, Dinger MK, Fields DA. The effect of the Thanksgiving holiday on weight gain. Nutr J. 2006;5:29–34. doi: 10.1186/1475-2891-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pearcey SM, de Castro JM. Food intake and meal patterns of weight-stable and weight-gaining persons. Am J Clin Nutr. 2002;76:107–12. doi: 10.1093/ajcn/76.1.107. [DOI] [PubMed] [Google Scholar]
- 50.Kulesza W. Dietary intake in obese women. Appetite. 1982;3(1):61–8. doi: 10.1016/s0195-6663(82)80037-8. [DOI] [PubMed] [Google Scholar]
- 51.Frayn K. Metabolic Regulation: a human perspective. 2. UK: Blackwell Sciences Ltd; 2003. Energy regulation and body weight regulation; pp. 300–19. [Google Scholar]
- 52.Blundell JE, Gillet A. Control of food intake in the obese. Obes Res. 2001;9:S263–70. doi: 10.1038/oby.2001.129. [DOI] [PubMed] [Google Scholar]
- 53.Tarasuk V, Beaon GH. The nature and individuality of within-subject variation in energy intake. Am J Clin Nutr. 1991;54(3):464–70. doi: 10.1093/ajcn/54.3.464. [DOI] [PubMed] [Google Scholar]
- 54.Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med. 2004;27(3):205–10. doi: 10.1016/j.amepre.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Mattes RD. Fluid calories and energy balance:the good, the bad, and the uncertain. Physiol Behav. 2006;89:66–70. doi: 10.1016/j.physbeh.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 56.Haber GB, Heaton KW, Murphy D, Burroughs LF. Depletion and disruption of dietary fibre:effects on satiety, plasma-glucose, and serum-insulin. Lancet. 1977;2:679–82. doi: 10.1016/s0140-6736(77)90494-9. [DOI] [PubMed] [Google Scholar]
- 57.Hulshof T, De Graaf C, Weststrate JA. The effects of preloads varying in physical state and fat content on satiety and energy intake. Appetite. 1993;21:273–86. doi: 10.1006/appe.1993.1045. [DOI] [PubMed] [Google Scholar]
- 58.Raben A, Tagliabue A, Christensen NJ, Madsen J, Holst JJ, Astrup A. Resistant starch:the effect on postprandial glycemia, hormonal response, and satiety. Am J Clin Nutr. 1994;60:544–51. doi: 10.1093/ajcn/60.4.544. [DOI] [PubMed] [Google Scholar]
- 59.Tournier A, Louis-Sylvestre J. Effect of the physical state of a food on subsequent intake in human subjects. Appetite. 1991;16:17–24. doi: 10.1016/0195-6663(91)90107-4. [DOI] [PubMed] [Google Scholar]
- 60.Westerterp-Plantenga MS, Wijckmans-Duijsens NA, Hoor FT. Food intake in the daily environment after energy-reduced lunch, related to habitual meal frequency. Appetite. 1994;22(2):173–82. doi: 10.1006/appe.1994.1017. [DOI] [PubMed] [Google Scholar]
- 61.Westerterp-Plantenga MS, Kovacs EM, Melanson KJ. Habitual meal frequency and energy intake regulation in partially temporally isolated men. Int J Obes Relat Metab Disord. 2002;26(1):102–10. doi: 10.1038/sj.ijo.0801855. [DOI] [PubMed] [Google Scholar]
- 62.Johnstone AM, Shannon E, Whybrow S, Reid CA, Stubbs RJ. Altering the temporal distribution of energy intake with isoenergetically dense foods given as snacks does not affect total daily energy intake in normal-weight men. Br J Nutr. 2000;83:7–14. [PubMed] [Google Scholar]
- 63.Speechly DP, Rogers GG, Buffenstein R. Acute appetite reduction associated with an increased frequency of eating in obese males. Int J Obes Relat Metab Disord. 1999;23(11):1151–9. doi: 10.1038/sj.ijo.0801046. [DOI] [PubMed] [Google Scholar]
- 64.Gibney M, Lee P. Patterns of food and nutrient intake in adults consuming high and low levels of table sugar in a Dublin suburb of chronically high unemployment. Proc Nutr Soc. 1989;48:A123. [Google Scholar]
- 65.Summerbell CD, Moody RC, Shanks J, Stock MJ, Geissler C. Relationship between feeding pattern and body mass index in 220 free-living people in four age groups. Eur J Clin Nutr. 1996;50:513–19. [PubMed] [Google Scholar]
- 66.Hampl JS, Heaton CL, Taylor CA. Snacking patterns influence energy and nutrient intakes but not body mass index. J Hum Nutr Diet. 2003;6(1):3–11. doi: 10.1046/j.1365-277x.2003.00417.x. [DOI] [PubMed] [Google Scholar]
- 67.Bérteus Forslund H, Torgerson JS, Sjöström L, Lindroos AK. Snacking frequency in relation to energy intake and food choices in obese men and women compared to a reference population. Int J Obes (Lond) 2005;29(6):711–9. doi: 10.1038/sj.ijo.0802950. [DOI] [PubMed] [Google Scholar]
- 68.Blundell JE, Stubbs RJ, Golding C, Croden F, Alam R, Whybrow S, Noury Le, Lawton CL. Resistance and susceptibility to weight gain: individual variability in response to a high-fat diet. Physiol Behav. 2005;86(5):614–22. doi: 10.1016/j.physbeh.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 69.Krantzler NJ, Mullen BJ, Schultz HG, Grivetti LE, Holden CA, Meiselman HL. The validity of telephoned diet recalls and records for assessment of individual food intake. Am J Clin Nutr. 1982;36:1234–42. doi: 10.1093/ajcn/36.6.1234. [DOI] [PubMed] [Google Scholar]
- 70.St Jeor ST, Guthrie HA, Jones MB. Variability in nutrient intake in a 28-day period. J Am Diet Assoc. 1983;83:155–62. [PubMed] [Google Scholar]
- 71.Bandini LG, Schoeller DA, Cyr HN, Dietz WH. Validity of reported energy intake in obese and nonobese adolescents. Am J Clin Nutr. 1990;52:421–25. doi: 10.1093/ajcn/52.3.421. [DOI] [PubMed] [Google Scholar]
- 72.Braam L, Ocke M, Bueno-de-Mesquita HB, Seidell J. Determinants of obesity-related underreporting of energy intake. Am J Epidemiol. 1998;147:1081–6. doi: 10.1093/oxfordjournals.aje.a009402. [DOI] [PubMed] [Google Scholar]