Abstract
Previous studies have demonstrated that heterotrimeric guanine nucleotide-binding regulatory (Gi) protein-deficient mice exhibit augmented inflammatory responses to lipopolysaccharide (LPS). These findings suggest that Gi protein agonists will suppress LPS-induced inflammatory gene expression. Lysophosphatidic acid (LPA) activates G protein-coupled receptors leading to Gi protein activation. We hypothesized that LPA will inhibit LPS-induced inflammatory responses through activation of Gi-coupled anti-inflammatory signaling pathways. We examined the anti-inflammatory effect of LPA on LPS responses both in vivo and in vitro in CD-1 mice. The mice were injected intravenously with LPA (10 mg/kg) followed by intraperitoneal injection of LPS (75 mg/kg for survival and 25 mg/kg for other studies). LPA significantly increased the mice survival to endotoxemia (P < 0.05). LPA injection reduced LPS-induced plasma TNF-α production (69 ± 6%, P < 0.05) and myeloperoxidase (MPO) activity in lung (33 ± 9%, P < 0.05) as compared to vehicle injection. LPS-induced plasma IL-6 was unchanged by LPA. In vitro studies with peritoneal macrophages paralleled results from in vivo studies. LPA (1 and 10 μM) significantly inhibited LPS-induced TNFα production (61 ± 9% and 72 ± 9%, respectively, P < 0.05) but not IL-6. We further demonstrated that the anti-inflammatory effect of LPA was reversed by ERK 1/2 and phosphatase inhibitors, suggesting that ERK 1/2 pathway and serine/threonine phosphatases are involved. Inhibition of phosphatidylinositol 3 (PI3) kinase signaling pathways also partially reversed the LPA anti-inflammatory response. However, LPA did not alter NFκB and peroxisome proliferator-activated receptor γ (PPARγ) activation. Inhibitors of PPARγ did not alter LPA-induced inhibition of LPS signaling. These studies demonstrate that LPA has significant anti-inflammatory activities involving activation of ERK 1/2, serine/threonine phosphatases, and PI3 kinase signaling pathways.
INTRODUCTION
Septic shock is initiated by the systemic inflammatory response syndrome (SIRS), which is the response to the overactivity of the innate immune system. The inflammatory process begins at the nidus of infection where bacteria proliferate and either invade the bloodstream or release various bacterial components, such as lipopolysaccharide (LPS), peptidoglycan, and lipoteichoic acid (1,2). The interaction of these microbial cellular components with macrophages, monocytes, or other host cells induces the release of inflammatory mediators that induce SIRS and the ensuing of septic shock (3).
Heterotrimeric guanine nucleotide-binding regulatory (Gi) proteins modulate LPS signaling pathways and downstream pro-inflammatory gene expression (4–7). In addition to toll-like receptor (TLR) 4, LPS also binds to a cluster of receptors in lipid rafts, some of which are Gi protein coupled (8). Our previous studies demonstrated that LPS-induced inflammatory cytokines and chemokines were augmented in vitro and in vivo in Gi protein-deficient mice compared with WT mice, suggesting an anti-inflammatory role of Gi proteins (5,6). Gi protein-coupled ERK 1/2 signaling pathway mediates LPS-induced signaling (4). Activation of Gi protein with transforming growth factor-β (TGF-β) activates ERK 1/2, which can suppress NFκB and p38 signaling and consequently negatively regulate LPS-induced inflammatory responses (9,10). It also has been reported that ERK 1/2 negatively regulates p38 signaling through upregulation of MAP kinase phosphatase (MKP)-1, a serine/threonine phosphatase (9). MKP-1 decreases pro- and anti-inflammatory cytokines in innate immune responses and endotoxic shock (11,12). These findings are consistent with our previous studies and the findings of others that serine/threonine phosphatase regulates LPS-induced signaling pathways (13–15). PI3 kinase signaling pathways have been shown to be regulated, in part, by Gi protein-dependent pathways. PI3 kinase negatively regulates LPS-induced inflammatory response (16,17). These studies suggest that ligands that activate Gi proteins, ERK 1/2, serine/threonine phosphatase, and PI3 kinase are candidates for negative regulation of LPS-induced signaling pathways.
Lysophosphatidic acid (LPA, 1-acyl-2-lyso-sn-glycero-3-phosphate) is an abundant extracellular metabolite of lyso-phosphatidylcholine generated by a variety of cells that is typically present in micromolar concentrations in biological fluids at sites of inflammation (18). It evokes various immunodulatory responses including prevention of apoptosis, chemotaxis, cytokine and chemokine secretion, platelet aggregation, and enhanced wound healing (19–21). LPA binds to at least four specific G-protein-coupled receptors (GPCRs): LPA1/EDG2, LPA2/EDG4, LPA3/EDG7, and LPA4/GPR23/p2y9 (22). Although LPA1 and LPA2 also activate the Gq and G12/13; LPA3 activates only Gi and Gq (22). Agonist coupling with LPA receptors activates signaling pathways such as adenylyl cyclase, phospholipase C, and Ras-MAP kinase (23). A fourth LPA receptor has been identified, although its biological significance remains to be established (24). In addition to these GPCRs, LPA also activates the cytoplasm/nuclear receptor PPARγ (25).
Recent studies demonstrated an anti-inflammatory effect of LPA in endotoxemia by reducing organ injury (26). However, the potential anti-inflammatory signaling pathway activated by LPA remains to be defined clearly. In the present study, we examined the effect of LPA on LPS responses in vivo by assessment of plasma cytokines, and pulmonary inflammation by assessment of tissue MPO activity and activation of NFκB and PPARγ. Because macrophages are regarded as key inflammatory cells in endotoxemia, we also examined the in vitro anti-inflammatory effect of LPA on LPS-induced TNFα and IL-6 production. Additionally, potential anti-inflammatory signaling pathways activated by LPA were examined with inhibitors of the ERK 1/2, serine/threonine phosphatase, and PI3 kinase signaling pathways.
MATERIALS AND METHODS
Reagents
LPS, LPA (Oleoyl-L-α-lysophosphatidic acid 18:1), okadaic acid, and calyculin A were purchased from Sigma (St. Louis, MO, USA). LPA was suspended in PBS with 2% BSA and sonicated for 15 s. Protein free S. minnesota R595 LPS was provided by Dr. Ernst Reitschel (Borstel, Germany). PD98059 and wortmannin were purchased from Calbiochem (San Diego, CA, USA). GW9662 was obtained from ALEXIS (San Diego, CA, USA).
Mice
Male CD-1 mice at 8 to 10 weeks of age were employed. Thirty mice were separated into two groups randomly. Fifteen mice in Group 1 were injected intravenously with LPA (10 mg/kg) followed by intraperitoneal injection of LPS (75 mg/kg). Fifteen mice in Group 2 were injected with PBS instead of LPA. Mouse survival was monitored every 24 h. CD-1 mice also were injected intravenously with LPA (10 mg/kg) followed by immediate intraperitoneal injection of LPS (25 mg/kg). Mice were killed at 1 h and 6 h after injection; plasma was collected from the vena cava for determination of TNF-α and IL-6 production. Lung tissue was collected for determination of tissue myeloperoxidase activity, NFκB, and PPARγ activity. The investigations conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and with the approval of the Institutional Animal Care and Use Committee.
Cell Stimulation
Four days prior to experiment, CD-1 mice were injected intraperitoneally with 1 ml of 1% Biogel (Bio-Rad, Hercules, CA, USA). Peritoneal macrophages were harvested by peritoneal lavages and maintained in RPMI 1640 medium (Cellgro Mediatech Inc, Herndon, VA, USA), supplemented with heat inactivated 1% fetal calf serum (FCS) (Sigma, St. Louis, MO, USA), 50 U/mL penicillin, 50 μg/mL streptomycin (Cellgro Mediatech). Peritoneal macrophages were pretreated with various inhibitors for 1 h followed by incubation with 10 μg/mL of LPS (from Salmonella enteritidis, Sigma, St. Louis, MO, USA or protein free S. minnesota R595 LPS provided by Ernst Reitschel, Borstel Germany) with or without LPA (10 μM) for 18 h. The supernatants were collected for assay of mediator production.
Assays for TNFα and IL-6 Production
TNFα and IL-6 production were measured using an enzyme-linked immunosorbent assay (ELISA) with mouse TNFα and IL-6 kits (eBioscience, San Diego, CA, USA).
Measurement of Myeloperoxidase Activity
Myeloperoxidase activity was determined in lung and gut as an index of neutrophil accumulation as described previously (27). Tissues were homogenized in a solution containing 0.5% hexa-decyl-trimethylammonium bromide dissolved in 10 mM potassium phosphate buffer (pH 7.0) and were centrifuged for 30 min at 20,000×g at 4° C. An aliquot of the supernatant was allowed to react with a solution of tera-methyl-benzidine (1.6 mM) and 0.1 mM H2O2. The rate of change in absorbance was measured by spectrophotometry at 650 nm. Myeloperoxidase activity was defined as the quantity of enzyme degrading 1 μmol hydrogen peroxide/min at 37° C and was expressed in units per 100 mg of tissue.
EMSA
Electrophoretic mobility shift assays (EMSAs) were performed as described previously (28). Oligonucleotide probes corresponding to the NFκB consensus sequence (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) and PPARγ consensus sequence (5′-GAA AAC TAG GTC AAA GGT CA-3′) were labeled with [γ32P]ATP using T4 polynucleotide kinase and were purified in Bio-Spin chromatography columns (Bio-Rad, Hercules, CA, USA). Ten micrograms of nuclear protein were preincubated with EMSA buffer (12 mM HEPES [pH 7.9], 4 mM Tris-HCl [pH 7.9], 25 mM KCl, 5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 50 ng/mL poly[d(I-C)], 12% glycerol [v/v], and 0.2 mM PMSF) on ice for 10 min before addition of the radiolabeled oligonucleotide for an additional 10 min. Protein-nucleic acid complexes were resolved using a nondenaturing polyacrylamide gel consisting of 5% acrylamide (29/1 ratio of acrylamide/bisacrylamide) and were run in 0.5× TBE (45 mM Tris-HCl, 45 mM boric acid, and 1 mM EDTA) for 1 h at constant current (30 mA). Gels were transferred to What-man 3M paper (Clifton, NJ, USA), dried under a vacuum at 80° C for 1 h, and exposed to photographic film at −70° C with an intensifying screen. Densitometric analysis was performed using ImageQuant (GE Healthcare, Piscataway, NJ, USA).
Statistical Analysis
Data are expressed as the mean ± standard error of the mean (SEM). Statistical significance was determined by analysis of variance (ANOVA) with Fisher’s probable least-squares difference test using GraphPad Prism software. Statistics of survival study were determined with Gehan-Breslow-Wilcoxon Test using GraphPad Prism software; P < 0.05 was used to reject the null hypothesis.
RESULTS
In Vivo Effects of LPA on Survival to Endotoxemia
LPA increased mice survival to endotoxemia
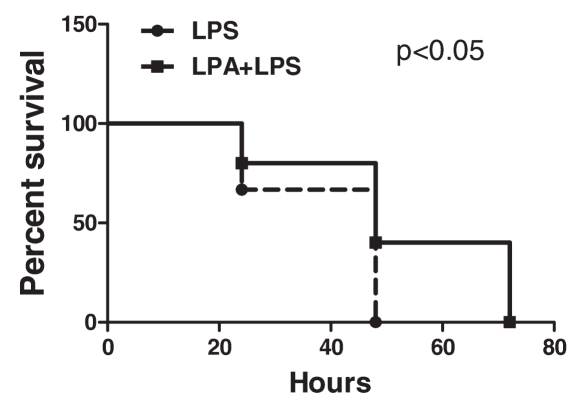
CD-1 mice were injected intravenously with LPA (10 mg/kg) or vehicle PBS followed by intraperitoneal injection of LPS (75 mg/kg). Survival was monitored for 72 h. LPA significantly increased survival to endotoxemia (Figure 1).
Figure 1.
Effect of LPA on mice survival to endotoxemia. 30 male CD-1 mice were separated into two groups randomly. Fifteen mice in Group 1 were injected intravenously with LPA (10 mg/kg) followed by intraperitoneal injection of LPS (75 mg/kg). Fifteen mice in Group 2 were injected with PBS instead of LPA. Mouse survival was monitored every 24 h. Statistics were determined with Gehan-Breslow-Wilcoxon Test using GraphPad Prism software. LPA injected mice exhibited significantly enhanced survival to LPS challenge (P < 0.05).
LPA inhibited LPS-induced TNFα production in vivo
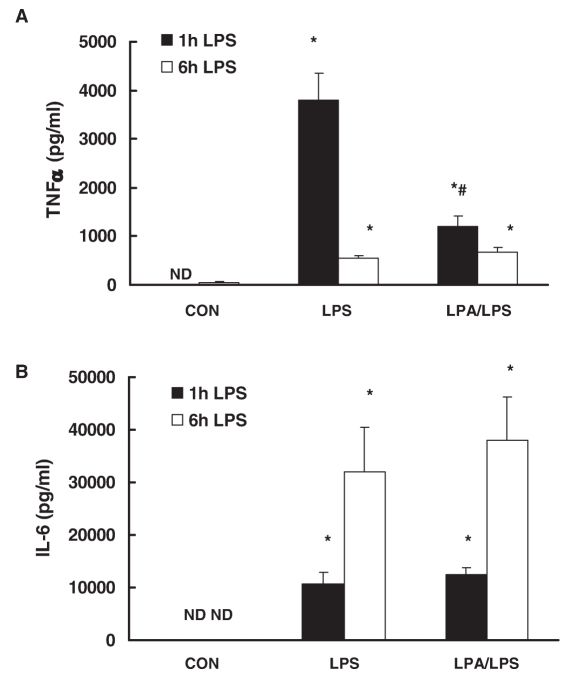
To examine the anti-inflammatory effect of LPA on LPS responses, CD-1 mice were injected intravenously with LPA (10 mg/kg) followed by intraperitoneal injection of LPS (25 mg/kg). After 1 h and 6 h, plasma TNFα and IL-6 production were examined by ELISA. LPS significantly induced TNFα and IL-6 production in plasma (Figure 2). LPA inhibited LPS-induced TNFα by 69 ± 6% (Figure 2A) 1 h after LPS challenge, but, interestingly, did not affect plasma IL-6 production (Figure 2B). Plasma TNFα level was decreased dramatically and IL-6 level was still elevated 6 h after LPS challenge. LPA have no effect on LPS-induced TNFα and IL-6 production.
Figure 2.
Effect of LPA on LPS-induced plasma TNFα and IL-6 production in vivo. CD-1 mice were injected intravenously with LPA (10 mg/kg) or vehicle PBS followed by intraperitoneal injection of LPS (25 mg/kg) or control PBS. After 1 h and 6 h, plasma TNFα (A) and IL-6 (B) production were examined by ELISA. N = 6; *, P < 0.05 compared with control; #, P < 0.05 compared with LPS group; ND, not detectable.
LPA inhibited LPS-induced tissue myeloperoxidase activity in vivo
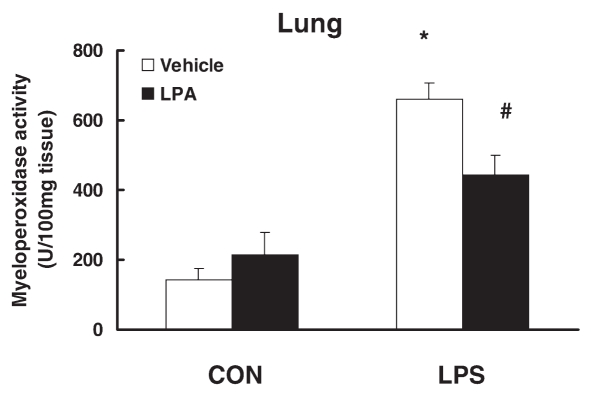
We further examined the effect of LPA injection on LPS-induced MPO activity as an index of neutrophil accumulation in the lung. LPS injection significantly increased lung MPO activity. LPA injection suppressed LPS-induced MPO activity in the lung (33 ± 9%, P < 0.05) (Figure 3).
Figure 3.
Effect of LPA on LPS-induced tissue myeloperoxidase activity in vivo CD-1 mice were intravenously injected with LPA (10 mg/kg) or vehicle PBS followed by intraperitoneal injection of LPS (25 mg/kg) or control PBS. After 6 h, lung was collected, and myeloperoxidase activity was examined. N = 3–4; *, P < 0.05 compared with control; #, P < 0.05 compared with LPS alone group.
NFκB and PPARγ signaling is not involved in the anti-inflammatory effect of LPA
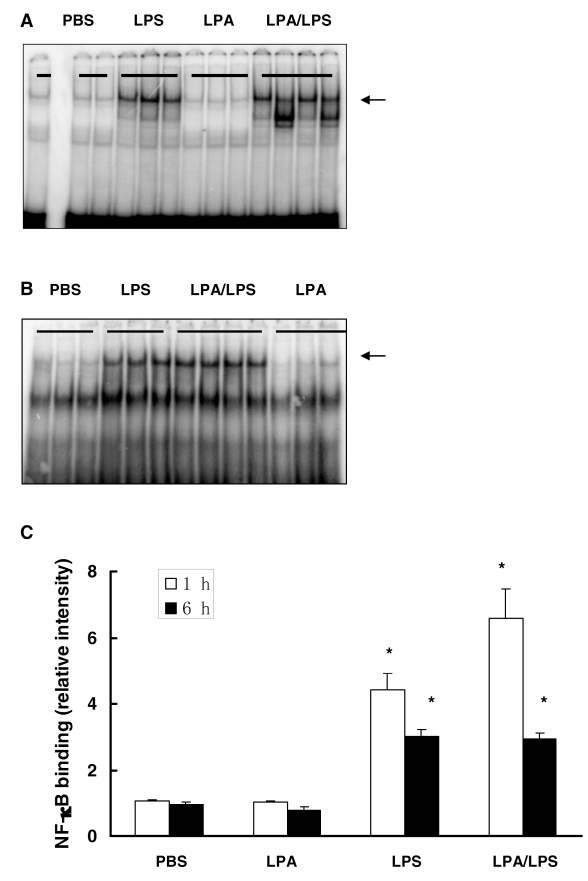
Because LPA is a weak activator of NFκB (29) and LPA also activates nuclear receptor PPARγ (25), the effect of LPA on LPS-induced NFκB and PPARγ activation were examined by EMSA in lung tissue 1 and 6 h after LPS administration. LPS significantly induced activation of NFκB; LPA had no effect on LPS-induced NFκB (Figure 4) and PPARγ (data not shown) activation.
Figure 4.
Effect of LPA on LPS-induced NFκB activation in vivo. CD-1 mice were intravenously injected with LPA (10 mg/kg) or vehicle PBS followed by intraperitoneal injection of LPS (25 mg/kg) or control PBS. After 1 h and 6 h, lung NFκB activation was examined by EMSA. Arrows indicate NFκB. (A) Representative gel of NFκB activation after 1 h LPS challenge. (B) Representative gel of NFκB activation after 6 h LPS challenge. (C) Densitometry analysis of NFκB activation. N = 3–4; *, P < 0.05 compared with control.
In Vitro Effect of LPA on LPS-Stimulated Macrophages
LPA inhibited LPS-induced TNFα production in vitro in peritoneal macrophage
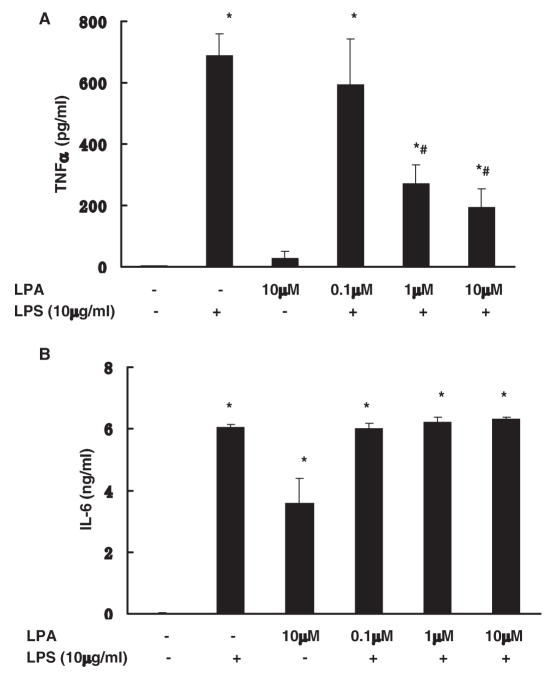
We next examined the anti-inflammatory effect of LPA on LPS responses in vitro in mouse peritoneal macrophages. Paralleling in vivo studies, LPA (1 to 10 μM) inhibited LPS stimulation of TNFα (61 ± 9% and 72 ± 9% respectively, P < 0.05) (Figure 5A). As with the in vivo response, LPA had no effect on LPS stimulation of IL-6 production. LPA alone did induce increase of IL-6 production (Figure 5B).
Figure 5.
Effect of LPA on LPS-induced TNFα and IL-6 production in vitro in peritoneal macrophage. Peritoneal macrophages were incubated with LPS (10 μg/mL) with or without LPA (0.1–10 μM) for 18 h. LPS-induced TNFα (A) and IL-6 (B) production were examined by ELISA. Data represent means ± SE from three independent experiments. *, P < 0.05 compared with basal; #, P < 0.05 compared with LPS alone group.
PPARγ is not involved in anti-inflammatory effect of LPA
To further examine the possible activation of PPARγ in mediating the anti-inflammatory effect of LPA, peritoneal macrophages were pretreated with the PPARγ inhibitor GW9662 (3 to 30 μM) for 1 h, followed by incubation with LPS with/without LPA. GW9662 had no effect on LPS-induced TNFα production (data not shown).
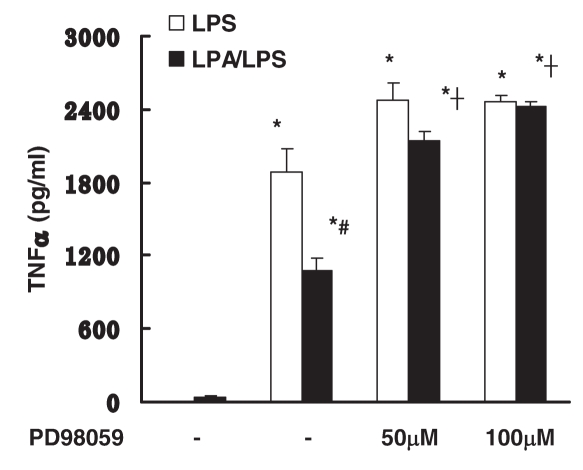
The ERK 1/2 signaling pathway mediates the anti-inflammatory effect of LPA
Previous studies suggested that Gi protein coupled the ERK 1/2 signaling pathway involved in TLR4 signaling (4). The involvement of the ERK 1/2 signaling pathway in anti-inflammatory effects of LPA in macrophages was examined. Peritoneal macrophages were pretreated with PD98059, a specific MEK-1/2, and downstream ERK 1/2 inhibitor for 1 h followed by incubation with LPS with/without LPA. Pretreatment of PD98059 (50 and 100 μM) effectively blocked the anti-inflammatory effect of LPA (Figure 6). This data suggests that the anti-inflammatory effect of LPA is mediated by ERK 1/2 signaling.
Figure 6.
Effect of ERK 1/2 pathways on anti-inflammatory effect of LPA. Peritoneal macrophages were pretreated with PD98059 (50–100 μM) for 1 h followed by incubation with LPS (10 μg/mL) with or without LPA (10 μM) for 18 h. LPS-induced TNFα production was examined by ELISA. Data represent means ± SE from three independent experiments. *, P < 0.05 compared with basal; #, P < 0.05 compared with LPS group; †, P < 0.05 compared with no pretreatment LPA/LPS group.
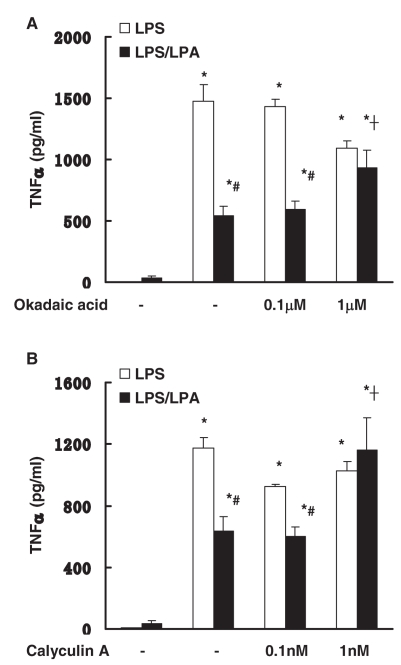
Serine/threonine phosphatase signaling mediate the anti-inflammatory effect of LPA
Serine/threonine phosphatase signaling negatively regulates LPS-induced signaling (11,12). The role of serine/threonine phosphatase signaling in the anti-inflammatory effect of LPA in macrophage was examined. Peritoneal macrophages were pretreated with okadaic acid or calyculin A (PP1 and PP2A serine/threonine phosphatase inhibitors) for 1 h followed by incubation with LPS with/without LPA. Pretreatment of okadaic acid (1 μM) or calyculin A (1 nM) reversed the anti-inflammatory effect of LPA (Figure 7). These data suggest that the anti-inflammatory effects of LPA also are mediated by serine/threonine phosphatase signaling.
Figure 7.
Effect of phosphatase pathways on anti-inflammatory effect of LPA. Peritoneal macrophages were pretreated with okadaic acid (0.1–1 μM, 7A) or calyculin A (0.1–1 nM, 7B) for 1 h followed by incubation with LPS (10 μg/mL) with or without LPA (10 μM) for 18 h. LPS-induced TNFα production was examined by ELISA. Data represent means ± SE from three independent experiments. *, P < 0.05 compared with basal; #, P < 0.05 compared with LPS group; †, P < 0.05 compared with no pretreatment LPA/LPS group.
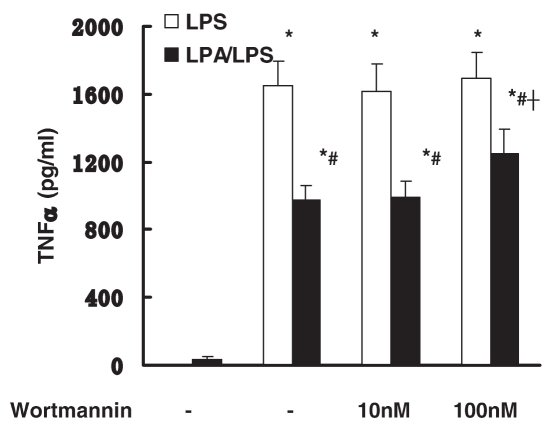
PI3 kinase signaling pathway mediates the anti-inflammatory effect of LPA
Previous studies suggested that PI3 kinase signaling negatively regulates LPS signaling (17). The involvement of PI3 kinase signaling in the anti-inflammatory effect of LPA was investigated. Peritoneal macrophages were pretreated with wortmannin, a PI3 kinase inhibitor, for 1 h, followed by incubation with LPS with/without LPA. Pretreatment of wortmannin (100 nM) partially blocked the anti-inflammatory effect of LPA (Figure 8).
Figure 8.
Effect of PI3 kinase pathways on anti-inflammatory effect of LPA. Peritoneal macrophages were pretreated with wortmannin (10–100 nM) for 1 h followed by incubation with LPS (10 μg/mL) with or without LPA (10 μM) for 18 h. LPS-induced TNFα production was examined by ELISA. Data represent means ± SE from six independent experiments. *, P < 0.05 compared with basal; #, P < 0.05 compared with LPS group; †, P < 0.05 compared with no pretreatment LPA/LPS group.
DISCUSSION
Our studies demonstrate that LPA significantly increased mice survival to endotoxemia and inhibited LPS-induced plasma TNF-α production and lung myeloperoxidase activity in vivo in CD-1 mice. LPS-induced plasma IL-6 was unchanged by LPA. Our in vitro studies with peritoneal macrophages parallel the in vivo studies demonstrating that LPA significantly inhibits LPS-induced production of TNFα but not IL-6. We further demonstrated that the anti-inflammatory effect of LPA is mediated by ERK 1/2 and serine/threonine phosphatase activation. Inhibition of serine/threonine phosphatase with okadaic acid and calyculin A reversed the LPA inhibition of LPS. Additionally, inhibition of the PI3 kinase pathway with wortmannin partially reversed the anti-inflammatory effects of LPA. By contrast, the PPARγ inhibitor GW9662 did not alter LPA inhibition. In addition, LPA did not alter LPS-induced activation of NFκB and PPARγ DNA binding. These data suggest that LPA activates GPCRs signaling pathways rather than cytoplasmic or nuclear PPARγ that inhibit LPS-induced TNFα.
Murch et al. (26) recently examined the effect of saturated LPA (18:0) and unsaturated LPA (18:1) on LPS shock in rats. Both LPAs (18:0 and 18:1) reduced plasma hepatic transaminase, creatine kinase, and IL-1β production induced by LPS shock. The protective effects of both forms of LPA were abrogated by a non-specific LPA antagonist, demonstrating that LPA signaling through a G protein-coupled pathway. Our studies are consistent with and extend these studies. The unsaturated forms of oleoyl LPA (18:1) selectively inhibited LPS-induced TNFα production without an effect on IL-6 production. Murch et al. also demonstrated that LPA suppressed LPS-induced IL-1β without an effect on IL-6. These observations of differential regulation of TNFα and IL-1β relative to IL-6 in mice and rats are intriguing, and demonstrate anti-inflammatory effects of LPA in the absence of IL-6 inhibition. LPA itself induces IL-6 production in macrophages, which may mask the anti-inflammatory effect of LPA on IL-6 production. The underlying mechanisms for such differential regulation remain unclear. Administration of LPS induced an increase in pulmonary infiltration of leukocytes and NFκB activation. LPA reduced LPS-induced pulmonary MPO without altering NFκB activation. LPA has been shown to activate cytoplasmic PPARγ and to induce PPARγ nuclear translocation and DNA binding (25). Our studies and others have demonstrated that PPARγ ligands are anti-inflammatory in models of polymicrobial sepsis, endotoxemia, zymosan-induced shock, and hemorrhagic shock (30). Thus it is reasonable to assume that LPA may suppress inflammation secondary to PPARγ activation. Indeed, previous studies demonstrated that the PPARγ antagonist GW 9662 partially attenuated unsaturated LPA (18:1) but not saturated LPA (18:0) inhibition of transaminase in rats subjected to endotoxemia (26). This suggests the unsaturated acyl fatty acid side chain of LPA (18:1) may be essential for PPARγ activation. However LPA (18:1) employed in vivo in the present study failed to alter LPS-induced decrease of PPARγ DNA binding in lung. Similarly, we found that GW9662 failed to reverse the inhibitory effect of LPA on LPS-induced TNFα production in vitro. It is possible that the disparity in LPA activation of PPARγ may be species dependent.
LPA binds to GPCRs that have the potential to modulate TLR4 activation. LPA has three characterized GPCRs designated as LPA1, LPA2, and LPA3. Studies demonstrated that the non-selective LPA antagonist Ki16425 blocked the beneficial response of LPA, suggesting that the anti-inflammatory effect of LPA was mediated through GPCRs (26). In our studies, LPA1 and LPA3 receptor antagonists, VPC12249 and VPC32179, failed to block the anti-inflammatory effect of LPA (data not shown), demonstrating the LPA2 may be involved in the effect. LPA2 is Gi, Gq, and G12/13 coupled (22). Our studies also demonstrated that the phospholipase C inhibitor Ro31-8425 failed to inhibit the LPA effect. Because phospholipase C activation occurs downstream of Gq, it is unlikely that Gq signaling is involved. On the other hand, LPA has been shown to be a potent activator of ERK 1/2. Activation of the Ras-ERK 1/2 signaling pathway by Gi protein is well established in many cells (32). We also have demonstrated that, in HEK 293 cells, the constitutively active TLR4-induced activation of ERK 1/2 is modulated by Gi proteins (4).
It is possible that LPA activation of ERK 1/2 is a mechanism for anti-inflammatory effects of LPA. To examine this possibility we employed MEK-1 inhibitor PD98059 that inhibits downstream activation of ERK 1/2. The effect of ERK 1/2 inhibition on the reversal of the LPA anti-inflammatory effects were striking. Thus despite the fact that LPS itself activates ERK 1/2, which can induce pro-inflammation secondary to transcription factor e.g. ELK and AP-1 (33), there are also anti-inflammatory responses linked to ERK 1/2 activation. Inhibition of ERK 1/2 by a specific ERK 1/2 inhibitor U0126 augments LPS induced nitric oxide production in RAW 264.7 cells (34). 15-Deoxy-Δ12,14-prostaglandin J2 suppresses LPS-induced pro-inflammatory mediators through ERK 1/2 signaling in epithelial cells (35). Activation of ERK 1/2 signaling pathway negatively regulates NFκB and p38 signaling in RAW 264.7 cells (36,37). Our data support the concept that ERK 1/2 signaling activated by LPA negatively regulates LPS signaling.
To further explore downstream signaling proteins that may play a role in LPA protection in endotoxemia, we examined the potential role of serine/threonine phosphatases. Okadaic acid and calyculin A are inhibitors of the PP1 and PP2A phosphatases in the serine/threonine phosphatase family. Inhibition of PP1 and PP2A has been shown to have a marked effect on macrophage regulation of gene expression, cytokine expression superanion generation and phagocytosis (13,15,38,39). Our previous studies and others also have demonstrated that serine/threonine phosphatase inhibition can reverse the anti-inflammatory effect of LPS tolerance (14). In the present study, we found that the two serine/threonine phosphatase inhibitors effectively reversed the inhibitory effect of LPA on LPS activation. Thus it appears that LPA activation of serine/threonine phosphatase is also important. Additionally, links between ERK 1/2 signaling and serine/threonine phosphatases have been demonstrated. The specific serine/threonine phosphatase MKP-1 has been implicated in the regulation of inflammation. Recent studies demonstrated that MKP-1 negatively regulates LPS-induced signaling. MKP-1 knockout mice exhibit a higher mortality rate and increased LPS response (12). ERK 1/2 signaling negatively regulates p38 pathway through MKP-1, indicating an MKP-1 pathway downstream of ERK 1/2 signaling (9). Thus, one could postulate that similar signaling cascades may exist in response to LPA. Future studies could examine the effect of LPA on LPS response in MKP-1 knockout mice.
The PI3 kinase pathway is another anti-inflammatory pathway that may be activated by LPA. Fukao and Koyasu proposed that PI3 kinase may act as a negative feedback mechanism that prevents excessive immune responses (40). Guha and Mackman have reported that activation of the PI3 kinase/Akt signaling pathway limits the pro-inflammatory effects of LPS in cultured monocytes (17). Our recent studies demonstrated PI3 kinase inhibitor, wortmannin, and LY294002 abolished the protective effect of endotoxin tolerance (41). Numerous studies have demonstrated that LPA can activate the PI3 kinase signaling pathway (42–45). The activation of PI3 kinase is in part regulated by Gαi and βγ subunits (46). LPA activation of ERK 1/2 has also been linked to activation of the PI3 kinase cascade (45). Evidence for an LPS-induced PI3 kinase activation in the present study was demonstrated by a partial reversal of LPA-induced inhibition of LPS with the PI3 kinase pathway inhibition wortmannin.
Our findings demonstrate an anti-inflammatory effect of LPA on endotoxin-induced systemic inflammation. Based on our in vitro studies with macrophages, this anti-inflammatory effect of LPA may be linked to a novel ERK 1/2 and serine/threonine phosphatase pathway and/or the PI3 kinase signaling cascades. Because LPA is an endogenously produced ligand that occurs at sites of inflammation in micromolar concentrations, it has potential therapeutic significance in treatment of sepsis.
ACKNOWLEDGMENTS
This work was supported by NIH GM27673 and NIH GM67202.
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Janeway CA, Jr, Medzhitov R. Lipoproteins take their toll on the host. Curr Biol. 1999;9:R879–82. doi: 10.1016/s0960-9822(00)80073-1. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 3.Glauser MP, Zanetti G, Baumgartner JD, Cohen J. Septic shock: pathogenesis. Lancet. 1991;338:732–6. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- 4.Fan H, Peck OM, Tempel GE, Halushka PV, Cook JA. Toll-like receptor 4 coupled GI protein signaling pathways regulate extracellular signal-regulated kinase phosphorylation and AP-1 activation independent of NF kappa B activation. Shock. 2004;22:57–62. doi: 10.1097/01.shk.0000129759.58490.d6. [DOI] [PubMed] [Google Scholar]
- 5.Fan H, et al. Differential regulation of lipopolysaccharide and Gram-positive bacteria induced cytokine and chemokine production in splenocytes by Galphai proteins. Biochim Biophys Acta. 2006;1763:1051–8. doi: 10.1016/j.bbamcr.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Fan H, et al. Lipopolysaccharide- and gram-positive bacteria-induced cellular inflammatory responses: role of heterotrimeric Galpha(i) proteins. Am J Physiol Cell Physiol. 2005;289:C293–301. doi: 10.1152/ajpcell.00394.2004. [DOI] [PubMed] [Google Scholar]
- 7.Lentschat A, et al. Mastoparan, a G protein agonist peptide, differentially modulates TLR4-and TLR2-mediated signaling in human endothelial cells and murine macrophages. J Immunol. 2005;174:4252–61. doi: 10.4049/jimmunol.174.7.4252. [DOI] [PubMed] [Google Scholar]
- 8.Triantafilou K, Triantafilou M, Dedrick RL. A CD14-independent LPS receptor cluster. Nat Immunol. 2001;2:338–45. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
- 9.Xiao YQ, et al. Oxidants selectively reverse TGF-beta suppression of proinflammatory mediator production. J Immunol. 2006;176:1209–17. doi: 10.4049/jimmunol.176.2.1209. [DOI] [PubMed] [Google Scholar]
- 10.Xiao YQ, et al. Cross-talk between ERK and p38 MAPK mediates selective suppression of pro-inflammatory cytokines by transforming growth factor-beta. J Biol Chem. 2002;277:14884–93. doi: 10.1074/jbc.M111718200. [DOI] [PubMed] [Google Scholar]
- 11.Chi H, et al. Dynamic regulation of pro-and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006;103:2274–9. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Q, et al. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med. 2006;203:131–40. doi: 10.1084/jem.20051794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barber SA, Perera PY, McNally R, Vogel SN. The serine/threonine phosphatase inhibitor, calyculin A, inhibits and dissociates macrophage responses to lipopolysaccharide. J Immunol. 1995;155:1404–10. [PubMed] [Google Scholar]
- 14.Fernando LP, Fernando AN, Ferlito M, Halushka PV, Cook JA. Suppression of Cox-2 and TNF-alpha mRNA in endotoxin tolerance: effect of cycloheximide, antinomycin D, and okadaic acid. Shock. 2000;14:128–33. doi: 10.1097/00024382-200014020-00009. [DOI] [PubMed] [Google Scholar]
- 15.Zhang P, Nelson S, Summer WR, Spitzer JA. Serine/threonine phosphorylation in cellular signaling for alveolar macrophage phagocytic response to endotoxin. Shock. 2000;13:34–40. doi: 10.1097/00024382-200013010-00007. [DOI] [PubMed] [Google Scholar]
- 16.Francois S, et al. Inhibition of neutrophil apoptosis by TLR agonists in whole blood: involvement of the phosphoinositide 3-kinase/Akt and NF-kappaB signaling pathways, leading to increased levels of Mcl-1, A1, and phosphorylated Bad. J Immunol. 2005;174:3633–42. doi: 10.4049/jimmunol.174.6.3633. [DOI] [PubMed] [Google Scholar]
- 17.Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 18.Eichholtz T, Jalink K, Fahrenfort I, Moolenaar WH. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993;291:677–80. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balazs L, Okolicany J, Ferrebee M, Tolley B, Tigyi G. Topical application of the phospholipid growth factor lysophosphatidic acid promotes wound healing in vivo. Am J Physiol Regul Integr Comp Physiol. 2001;280:R466–72. doi: 10.1152/ajpregu.2001.280.2.R466. [DOI] [PubMed] [Google Scholar]
- 20.Graler MH, Goetzl EJ. Lysophospholipids and their G protein-coupled receptors in inflammation and immunity. Biochim Biophys Acta. 2002;1582:168–74. doi: 10.1016/s1388-1981(02)00152-x. [DOI] [PubMed] [Google Scholar]
- 21.Gueguen G, et al. Structure-activity analysis of the effects of lysophosphatidic acid on platelet aggregation. Biochemistry. 1999;38:8440–50. doi: 10.1021/bi9816756. [DOI] [PubMed] [Google Scholar]
- 22.Contos JJ, Ishii I, Chun J. Lysophosphatidic acid receptors. Mol Pharmacol. 2000;58:1188–96. doi: 10.1124/mol.58.6.1188. [DOI] [PubMed] [Google Scholar]
- 23.Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26:870–81. doi: 10.1002/bies.20081. [DOI] [PubMed] [Google Scholar]
- 24.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–6. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 25.McIntyre TM, et al. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc Natl Acad Sci U S A. 2003;100:131–6. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murch O, Collin M, Thiemermann C. Lysophosphatidic acid reduces the organ injury caused by endotoxemia-a role for G-protein-coupled receptors and peroxisome proliferator-activated receptor-gamma. Shock. 2007;27:48–54. doi: 10.1097/01.shk.0000235086.63723.7e. [DOI] [PubMed] [Google Scholar]
- 27.Zingarelli B, et al. Absence of inducible nitric oxide synthase modulates early reperfusion-induced NF-kappaB and AP-1 activation and enhances myocardial damage. Faseb J. 2002;16:327–42. doi: 10.1096/fj.01-0533com. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan JM, et al. 15-Deoxy-delta(12,14)-prostaglandin J(2) (15D-PGJ(2)), a peroxisome proliferator activated receptor gamma ligand, reduces tissue leukosequestration and mortality in endotoxic shock. Shock. 2005;24:59–65. doi: 10.1097/01.shk.0000167108.88376.f2. [DOI] [PubMed] [Google Scholar]
- 29.Shahrestanifar M, Fan X, Manning DR. Lysophosphatidic acid activates NF-kappaB in fibroblasts. A requirement for multiple inputs. J Biol Chem. 1999;274:3828–33. doi: 10.1074/jbc.274.6.3828. [DOI] [PubMed] [Google Scholar]
- 30.Zingarelli B, Cook JA. Peroxisome proliferator-activated receptor-gamma is a new therapeutic target in sepsis and inflammation. Shock. 2005;23:393–9. doi: 10.1097/01.shk.0000160521.91363.88. [DOI] [PubMed] [Google Scholar]
- 31.Gesty-Palmer D, El Shewy H, Kohout TA, Luttrell LM. beta-Arrestin 2 expression determines the transcriptional response to lysophosphatidic acid stimulation in murine embryo fibroblasts. J Biol Chem. 2005;280:32157–67. doi: 10.1074/jbc.M507460200. [DOI] [PubMed] [Google Scholar]
- 32.Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999;11:177–83. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- 33.Yang H, Young DW, Gusovsky F, Chow JC. Cellular events mediated by lipopolysaccharide-stimulated toll-like receptor 4. MD-2 is required for activation of mitogen-activated protein kinases and Elk-1. J Biol Chem. 2000;275:20861–6. doi: 10.1074/jbc.M002896200. [DOI] [PubMed] [Google Scholar]
- 34.Koide N, et al. Inhibition of extracellular signal-regulated kinase 1/2 augments nitric oxide production in lipopolysaccharide-stimulated RAW264.7 macrophage cells. FEMS Immunol Med Microbiol. 2005;45:213–9. doi: 10.1016/j.femsim.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz PA, Kim SC, Sartor RB, Haller D. 15-deoxy-delta12,14-prostaglandin J2-mediated ERK signaling inhibits gram-negative bacteria-induced RelA phosphorylation and interleukin-6 gene expression in intestinal epithelial cells through modulation of protein phosphatase 2A activity. J Biol Chem. 2004;279:36103–11. doi: 10.1074/jbc.M405032200. [DOI] [PubMed] [Google Scholar]
- 36.Aga M, et al. Evidence for nucleotide receptor modulation of cross talk between MAP kinase and NF-kappa B signaling pathways in murine RAW 264.7 macrophages. Am J Physiol Cell Physiol. 2004;286:C923–30. doi: 10.1152/ajpcell.00417.2003. [DOI] [PubMed] [Google Scholar]
- 37.Watters JJ, et al. A differential role for the mitogen-activated protein kinases in lipopolysaccharide signaling: the MEK/ERK pathway is not essential for nitric oxide and interleukin 1beta production. J Biol Chem. 2002;277:9077–87. doi: 10.1074/jbc.M104385200. [DOI] [PubMed] [Google Scholar]
- 38.Mayer AM, Brenic S, Stocker R, Glaser KB. Modulation of superoxide generation in in vivo lipopolysaccharide-primed rat alveolar macrophages by arachidonic acid and inhibitors of protein kinase C, phospholipase A2, protein serine-threonine phosphatase(s), protein tyrosine kinase(s) and phosphatase(s) J Pharmacol Exp Ther. 1995;274:427–36. [PubMed] [Google Scholar]
- 39.Sung SJ, Walters JA. Stimulation of interleukin-1 alpha and interleukin-1 beta production in human monocytes by protein phosphatase 1 and 2A inhibitors. J Biol Chem. 1993;268:5802–9. [PubMed] [Google Scholar]
- 40.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–63. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 41.Peck OM, et al. The phosphatidylinositol 3 kinase pathway regulates tolerance to lipopolysaccharide and priming responses to Staphylococcus aureus and lipopolysaccharide. Shock. 2006;26:31–6. doi: 10.1097/01.shk.0000223128.79759.3d. [DOI] [PubMed] [Google Scholar]
- 42.Chou CH, et al. Up-regulation of interleukin-6 in human ovarian cancer cell via a Gi/PI3K-Akt/NF-kappaB pathway by lysophosphatidic acid, an ovarian cancer-activating factor. Carcinogenesis. 2005;26:45–52. doi: 10.1093/carcin/bgh301. [DOI] [PubMed] [Google Scholar]
- 43.Deng W, et al. The lysophosphatidic acid type 2 receptor is required for protection against radiation-induced intestinal injury. Gastroenterology. 2007;132:1834–51. doi: 10.1053/j.gastro.2007.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fromm C, Coso OA, Montaner S, Xu N, Gutkind JS. The small GTP-binding protein Rho links G protein-coupled receptors and G alpha12 to the serum response element and to cellular transformation. Proc Natl Acad Sci U S A. 1997;94:10098–103. doi: 10.1073/pnas.94.19.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sautin YY, Crawford JM, Svetlov SI. Enhancement of survival by LPA via Erk1/Erk2 and PI 3-kinase/Akt pathways in a murine hepatocyte cell line. Am J Physiol Cell Physiol. 2001;281:C2010–9. doi: 10.1152/ajpcell.00077.2001. [DOI] [PubMed] [Google Scholar]
- 46.Bommakanti RK, Vinayak S, Simonds WF. Dual regulation of Akt/protein kinase B by heterotrimeric G protein subunits. J Biol Chem. 2000;275:38870–6. doi: 10.1074/jbc.M007403200. [DOI] [PubMed] [Google Scholar]