Abstract
In this experimental study, we aimed to examine the ability of absorbable oxidised regenerated cellulose (Interceed, TC-7, Johnson & Johnson, USA) to inhibit the formation of peritendinous fibrotic adhesions after tendon repair in rats. Both Achilles tendons of 23 female Wistar-Albino rats weighing between 350 and 450 grams were cut and repaired. On the right side, Interceed absorbable adhesion barriers were wrapped around the repaired tendon (group I). On the left, the same procedures were applied except for the Interceed wrapping and these were grouped as control (group II). Animals were sacrificed at postoperative day 28 and macroscopic and histological examination was performed. All the animals survived and no tendon rupture was observed. No wound dehiscence, wound infection or exposure of repaired tendons occurred. Macroscopically, there were three (13.1%) tendons without adhesion formation and 20 (86.9%) tendons with inferior adhesion formation in group I; on the other hand, there were 16 tendons (69.5%) with medium grade adhesion formation and seven tendons (30.5%) with severe peritendinous adhesion formation in group II (control group) (p < 0.05). Histologically, adhesion formation was absent in 11 tendons (47.8%) and slight in 12 tendons (52.2%) in group I; while in group II, it was slight in two (8.6%), moderate in 15 (65.2%) and severe in six tendons (26.2%) (p < 0.05). Sixteen (69.5%) of 23 tendons in group I and 11 (47.8%) of 23 tendons in group II showed no inflammatory reaction (p < 0.05). Nineteen (82.6%) tendons in group I and only one tendon in group II showed excellent to good tendon healing (p = 0.00). According to our results, we feel that Interceed may have an intraoperative role to play in the reduction of adhesions after surgical tendon repair. This study suggests that absorbable oxidised regenerated cellulose merits further evaluation as a potential treatment to inhibit the formation of peritendinous adhesions. Rigorous and extensive controlled trials should be undertaken on patients undergoing tendon repair with or without this barrier.
Résumé
Cette étude expérimentale a pour but de mettre en évidence l’action de l’Interceed TC-7 de Johnson & Johnson afin d’éliminer la formation d’adhérence péri-tendineuse dans la réparation de tendons chez le rat. Les deux tendons d’Achilles de 23 rats albinos femelles pesant entre 350 et 450 grammes ont été sectionnés puis réparés. Du côté droit, le produit Interceed a été appliqué autour du tendon (groupe I), le côté gauche a constitué le groupe contrôle. Les animaux ont été sacrifiés à J28 avec un examen macroscopique et histologique. Tous les animaux ont survécu à la réparation et aucun n’a présenté de rupture du tendon. Il n’y a pas eu d’infection. Macroscopiquement, dans le groupe I, 3 tendons (13.1%) n’ont pas présenté d’adhérence péri-tendineuse et 20 (86.9%) ont présenté des adhérences inférieures. Dans le groupe contrôle 16 rats (69.5%) présentaient des adhérences de grade moyen et 7 (30.5%) des adhérences importantes, cette différence était significative (p < 0.05). Histologiquement, la formation d’adhérences est nulle chez 11 rats (47.8%) et légère chez 12 (52.2%). Dans le groupe I, en ce qui concerne le groupe contrôle, les adhérences sont présentées chez deux rats (8.6%) sont modérées chez 15 rats (65.2%) et sévères chez 6 rats (26.2%) dans le groupe II (p < 0.05). 16 (69.5%) des 23 tendons du groupe I et II (47.8%) des 23 tendons du groupe II ne montrent pas de réaction inflammatoire (p < 0.05). 19 (82.6%) tendons du groupe I et seulement 1 tendon du groupe II montrent une excellente cicatrisation. Ces résultats démontrent que l’Interceed peut avoir un rôle important dans la réduction des adhérences après réparation chirurgicale des tendons. Après cette étude, nous pouvons penser que ce produit nécessite d’autres évaluations quant à son potentiel d’inhibition dans la formation d’adhérences péri-tendineuses.
Introduction
Despite the improvements in surgical techniques and postoperative rehabilitation programmes, the formation of fibrous adhesions between the healing tendon and the surrounding tissues is still the most common complication after lacerated tendon repair. It is believed that sutures, tendon sheath damage and immobilisation all contribute to the adhesion formation [9]. The subcutaneous tissue and the skin act as a single compartment during the healing process, and adhesion around the repair site interferes with tendon gliding, which results in contracture and decreased range of motion of the digit [20]. The control of excessive scar formation is essential to restore the functional integrity of a healing tendon after surgery. Continuous efforts are being made to reduce postoperative peritendinous adhesions in damaged tendons.
The main techniques for reducing adhesions without adversely affecting the healing process are mechanical and biochemical. Mechanical barriers like alumina sheaths, polyethylene membranes, cellophane, sterispon wrapping, stainless steel or silicone sheeting, amniotic membranes, chitosan membranes, silicone rubber envelopes, polytetrafluoroethylene surgical membranes, seprafilm, hydrogel sealant, chondritin sulphate coated polyhydroxyethyl metacrylate membrane, and autogenous vein graft may be either biological or synthetic [3, 6, 11, 16, 19, 20, 25]. The ideal barrier would be easy to use, biocompatible, allow tendon movement, not add undue bulk, remain at the site of repair long enough to allow tendon healing (extrinsic healing), absorbable, and cheap. There are some studies that have concentrated on reducing peritendinous adhesions through biochemical means by controlling the size and quality of newly formed collagenous scar. They include cortisone [24], dextran 70 [10], fibrin [7, 13], collagen inhibitor [2], aprotinin—a proteinase inhibitor [21], antihistamine [23], indomethacin [21], hyaluronic acid [12, 19] and 5-fluorouracil [1].
The use of a barrier during surgery to protect raw tissue surfaces as they heal has been shown to be one of the most effective methods of reducing adhesions. Interceed is a product that is used to reduce the likelihood of adhesions developing. It is a lightweight tissue-like “fabric” that can be placed at the surgical site. The fabric helps to prevent post-operative adhesions by protecting and separating the surfaces where adhesions are likely to form. The Interceed is laid over the raw areas at the completion of surgery and is absorbed over the next 2–4 weeks, during which time the tissues have the opportunity to heal themselves, thus reducing the risk of adhesions. The absorption time depends on the surgical site applied and size of applied material. It is mostly used as an adjuvant in open (laparotomy) gynaecological pelvic surgery to reduce the incidence of postoperative pelvic adhesions after meticulous haemostasis is achieved consistent with microsurgical principles. Its effect on prevention of peritoneal adhesions was reported in some experimental and clinical studies [14]. However, there was no report in the literature regarding its use in prevention of peritendinous adhesions after tendon repair.
In this experimental study, we aimed to examine the ability of absorbable oxidised regenerated cellulose (Interceed, TC-7, Johnson & Johnson, USA) to inhibit the formation of peritendinous fibrotic adhesions after tendon repair in rats.
Materials and methods
Twenty-three female Wistar-Albino rats weighting between 350 and 450 grams were used for this study after approval was obtained from the University Committee on Animal Resources. Before surgery, the rats were allowed to walk on a plain platform for walking track analysis.
Anaesthesia was performed with an intraperitoneal injection of pentothal (50 mg/kg). A preoperative dose of intramuscular cefazolin sodium (0.1 mg/kg) was administered for infection prophylaxis. The rat was positioned prone on the operating table and, in order to standardise the surgical technique, the same surgeon performed the operations.
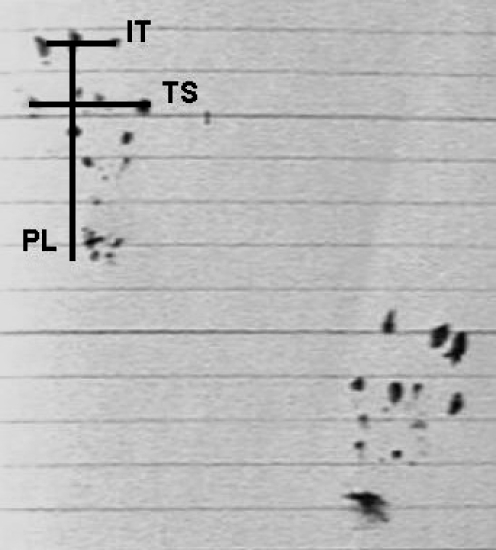
Both hind limbs of the rats were shaved. The surgical field was prepared with povidone-iodine (Betadine) soap and solution. The area was draped in an aseptic fashion. A posterior longitudinal skin incision of 2 cm was made in both legs sequentially. After dissection of skin and subcutaneous tissues, the Achilles tendon was exposed and full thickness transverse tenotomy with a number 11 blade was made at a point 0.5 proximal to distal insertion of the tendon. Ruptured tendons were repaired with modified Kessler technique by using 5/0 monofilament polypropylene (Prolene; Ethicon, Somerville, NJ) sutures. Tendon sheaths were not repaired in any rats. On the right side, Interceed absorbable adhesion barriers were wrapped around the repaired tendons and recorded as group I (Fig. 1). On the left Achilles tendons, the same procedures were applied without the Interceed wrapping and they were grouped as controls (group II). The skin was closed in interrupted fashion with 5/0 silk suture. Following surgical intervention, no wound dressing was applied. The mobilisation was not limited after surgery. All rats lived for 28 days in room temperature and at the end of the first postoperative month, postoperative walking analysis was done. From this analysis, preoperative and postoperative foot sign length (PL), distance between 1st and 5th digits (TS) and distance between 2nd and 4th digits (IT) were measured (Fig. 2).
Fig. 1.
On the right side, Interceed absorbable adhesion barriers were wrapped around the repaired tendons and recorded as group I. On the left Achilles tendons, the same procedures were applied without the Interceed wrapping and they were grouped as control (group II)
Fig. 2.
All the rats lived for 28 days in room temperature and at the end of the first postoperative month, postoperative walking analysis was done. From this analysis, preoperative and postoperative foot sign length (PL), distance between 1st and 5th digits (TS) and distance between 2nd and 4th digits (IT) were measured
Animals were sacrificed at postoperative day 28 by a lethal dose of pentobarbital (150 mg/kg) and knee disarticulation was done in both hind limbs. The criteria described by Tang et al. [22] were used for quantitative evaluation of peritendinous adhesions macroscopically. The adhesion length, density, and motion capacity of the repaired tendons were evaluated.
The Achilles tendons in both hind limbs were excised en bloc with origin and insertion. They were fixed in neutral buffered formalin for at least 48 hours and embedded in paraffin. Permanent sections of 5–7 μm were stained with haematoxylin and eosin. The histological sections (stained with haematoxylin and eosin) were evaluated microscopically (Olympus BX-50, Japan) by two independent observers (double blinded fashion) for the presence of fibrosis, the density of fibrosis, the vascularity at the fibrosis, the presence or absence of foreign body response and inflammation, and for the extent of normal tendon healing. For the grading of peritendinous fibrous tissue development and tendon healing, the criteria described by Tang et al. [22] were used (Tables 1 and 2). The criteria described by Moran et al. [18] were used to evaluate peritendinous inflammatory reaction (Table 3).
Table 1.
Criteria described by Tang et al. [22]; a histological evaluation of adhesions
| Points | Features of adhesion |
|---|---|
| Quantity | |
| 0 | No apparent adhesions |
| 1 | A number of scattered filaments |
| 2 | A large number of filaments |
| 3 | Countless filaments |
| Quality | |
| 0 | No apparent adhesions |
| 1 | Regular, elongated, fine, filamentous |
| 2 | Irregular, mixed, shortened, filamentous |
| 3 | Dense, not filamentous |
| Grading of adhesions | |
| 0 | None |
| 1–2 | Slight |
| 3–4 | Moderate |
| 5–6 | Severe |
Table 2.
Criteria described by Tang et al. [22]; a histological grading system for tendon healing
| Grade | Description |
|---|---|
| Excellent | Continuity of tendon was re-established and the epitenon was smooth |
| Good | Regular intratendinous collagen bundles, but the epitenon was destroyed by adhesions |
| Fair | Irregular intratendinous collagen bundles, and partly interrupted by adhesions |
| Poor | Disconnection of the repair site by adhesion tissues |
Table 3.
Criteria described by Moran et al. [18]; a grading system for evaluation of peritendinous inflammatory reaction
| Grade | Inflammatory reaction |
|---|---|
| 0 | No reaction |
| 1 | Minimal leukocytes infiltration into fibroosseous tendon sheath |
| 2 | Leukocyte infiltration in synovium and epitenon |
| 3 | Leukocyte infiltration in synovium and endotenon |
| 4 | Diffuse leukocyte infiltration |
For statistical analysis, Wilcoxon’s signed rank test, the Kruskal-Wallis test and the Mann-Whitney U test were used. A p value of less than 0.05 was considered statistically significant.
Results
All animals survived and no tendon rupture was observed. They walked in their cages without any difficulty. No wound dehiscence, wound infection or exposure of repaired tendons occurred.
Walking analysis The average values of IT, TS and PL were 9.3 mm (range 7 to 12 mm), 16.1 mm (range 12 to 22 mm) and 32.52 mm (range 29 to 40 mm) in group I, respectively. For group II, they were 8.82 mm (range 6 to 11 mm), 14.9 mm (range 12 to 17 mm) and 30.3 mm (range 27 to 40 mm), respectively. The statistical significance was only present in PL measurements between both groups (p < 0.05).
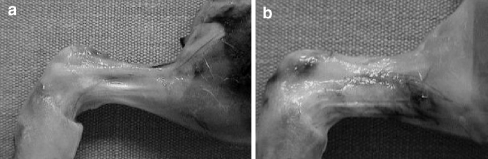
Macroscopic evaluation of peritendinous adhesion formation There were three tendons (13.1%) with absent adhesion formation and 20 tendons (86.9%) with inferior adhesion formation in group I; on the other hand, there were 16 tendons (69.5%) with medium grade adhesion formation and seven tendons (30.5%) with severe peritendinous adhesion formation in group II (control group) (Fig. 3). The difference between both groups was statistically significant (p < 0.05).
Fig. 3.
Macroscopic appearance of healed tendons. a Experiment group. b Control group
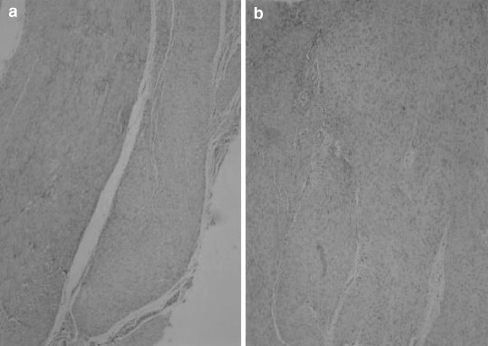
Histological evaluation of peritendinous adhesion formation Adhesion formation was absent in 11 tendons (47.8%) and slight in 12 (52.2%) in group I; while in group II, it was slight in two tendons (8.6%), moderate in 15 (65.2%) and severe in six (26.2%). The difference between groups was statistically significant (p < 0.05) (Fig. 4).
Fig. 4.
Histological examination showed minimal peritendinous fibrosis in group I (a) and significant fibrosis in group II (b)
Presence or absence of inflammatory reaction Sixteen (69.5%) of 23 tendons in group I and 11 (47.8%) of 23 tendons in group II showed no inflammatory reaction (p < 0.05).
Tendon healing Nineteen (82.6%) tendons in group I and only one tendon in group II showed excellent–good tendon healing and this difference was statistically significant (p = 0.00).
Discussion
This study was designed to assess the effect of Interceed, an absorbable adhesion barrier of oxidised regenerated cellulose, on peritendinous adhesion formation prevention in a rat experimental model.
Peritendinous adhesions are the most important postoperative complications following tendon repair in the hand. Development of dense adhesions between the tendons and the surrounding structures causes impairment in digital function.
The hand is the most commonly injured part of the body with hand injuries representing about 7.5–10% of all emergency room visits [6]. Despite advances in surgical techniques and postoperative therapy, the results of tendon repair remain largely unpredictable, and adhesion formation remains an aggravating problem for the hand surgeon and the patient [15].
Injured tendons are capable of intrinsic healing when surgery is not available provided that the damaged tissue can obtain the necessary nutrients. In such cases, to maximise the chance of complete recovery, it is essential that the formation of adhesions in the healing area be avoided to the greatest possible extent; toward this end, methods of separating the healing tendon from its surrounding tissues have been advocated [11]. Although it is generally agreed that primary repair of flexor tendons, with accompanying preservation or restoration of the tendon sheath, is the treatment of choice, pursuing this course can create problems. The surgical sheath closure may narrow the diameter of the gliding tunnel. In two studies repaired tendons with enlarged sheath tunnel had greater tendon movement than those within a narrowed sheath [22]. At other times surgical repair of the sheath is not possible because of major defects in its substance after injury or the sheath is crushed so badly that it must be resected [20]. In such cases attempts are made to restore the integrity of the sheath or to replace it with various biological or synthetic materials [11].
The idea of a mechanical barrier to adhesion formation is not new. In previous decades, many different materials, both biological and synthetic, have been tested [3, 6, 11, 16, 19, 20, 25]. Biological barriers have met with variable success and add donor site morbidity and surgical complexity to the procedure. On the other hand, some synthetic materials failed because they stimulated a severe inflammatory response or allowed ingrowths of adhesions around the edges of the material. Other materials prevented nutrient diffusion to the healing tendon leading to tendon necrosis. In recent years, the focus has turned to diffusible membranes. Isik et al. [17] used a hyaluronic acid membrane in a chicken model and showed adhesions were inhibited. This has not found wide clinical application, however, because the material is difficult to prepare and must be sewn around the tendon repair. Merle et al. [16] studied a bioresorbable gel composed of a carbohydrate polymer (ADCON-T/N). Although a decrease in tendon adhesion formation was demonstrated in an animal model, clinical trial results have been disappointing, and there is some question as to whether the material migrates from the repair site [17]. In our study, we also determined that normal walking analysis of the experimental side was better compared to the control side in which no adhesion barrier had been used after tendon repair surgery.
In our study, we tested the hypothesis that the use of absorbable oxidised regenerated cellulose barrier will be safe and significantly reduce the extent of peritendinous adhesion formation after tendon repair. For this purpose, a bilateral Achilles tendon repair rat model was employed with efficacy of the barrier assessed using qualitative and quantitative histological methods. Oxidised regenerated cellulose (Interceed) is a barrier that adheres to the site of injury, gelatinises, and purportedly keeps the opposing tissue layers separated during the healing process. Interceed was approved by the FDA in 1989 as the first product specifically indicated for reduction of post-surgical adhesions especially in pelvic and abdominal operations. Many studies since then have shown that the proper use of Interceed is useful in reducing formation of adhesions after abdominal and pelvic surgery [4, 5, 14]. It has been recently reported that Interceed influences expression of factors commonly accepted to be associated with adhesiogenesis, especially tending to decrease TGF-b1 expression in adhesion fibroblasts [8].
In conclusion, prevention of peritendinous adhesions following tendon repair represents a major challenge in hand surgery. This study suggests that absorbable oxidised regenerated cellulose merits further evaluation as a potential treatment to inhibit the formation of peritendinous adhesions. However, it should be borne in mind that tendon healing potential, tolerance to infection and the tolerance or immune response to foreign materials in rats are somewhat different to that human beings. Rigorous and extensive controlled trials should be undertaken on patients undergoing tendon repair with or without this barrier. Such trials may help determine its ultimate value. According to our results, we feel that Interceed may have an intraoperative role to play in the reduction of adhesions after surgical tendon repair.
References
- 1.Akali A, Khan U, Khaw PT, McGrouther AD (1999) Decrease in adhesion formation by a single application of 5-fluorouracil after flexor tendon injury. Plast Reconstr Surg 103:151–158 [DOI] [PubMed]
- 2.Bora Jr FW, Lane JM, Prockop DJ (1972) Inhibitors of collagen biosynthesis as a means of controlling scar formation in tendon injury. J Bone Jt Surg Am 54:1501–1508 [PubMed]
- 3.Demirkan F, Colakoglu N, Herek O, Erkula G (2002) The use of amniotic membrane in flexor tendon repair: an experimental model. Arch Orthop Trauma Surg 122:396–399 [DOI] [PubMed]
- 4.Diamond MP, DeCherney AH (1987) Pathogenesis of adhesion formation/reformation: application to reproductive pelvic surgery. Microsurgery 8:103–107 [DOI] [PubMed]
- 5.Diamond MP (2001) Prevention of adhesions. In: Gershensopn DM, DeCherney AH (eds) Operative gynecology, 2nd edn. Saunders, Philadelphia, pp 211–222
- 6.Ferguson REH, Rinker B (2006) The use of hydrogel sealant on flexor tendon repairs to prevent adhesion formation. Ann Plast Surg 56:54–58 [DOI] [PubMed]
- 7.Frykman E, Jacobson S, Widenfalk B (1973) Fibrin sealant in prevention of flexor tendon adhesions: an experimental study in the rabbit. J Hand Surg Am 18:68–75 [DOI] [PubMed]
- 8.Gago LA, Saed GM, Wang RX (2003) Effects of oxidized regenerated cellulose on the expression of extracellular matrix and transforming growth factor-beta1 in human peritoneal fibroblasts and mesothelial cells. Am J Obstet Gynecol 189:1620–1625 [DOI] [PubMed]
- 9.Gelberman RH, Woo SLY, Amiel D, Horibe S, Lee D (1990) Influences of flexor sheath continuity and early motion on tendon healing in dogs. J Hand Surg Am 15:69–77 [DOI] [PubMed]
- 10.Green S, Szabo R, Langa V, Klein M (1986) The inhibition of flexor tendon adhesions. Bull Hosp Joint Dis 46:16–21 [PubMed]
- 11.Gudemez E, Eksioglu F, Korkusuz P, Asan E, Gursel I, Hasirci V (2002) Chondroitin sulfate-coated polyhydroxyethyl methacrylate membrane prevents adhesion in full-thickness tendon tears of rabbits. J Hand Surg Am 27:293–306 [DOI] [PubMed]
- 12.Isik S, Ozturk S, Gurses S, Yetmez M, Guler MM, Selmanpakoglu N, Gunhan O (1999) Prevention of restrictive adhesions in primary tendon repair by HA-membrane: experimental research in chickens. Br J Plast Surg 52:373–379 [DOI] [PubMed]
- 13.Jones MM, Burnett S, Southgate A, Sibbons P, Grobbelaar AO, Green CJ (2002) The role of human-derived fibrin sealant in the reduction of postoperative flexor tendon adhesion formation in rabbits. J Hand Surg Br 27:278–282 [DOI] [PubMed]
- 14.Linsky C, Diamond M, Cunningham T, Constantine B, De Cherney A, di Zerega G (1987) Adhesion reduction in the rabbit uterine horn model using an absorbable barrier, TC-7. J Reprod Med 32:17–21 [PubMed]
- 15.Matloub HS, Dzwierzynski WW, Erickson S (1996) Magnetic resonance imaging scanning in the diagnosis of zone II flexor tendon rupture. J Hand Surg Am 21:451–455 [DOI] [PubMed]
- 16.Menderes A, Mola F, Tayfur V, Vayvada H, Barutcu A (2004) Prevention of peritendinous adhesions following flexor tendon injury with seprafilm. Ann Plast Surg 53:560–564 [DOI] [PubMed]
- 17.Mentzel M, Hoss H, Keppler P (2000) The effectiveness of ADCON-T/N, a new anti-adhesion barrier gel, in fresh divisions of the flexor tendons in zone II. J Hand Surg Br 25:590–592 [DOI] [PubMed]
- 18.Moran SL, Ryan CK, Orlando GS, Pratt CE, Michalko KB (2000) Effects of 5-fluorouracil on flexor tendon repair. J Hand Surg Am 25:242–251 [DOI] [PubMed]
- 19.Ozgenel GY (2004) The effects of a combination of hyaluronic and amniotic membrane on the formation of peritendinous adhesions after flexor tendon surgery in chickens. J Bone Jt Surg Br 86:301–307 [DOI] [PubMed]
- 20.Siddiqi NA, Hamada Y, Ide T, Akamatsu N (1995) Effects of hydroxyapatite and alumina sheaths on postoperative peritendinous adhesions in chickens. J Appl Biomat 6:43–53 [DOI] [PubMed]
- 21.Szabo RM, Younger E (1990) Effects of indomethacin on adhesion formation after repair of zone II tendon lacerations in the rabbit. J Hand Surg Am 15:480–483 [DOI] [PubMed]
- 22.Tang JB, Shi D, Zhag QG (1996) Biochemical and histologic evaluation of tendon sheath management. J Hand Surg Am 21:900–908 [DOI] [PubMed]
- 23.Walker FG, Bensley SH, Lindsay WK (1961) The effects of an antihistamine (promethazine) on the reaction of the tendons to trauma: a histological study. Can J Biochem Physiol 39:89–101 [PubMed]
- 24.Wrenn RN, Golduer JL, Markee JL (1954) An experimental study of the effect of cortisone on the healing process and tensile strength of tendons. J Bone Jt Surg Am 36:588–601 [PubMed]
- 25.Zhang H, Sheng ZJ, Hou CL (1997) Effect of chitosan membrane on tendon adhesion and healing. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 13:382–387 [PubMed]