Abstract
Aging is a complex process involving intracellular changes and, notably, modifications in intercellular communications, required for coordinated responses to internal and external events. One of the potential reasons for such changes is an age-dependent failure of the integrating systems, including the circadian clock. Here we demonstrate that aging in a diurnal vertebrate, zebrafish (Danio rerio), is associated with major but selective circadian alterations. By 3–5 years of age, zebrafish have reduced amplitude and increased fragmentation of entrained circadian rhythms of activity, with fast desynchronization of the rhythms in the absence of environmental time cues. Aging in zebrafish is also associated with a reduction in the overall duration of nighttime sleep, followed by lower activity levels and a higher arousal threshold during the day. The production of the principal circadian hormone, melatonin, progressively declines during zebrafish aging. However, the ability of melatonin to acutely promote sleep and entrain circadian rhythms of activity remains robust until at least 4–5-years of age, consistent with the preserved levels of mRNA expression for melatonin receptors. Aged zebrafish have altered expression of the circadian genes zBmal1 and zPer1 but not zClock1. A lack of circadian time cues alters cognitive performance in aged more than in young zebrafish and this can be partially attenuated by daily melatonin administration. The advantages of zebrafish as a diurnal, small, prolific and genetically well-characterized vertebrate model provide new opportunities to clarify the intrinsic circadian factors involved in human aging and promote the search for prophylactic and treatment strategies.
Keywords: zebrafish, aging, circadian, locomotor activity, sleep, melatonin
1. Introduction
The circadian clock system is a phylogenetically highly conserved mechanism of temporal synchronization of the biological processes with the regular 24-hour changes of our planet environment. Importantly, it is also a principal integrating mechanism for internal coordination, allowing individual intracellular, physiological and behavioral events to be initiated and terminated at the right time, without mutual conflict. A recent progress in understanding the molecular mechanisms of the circadian clock revealed autoregulatory transcription–translation feedback loops involving several mutually-dependent circadian genes of Clock, Bmal, Period, Cry and Rev-Erb families [for reviews, 9, 26].
The results of multiple studies in humans and animal models suggest that aging alters the circadian clock. This involves changes in the master clock, peripheral oscillators or clock-controlled processes [for review, 11, 18]. As a result, the integrity of multiple rhythms, including sleep, body temperature, hormonal secretion, gastrointestinal, cardiovascular or kidney functions is jeopardized, contributing to increased probability of age-related disease states [1, 3, 12, 19, 21, 28]. Studies in diurnal insects (Drosophila) and nocturnal mammals (hamsters and mice) show age-dependent changes in the expression of a number of core circadian genes [2, 7, 15, 16, 17, 24, 29]. Moreover, mutations in circadian genes can lead to premature aging [for review, 18]. However, since not all the circadian gene mutations associated with alterations in circadian patterns of behavior can cause early aging, it appears that only some circadian factors are able to accelerate or inhibit the aging process. This phenomenon is likely a result of the circadian gene products serving as transcription factors for diverse non-circadian genes, only some of which affect the course of aging [for review, 22]. This area requires further in-depth investigation and diverse animal models could help to elucidate the mechanisms involved in the role of the circadian factors in aging.
In spite of highly conserved principal mechanisms of the circadian system, an animal’s life style defines certain differences between the nocturnal and diurnal species. This is especially evident in terms of mutual synchronization of specific behavioral, physiological and circadian events. For example, in mammals, the SCN neuronal activity is high at daytime, corresponding to a daily rest period in nocturnal species and an activity period in diurnal animals. Similarly, an opposite behavioral state corresponds to exclusively nighttime secretion of the principal circadian hormone, melatonin, in either diurnal or nocturnal animals. As a result, the circadian factors may have quite different effects on behavior, e.g., melatonin promotes sleep in diurnally-active humans, macaques, birds or zebrafish, in contrast to lack of such effect of melatonin in nocturnal rats, mice or owls [20, 23, 32, 33, 35, 36]. It is thus important to evaluate the extent to which the circadian system and aging are linked in diurnal vertebrates. We propose zebrafish as a model for such studies.
Zebrafish is a diurnal vertebrate and a popular organism for developmental biology and genetics. Recently, it has also attracted attention as a model for studying aging [8, 13, 14, 27, 31]. Under laboratory conditions, zebrafish mature within the first 6 months and survive for up to 6 years. They show age-dependent changes in multiple physiological and cognitive parameters by 2 years of age [27, 31]. The presence of both central circadian oscillators (the pineal gland and eyes) and peripheral oscillators in multiple tissues [for review, 4, 6, 25] makes zebrafish a potentially outstanding source of information on the intrinsic and environmental factors involved in coordinating these circadian elements and their impact on aging.
Here we show that zebrafish aging is associated with significant changes in rhythms of activity, sleep, melatonin production and expression of core circadian genes, zBmal1 and zPer1, but not zClock1. The level of expression for melatonin receptor genes and melatonin efficacy to promote sleep or entrain circadian rhythms of activity is preserved in aged zebrafish. We also demonstrate that cognitive changes in the absence of environmental circadian time cues are more pronounced in aged than in young zebrafish and can be attenuated by regular melatonin administration. These findings suggest that zebrafish is a promising animal model for studying the molecular mechanisms of circadian aging and their role in the overall aging process in diurnal vertebrates.
2. Materials and methods
2.1 Animals and housing conditions
Adult male zebrafish (Danio rerio, AB wild type strain) of five age groups were studied [mean (SEM)]: young, 1-year old [0.9 (± 0.04)]; 2-years old [2.1 (± 0.07)]; 4-years old [3.9 (± 0.09)], and 5 years old [5.3 (± 0.08)]. Prior to and between experiments, fish were maintained in 3-L tanks (5–7 fish/tank) in a temperature-controlled (26.5°C) multi-tank re-circulating water system (Aquaneering, San Diego, CA, USA) in 14L:10D light:dark cycle (LD; 400 lux vs. <1 lux). Animals were fed twice a day with live brine shrimp (Brine Shrimp Direct, Ogden, UT, USA) and flake food (TetraMin, Tetra Blacksburg, VA, Germany). The protocol was approved by the Boston University School of Medicine animal care and use committee and complied with the NIH Guide for the Use and Care of Laboratory Animals.
2.2 Locomotor activity and sleep recordings
Individual zebrafish locomotor activity was continuously documented using automatic animal tracking software (Video-track, View Point Inc, France). Fish, 10–24 recorded in parallel, were placed in individual compartments of the circadian system (long-term recording) or individual tanks for short-term recordings. Each behavioral system had one camera placed above the tanks and illuminated floor to provide back-light for the camera recordings. An additional light source was placed next to the tanks for providing the LD cycle. Under dim light contained twelve individual home/experimental compartments (round, 95 mm in diameter, or rectangular, 80 × 80 mm), with mesh-covered bottom and top, maintained in a rack within a common water tank. Continuous water filtration, bottom-wash recirculation and fresh water supplementation, temperature and conductivity control assured constant optimal water conditions and consistent image quality. Feeding was provided at certain times of day (in LD) or was continuously available in the individual feeders (in dL). While in the circadian system, fish were fed decapsulated brine shrimp eggs (Brine Shrimp Direct, Ogden, UT, USA). During the short-term recordings (minutes-hours) in individual tanks, water was not changed and fish were not fed.
Young and aged zebrafish were recorded in parallel and their individual tanks or compartments were within the same behavioral recording system to avoid potential small differences in their environment. The data acquisition speed was set at 30 frames/sec. The data integration period was 30–900 sec, depending on the type of experimental procedure and duration of the behavioral recordings. Individual distance traveled, time moving, inactivity time and number of inactivity bouts was registered. Inactivity threshold was defined as equal or below 0.1 cm/sec. Consistent environmental conditions and thorough pre-recording calibration assured lack of recording artifact. Prior to completing a recording period, visual observation of automatic tracking was conducted for at least 15 min. In rare cases when a recording artifact was observed at the end of the recording, i.e., a tracking line did not follow the fish, the data for this fish/period were removed from further analysis. The circadian amplitude of activity was estimated as half of the distance between the peak and the trough of the sine wave with a period of 24 h fitted to activity data using a nonlinear least squares analysis (Mathematica, Wolfram Research, Champaign, IL).
Video recordings were conducted for sleep evaluation, with further manual scoring of the duration of individual inactivity periods in individually housed fish. In different experiments, the camera was placed either in front of the tanks, allowing us to evaluate the depth at which the animals stayed, or above the fish tanks, providing a better view of horizontal movement. Based on the increase in arousal threshold following at least 5 sec of inactivity (see Results), this interval was used to define a sleep-like state episode. Total number and duration of inactivity episodes of 5 sec or more during the daytime and nighttime hours was used to estimate the total sleep duration.
2.3 Arousal threshold evaluation
To assess the arousal threshold to electric stimulation, individual zebrafish were adapted to a tank containing flat electrodes along the tank walls. Following complete motionlessness of 1 sec or more, fish were stimulated with an increasing current (in 0.1 mA steps; Master-8cp, A.M.P. Instruments, Jerusalem, Israel), until a consistent minimal startle or escape response to the stimulation was observed, defined as an immediate initiation of fast activity following stimulation, typically associated with a bend and change in prior heading of around 30°. At each time period (day or night), at least three independent evaluations were conducted in each fish.
2.4 Cognitive test: Generalization of the Conditioned Stimulus
Recognition of an earlier learned conditioned stimulus (CS) in a different environment, where such CS has not been reinforced, i.e., generalization of a CS to new environment, is an important cognitive ability, which can increase individual survival. The experimental procedures were conducted similar to that described in the earlier study in young and aged zebrafish [31], with some modifications. In brief, prior to the experiments, fish were adapted to the red color filter placed daily next to the wall of the housing tank, at random hours and without association with other procedures. At Baseline, 1- and 4-years old fish were evaluated in the T-maze with the white/white and red/white short arms, to control for the behavioral asymmetry and red color preference or avoidance. Each T-maze procedure included 14 consecutive trials. During the white/red trials, the red filter was introduced randomly either on the left or right arm of the maze at each consecutive trial. Fish were then conditioned to the red color by presenting it in the housing tank 5 min prior to the restricted once-a-day food administration over 12 daily sessions under 100 lux illumination. By the end of the conditioning period, color preference in the tank was documented as reported earlier [31], with over 80% of the fish of either age group preferring to stay near the red color filter, idependent of the side of the tank at which it was placed.
Fish were then maintained either in LD or dL for 3 consecutive 24-h periods, after which they were again tested in the T-maze paradigm (“post-conditioning” T-maze test) at ZT 5–8 (according to LD), under 100 lux illumination. Since LD and dL conditions were associated with different light intensity during the day (400 vs. 5 lux), a 2-hour adaptation period under 100 lux illumination was applied to both groups prior to testing. Increase in the number of the red-color arm choices during the second “postconditioning” T-maze test, compared to baseline test, was a measure of CS generalization. The control animals were conditioned to blue color and this did not significantly affect their performance in the red-white T-maze. Similarly, no significant side (T-maze arm) preference was found at baseline or during the white/white condition.
2.5 Brain melatonin measurements
Zebrafish of different age were frozen by immersion in liquid nitrogen at daytime (ZT 1 and ZT 13; zeitgeber time, ZT 0=lights on time) and at night (ZT 16, 19 and 22). At night, samples were collected under red light (5–10 lux) illumination. The daytime melatonin levels were assessed following a 1-hour exposure to dim light (5 lux), an hour after or an hour before habitual night period, i.e., at ZT 1 and ZT 13, respectively. This avoided suppression of melatonin production by bright light. To control for the potential effects of the dim light conditions used in the behavioral recordings, 2 young and 2 old fish were exposed to 5 lux illumination for 2 hours, at ZT 17–19. Brain tissue was dissected while frozen, sonicated in 96% ethanol and centrifuged. The supernatant was removed and diluted to a concentration of 10% ethanol. Melatonin was extracted from the diluted supernatant through C18 columns and measured using a radio-immunoassay (ALPCO, Windham, NH, USA). The pellet was solubilized in 0.1N NaOH and assessed for protein content (BCA Protein Assay, Pierce Biotechnology, Rockford IL, USA). Melatonin concentrations in each sample were then adjusted for protein level.
2.6 Melatonin treatment
For stock solution, melatonin (Sigma, St. Louis, MO, USA) was directly dissolved to 10 μM concentration in water. The actual levels of melatonin were confirmed by radioimmunoassay (ALPCO, Windham, NH, USA), using serial dilutions, in three independent experiments. The treatment or control solution (water) was administered directly into the fish tank. The final concentration of melatonin in the tank was 100 nM. The duration and time of treatment is described in the Results.
2.7 Sample collection for the circadian gene expression analysis
The eyes, as well as the pineal gland, are prominent circadian oscillators in zebrafish [for review, 4, 6, 25]. Our preliminary data showed principal similarities between the patterns of circadian gene expression in the eyes and brain tissue of adult zebrafish. Eye dissection from an express frozen fish is easy, especially compared to the pineal dissection, helping to preserve the tissue for efficient mRNA recovery. We thus used the eyes of individual fish to determine a circadian pattern of gene expression.
Zebrafish of different age were maintained in the main housing, i.e., temperature-controlled (26.5°C) multi-tank re-circulating water system in 14:10 LD, and fed at ZT 3. Each age group (N=49) was housed in seven 3-L tanks, 7 fish per tank. At specific time points, all the fish within one tank of each age group were simultaneously frozen by immersion in liquid nitrogen. All the samples were collected within the same 24-h period, starting ZT 5, and at 2–4 hour intervals thereafter until ZT 2 next morning. The samples were stored at −80°C.
2.8 Real-time quantitative RT-PCR (QPCR)
Fish were dissected on dry ice and total RNA was extracted from individual eyes using RNeasy kit (Qiagen, Chatsworth, CA, USA), according to the manufacturer’s protocol. The quantity and quality of RNA was determined spectrophotometrically at 260 nm and 260/280 nm. The same amount of RNA from each sample was converted into cDNA using the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA), according to the manufacturer’s instruction. QPCR was performed using a TaqMan® Universal PCR Master Mix and ABI Prism 7300 Real Time PCR System (ABI, Foster City, CA, USA). The TaqMan® primers and probes {5′ FAM, 3′ TAMRA} were designed based on previously reported sequences of zebrafish genes and obtained from ABI, including zPer1 (accession number NM_001030183): Forward, 5′-GAA AAGGCTCAGCCACAGAGA -3′, Reverse, 5′-CGCTCAAAAGACTGAATGACACTGA-3′, {CATT TGAGCTCTTGCTTTC}; zBmal1 (NM_131577): Forward, 5′-CAGAGCTTCGCCACAAACC-3′, Reverse, 5′-CTGTGATCAATGCATGTCCTTTCA-3′ {CTCGATGTGAGGATCTG}; β-actin (AF057040): Forward 5′-GCTGTTTTCCCCTCCATTGTTG-3′, Reverse, 5′-TTTCTGTCCCATGCC AACCAT-3′, {CCCAGACATCAGGGAGTG}; Clock1 (NM_130957): Forward, 5′-CATCCTACAGA AGAGCATCGACTT-3′; Reverse, 5′-GATTTCACTCGACTCCGACTGT-3′,{AAGCACAAAGAAAT TG}; Mel1a1 (NM_131393): Forward, 5′-CTGGTGATTTTCTCCGTCTACAGA-3′, Reverse, 5′-CCG CCACTGCCAAACTC-3′ {AACGCAGGTAACATTTT}; Mel1c (XM_687104): Forward, 5′-CCGTC TACAGGAACAAGAAACTGA-3′, Reverse, GGTCAGCCACAGAAAGACTCA-3′, {AATGCAGG TAACATCTTTG}. Gene expression was normalized using β-actin expression level for each individual fish sample. Relative mRNA expression level was calculated using the standard comparative delta-Ct method. For each gene, samples collected at the same time point for both age groups were processed in parallel and the expression was measured within the same microplate, in triplicate. Due to technical error, the ZT 5 set of fish samples for the aged group was lost.
2.9 Statistical analysis
A mixed-model analysis (Proc Mixed procedure; SPSS 15.0; SPSS Inc., Chicago, IL, USA) was used to examine the effects of age, time and interaction of these factors on behavioral parameters measured over prolonged periods, the levels of melatonin and the mRNA expression. Where the data are presented as the percentage change from control values for easier appreciation of differences, the statistical analysis reported was based on the original data. The data analysis was based on 9–12 fish per group for activity and sleep tests, 4–7 fish per group per time point for melatonin measurements and 4–6 fish per group per time point for gene expression. The data are presented as mean±SEM. For all individual comparisons, difference was considered significant if p<0.05, with non-significant (n/s) difference noted for specific factors. The Bonferroni multiple-comparison correction was used where appropriate.
3. Results
3.1. Circadian patterns of locomotor activity deteriorate in aged zebrafish
During the day in LD, zebrafish typically maintained slow speed motion (typically, 5–8 meters per min), with bouts of fast movement (above 14 meters/min) and inactivity. Under these conditions of entrainment to light and regular feeding, young and aged individually-housed zebrafish showed a nighttime reduction in activity, consistent with their diurnal life-style (Fig. 1A). Aging was associated with a significant reduction in the daily amplitude of activity (p<0.01, for aging factor, Proc Mixed) due to the reduction in daytime activity levels in 3-year old and 4-year-old, compared to 1-year old zebrafish (p<0.05 for either age comparison, Proc Mixed; Fig. 1A). At night, aged animals showed an increase in the number of inactivity bouts (17.4±0.83 in aged vs. 14.9 ±0.97 in young, per 15 min periods, p<0.05, Proc Mixed) but a reduction in their nighttime duration (1.26±0.16 vs. 1.72±0.11 sec per 15 min periods; p<0.05, Proc Mixed), compared to young zebrafish.
Figure 1. Aging in zebrafish results in reduced daytime activity and lower nighttime melatonin production.
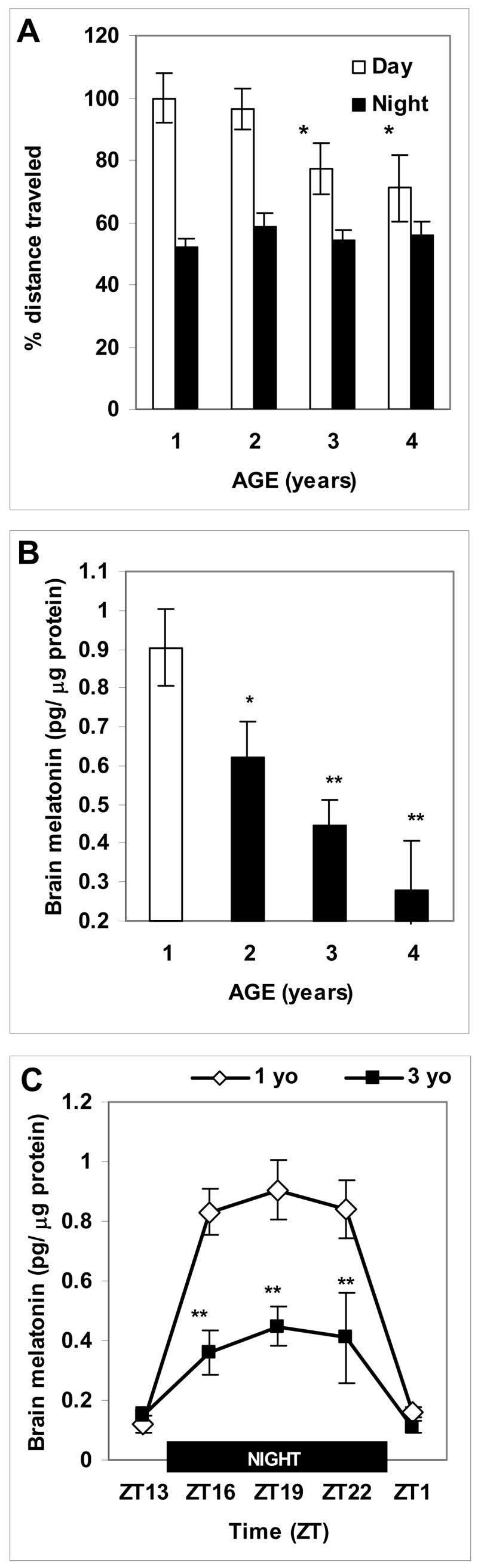
A: Relative percent distance traveled in LD during the day (white) and at night (black) in zebrafish of four age groups (n=11–12 per group; same groups at daytime and at night), with daytime distance traveled by 1-year old fish represented as 100%. B: Brain melatonin levels (pg/μg protein) in the middle of the dark period in young (white) and aged (black) zebrafish (n=5–7 fish per group). C: Comparison of daily patterns of brain melatonin levels (pg/μg protein) in 1-year old (white diamond) and 3-year old (black square) zebrafish (4–7 fish per group per time point). Horizontal black bar represents the night period. Mean (SEM); * p < 0.05,** p < 0.001, compared to 1-year old zebrafish.
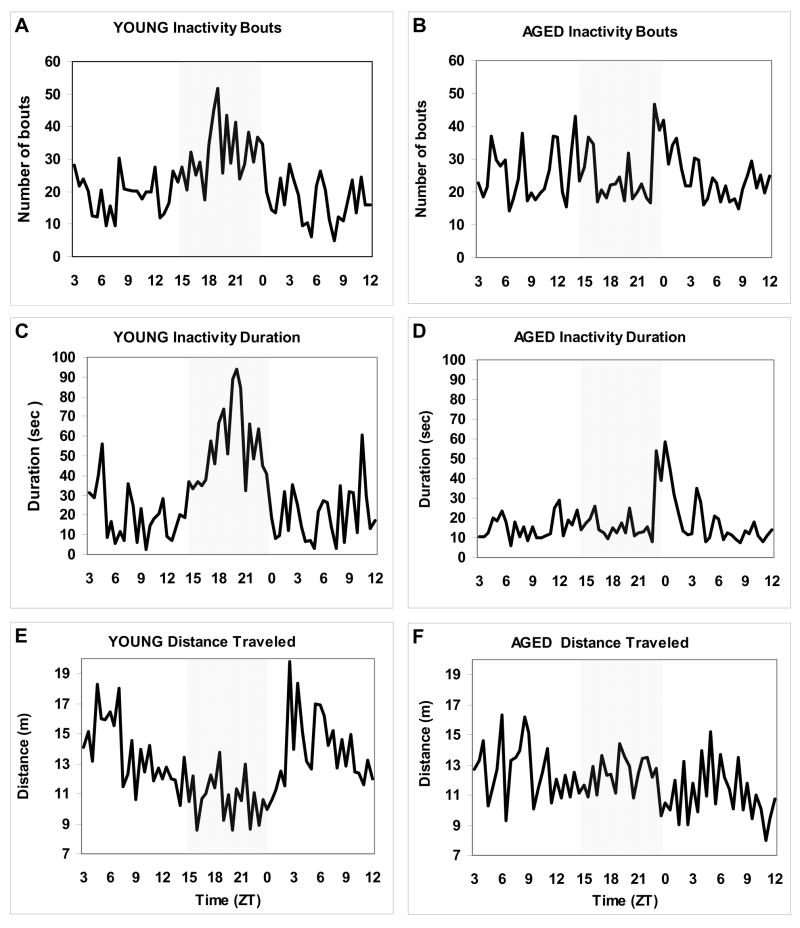
Under constant dim light (dL) conditions, circadian variation in locomotor activity was age-dependently reduced in zebrafish. The results of a representative parallel recording of two individual zebrafish, 1-and 4-year old, following two days in dL are shown in Figure 2. While the majority of the young animals preserved a 24-h pattern of locomotion, it quickly deteriorated in aged zebrafish. This was associated with a higher number of inactivity bouts in aged animals over a 24-h period (24.9±0.96 in aged vs. 22.0 ±1.17 in young per 15 min; p=0.055, Proc Mixed). However, the group mean duration of these bouts was much shorter in aged animals than in the young ones (0.66±0.03 vs. 1.59±0.19 sec, respectively; p<0.0001, Proc Mixed, N=20 each group), resulting in the overall reduction in inactivity duration (17.1 ± 1.27 vs. 31.0 ±2.79 sec per 15 min; p<0.0001, Proc Mixed). In spite of this, aged animals, on average, also had a lower distance traveled over a 24-period (12.0± 0.19 vs. 12.9±0.27 meters per 15 min; p<0.05, Proc Mixed), reflecting a lower speed of movement. Thus, in aged zebrafish, the lack of entraining environmental cues under constant conditions reduced both rest (based on their duration of inactivity) and alertness (based on their distance traveled), promoting slow speed frequent movement episodes instead. An absence of a clear daily pattern of locomotion in dL did not allow us to evaluate an intrinsic circadian period of activity in aged zebrafish and compare it to that in young animals.
Figure 2. Example of the circadian patterns of activity in constant dim light in young (1-year old) and aged (4-year old) zebrafish.
A, B: Number of inactivity bouts (per 15 min); C, D: Inactivity duration (sec/15 min); E,F: Distance traveled (meters/15 min) in young and aged fish, respectively, over a 33-hour period after being maintained in dL for 2 days. Gray area of each plot corresponds to night period in LD.
3.2 Melatonin production is reduced in aged zebrafish
In LD, the daily pattern of melatonin production was observed in both young and aged zebrafish, with gradual age-dependent decline in nighttime brain melatonin levels from 1 to 4 years of age (at ZT 19, p<0.0001, Proc Mixed; Fig. 1B). A significant difference in peak melatonin levels between the 1- and 3-year old groups (at ZT 19) was consistent when the results of three nighttime measurements were compared (p<0.0001, Proc Mixed; Fig. 1C). No difference in low daytime melatonin levels was found between the different age groups. Similarly, no circadian phase shift in melatonin production was found in the aged group, confirming that the level of melatonin production rather than its circadian phase was changed in aged zebrafish. The 5 lux illumination at night, used for studying the circadian rhythms of activity, did not significantly change the nighttime melatonin levels in young or aged zebrafish, compared to ~1 lux (data not shown).
3.3 Aging in zebrafish is associated with reduced sleep time
Similar to findings in zebrafish larvae [36], adult zebrafish maintained in LD demonstrate periods of prolonged quiescence at night, with increased arousal threshold, and their cognitive performance deteriorates when they are deprived of such periods of behavioral quietness [34]. This suggests that adult zebrafish experience a behavioral and physiological state analogous to sleep.
Specific sleep postures are characteristic of many species [5]. In adult zebrafish, the prolonged periods of inactivity (over 5 sec) are typically spent at the top one third of the tank, in contrast to the daytime behavior when fish swim throughout the tank. At night, some fish alternate between the top and bottom of the tank, staying quiet at both locations. Inactivity in adult zebrafish is often, though not always, associated with maintaining a slightly head up position. During prolonged periods of inactivity, zebrafish may stay completely motionless or occasionally perform slow movements of the dorsal and pectoral fins that can move the fish slightly forward or backward, or not affect their location.
An important parameter differentiating sleep and quiet wakefulness in zebrafish, as well as other species, is their increase in arousal threshold [34, 36]. An arousal threshold to electric stimulation after a 1–2 sec period of inactivity, required to reliably document a behavioral response to stimulation, was considered as basal threshold. This basal daytime arousal threshold was 18.3±2.1% and 23.1±3.5 % higher in 4-and 5- year old fish, respectively, when compared to mean arousal threshold in young, 1-year old fish tested in parallel (p<0.001, for young versus aged comparison, n/s for between aged group comparison, Proc Mixed). The increase in basal arousal threshold at night, compared to daytime, was significant for 11 both young and aged zebrafish (12.5±1.4 % and 14.1±2.1% increase above the daytime threshold for 1-and 4-year old fish, respectively; p<0.01 for both within-age comparisons; n/s for age factor, Proc Mixed).
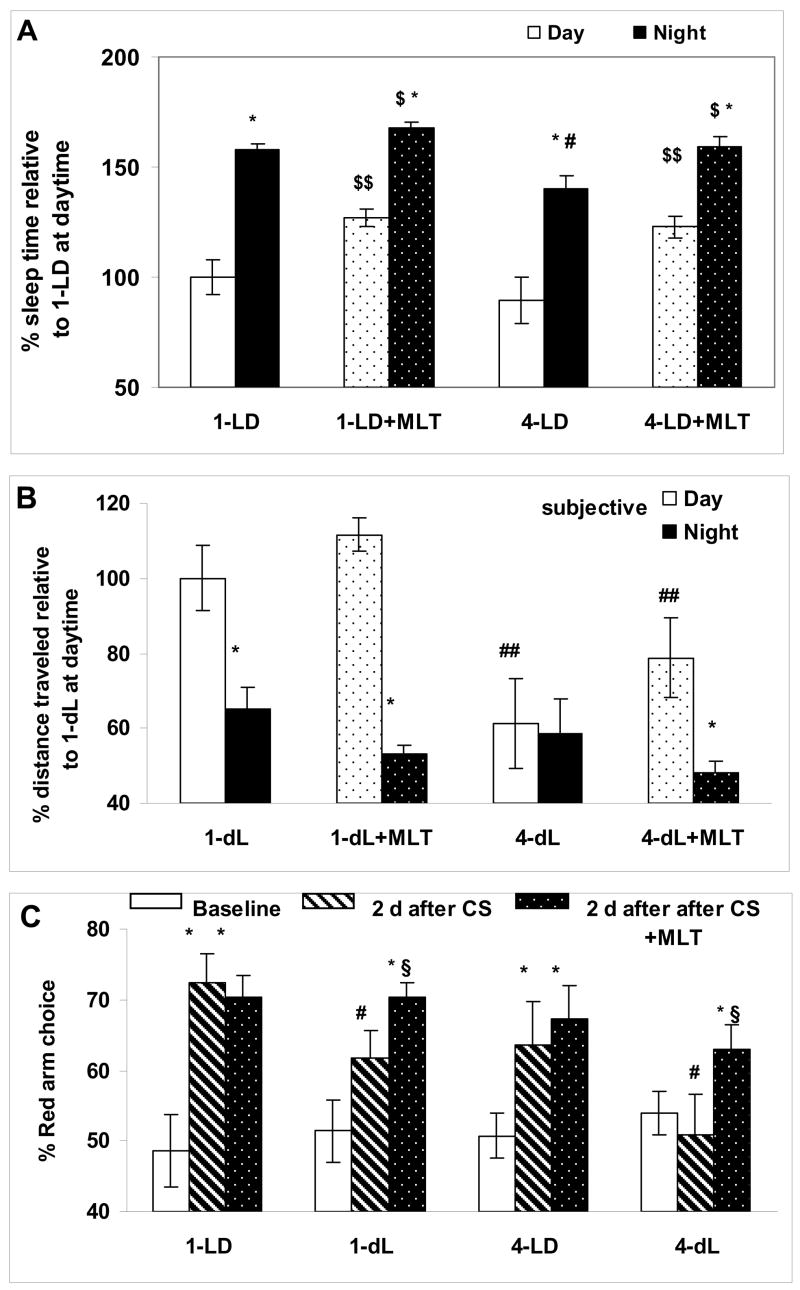
A significant increase in individual arousal threshold following 4–7 seconds of inactivity was found in both 1-year old (19.2±3.2%; p<0.001, Proc Mixed) and 4-years old (17.8±4.1%; p<0.001, Proc Mixed) zebrafish at either daytime or at night [5.3±0.76 sec for two ages combined, p<0.01, for time inactive (below and above 4 sec) factor, Proc Mixed), with no significant difference in this period for the age, time-of-day or time-of-day by age factors. Further increases in inactivity duration (i.e., over 7 sec) showed a tendency toward higher arousal threshold in both age groups but this did not reach the level of significance. Therefore, a 5-sec or longer period of inactivity was considered as representative of the sleep state in young and aged zebrafish [34] and was used for manual scoring of total sleep duration. The total duration of sleep was lower in 4-year old, compared to 1-year old zebrafish, at night (p<0.05, Proc Mixed) but not at daytime (Fig. 3A, 1-LD vs. 4-LD).
Figure 3. Aging in zebrafish is associated with sleep alterations, reduced intrinsic circadian rhythm of activity and cognitive performance, and these effects are counteracted by repeated overnight melatonin administration.
A: Change in percentage of sleep time at daytime (ZT 5–7) and at night (ZT 18–20), with or without 30-min melatonin (+MLT) pre-treatment in 1- and 4-year old zebrafish, relative to mean sleep time in 1-year old zebrafish during the day represented as 100%. B: Percentage of distance traveled at daytime and at night under constant dim light conditions in 1-year old (1-dL) and 4-year old (4-dL) zebrafish at baseline and after melatonin administration (1-dL-MLT and 4-dL-MLT), relative to 1-dL at daytime, represented as 100%. In A and B:* p< 0.05 relative to the same age and treatment condition at daytime; $ p<0.05 and $$ p<0.001, relative to the same age and time in the absence of melatonin treatment; # p< 0.05 and ## p<0.001, relative to the same time and treatment condition in the young group. C: Generalization of conditioned response after exposure to 2 days of different light conditions (LD or dL), with or without overnight melatonin administration in 1- and 4-year old zebrafish. Percentage of choosing the red arm of the T-maze at baseline (white), 2 days after the end of conditioning (diagonal) and 2 days after the end of conditioning with overnight melatonin treatment (black with dots). * p< 0.0001 relative to the same age and light condition at baseline; $ p<0.01, relative to the same age and light condition without melatonin treatment, # p<0.05 relative to the same age and treatment condition in LD. Data presented as group mean (SEM); N=8–10 fish per data point; Proc Mixed for all comparisons.
3.4 Sleep-promoting and entraining effects of melatonin are preserved in aged zebrafish
Melatonin administration increased sleep time in young and aged zebrafish in LD (Fig. 3A), typically within 20 min after treatment. In both age groups (1- and 4-year old), the effect was significant at daytime and at night (p<0.001 for day and p<0.01 for night, n/s for age or time by age factors; Proc Mixed). The results of the automatic locomotor activity recordings in LD also showed a reduction in distance traveled and an increase in inactivity duration in 1-, 4- and 5-years old zebrafish, compared to age-matched control (p<0.001 for each of three age groups, Proc Mixed), with no significant age difference in melatonin efficacy. An arousal threshold during sleep (i.e., after at least 5-sec inactivity) was not significantly increased by melatonin in young or aged zebrafish, compared to their sleep in the absence of melatonin treatment. The daytime arousal threshold following overnight melatonin treatment and 4-hour daytime washout period was significantly reduced in 4-year old zebrafish, compared to the same fish arousal threshold in the absence of prior nighttime melatonin treatment (p<0.05, Proc Mixed). Although a similar tendency was observed in young zebrafish, the effect did not reach the level of significance (p=0.09).
Repeated overnight (ZT 14–24, for 3 consecutive nights) melatonin treatment in dL resulted in increased circadian amplitude of activity in young zebrafish and presence of circadian variation in activity in 4-year old individuals (p<0.001 for change in daily amplitude of activity, Proc Mixed; Fig. 3B). The latter was associated with reduced nighttime activity (p<0.01, Proc Mixed) and tendency toward increased daytime activity, when compared to the same time period without prior melatonin administration (Fig. 3B).
3.5 Lack of circadian entrainment is associated with reduced cognitive performance and daily melatonin treatment can attenuate this effect
In zebrafish, aging is associated with reduced cognitive performance in LD [31]. We have used one of the evaluated experimental paradigms, generalization of adaptive association to a new environment, to determine the effects of constant dim light on cognitive performance. Recognition of an earlier learned CS (in this case, a red color filter in the fish tank was associated with food) in a different environment, a T-maze, where the CS has not been reinforced (i.e., generalization of a CS) was tested.
In LD, generalization of the CS, i.e., increased choice of the red-colored arm of the maze in the new environment of a T-maze at a “post-conditioning” period compared to baseline behavior in the same T-maze, was reduced in the control aged zebrafish group, although this did not reach the level of significance (p=0.072, Proc Mixed), unlike in the earlier report [31]. Cognitive performance was altered in both 1- and 4-year old zebrafish maintained in dL, compared to LD (p<0.001 for environmental illumination factor, Proc Mixed), with significantly more robust effect in aged zebrafish (p<0.05 for age and age by illumination factors, Proc Mixed; Fig. 3C).
In LD, overnight melatonin treatment did not significantly change daytime performance in young or aged zebrafish. After administration during three consecutive nights in dL, melatonin significantly improved daytime CS generalization in both young and aged zebrafish (p<0.01 for treatment factor, n/s for age or age by treatment factor, Proc Mixed; Fig. 3C).
3.6 Expression of core circadian genes is altered during zebrafish aging
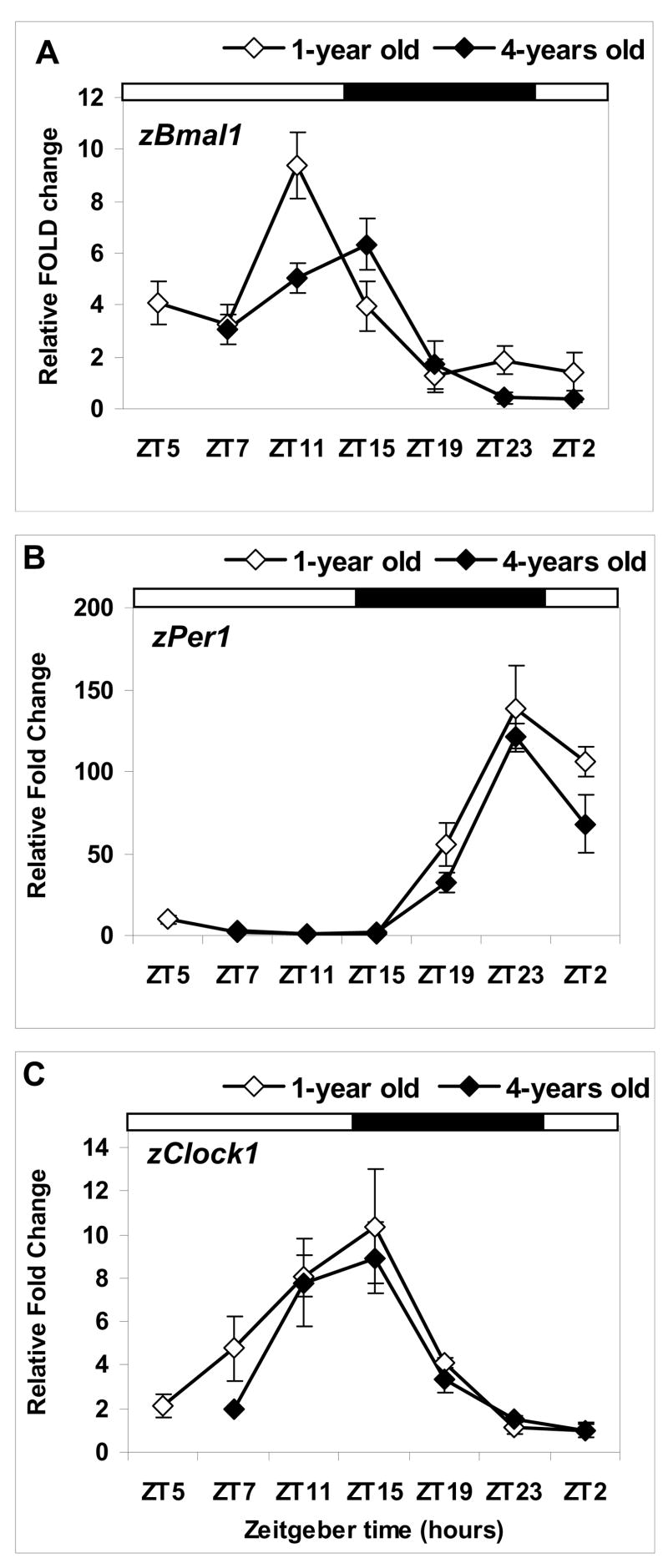
Quantitative assessment of the daily pattern of circadian gene expression in zebrafish eyes showed distinct daily variation in mRNA levels. The time of the daily peak and trough (in parenthesis) in 1-year old zebrafish was found at ZT 11 (ZT 19) for zBmal1, ZT 23 (ZT 11) for zPer1 and ZT 15 (ZT 2) for zClock1 (Fig. 4). An age-dependent reduction in mRNA expression was found for zBmal1 and zPer1 genes (Fig. 4A,B). For both genes, the effect was significant for the time and time by age factors (p<0.0001 for both comparisons, Proc Mixed), with the age factor being significant for zPer1 (p<0.05, Proc Mixed). Peak zBmal1 level was significantly lower in the aged group (p<0.01, for age factor, Proc Mixed) and associated with an apparent phase delay in the expression of this gene in 4-year old zebrafish (Fig. 4A). While changes in zClock1 were significant for the time factor (p<0.0001, Proc Mixed), no age-related changes were found for this gene’s mRNA levels (Fig. 4C). No significant time, age or time by age effects were found for either zMel1a1 or zMel1c genes, encoding for two melatonin receptors (data not shown).
Figure 4. Daily pattern of circadian gene expression is changed in aged compared to young zebrafish maintained in LD.
Relative mRNA levels for zBmal1, zPer1 and zClock1 in the eyes of 1-year old (white) and 4-year old (black) zebrafish are presented as fold change relative to the mean mRNA level of expression in 1-year old zebrafish at the time of the daily trough (see Results), represented in each plot (A–C) as 1 on Y axis. Each data point represents the mean (SEM) of relative mRNA expression in 4–6 individual fish samples, each measured in triplicate. The results of the statistical analysis using Proc Mixed are listed in the text. X-axis: zeitgeber time (ZT 0 = lights on, ZT14-lights off), with light-dark cycle represented by horizontal bars above each plot.
4. Discussion
Our results show that in a diurnal vertebrate, zebrafish, aging is characterized by the disruption of circadian functions, including activity, sleep and melatonin production. This manifests under the regular light-dark cycle and especially in the absence of regular time cues. Aging in zebrafish is also associated with a reduction in the expression of mRNA for two core clock genes, zBmal1 and zPer1, and changes in the pattern of zBmal1 expression, with no significant differences in zClock1 expression. In spite of the deficient production of the principal circadian hormone melatonin in aged zebrafish, the expression of mRNA for melatonin receptors is not altered at old age. Consistent with this, administration of melatonin to aged zebrafish continues to promote daytime or nighttime sleep, augments their circadian rhythmicity and improves cognitive performance under constant environmental conditions. Thus, this well-characterized diurnal vertebrate model of development and genetics can be successfully applied to investigate an impact of aging on the circadian system and, conversely, a role of the circadian alterations in the process of aging.
Change in sleep and activity patterns is one of the hallmarks of human aging, however the underlying mechanisms are yet to be fully understood [21]. Previous research from our laboratory has shown that larval zebrafish display the behavioral features of sleep [36]. The present study demonstrates that this phenomenon is preserved in adult zebrafish. Aging, however, modifies zebrafish sleep, resulting in the reduction of its overall duration, especially at night, and an increase in sleep fragmentation. Interestingly, basal daytime arousal thresholds during wakefulness are increased in aged zebrafish, potentially reflecting a lower level of alertness due to reduced circadian amplitude and/or a sleep deficiency. This is further supported by the finding that overnight melatonin treatment in LD promotes sleep and results in a reduction in daytime arousal threshold in aged fish. The ability of melatonin to improve cognitive performance in aged zebrafish following overnight administration under constant conditions (dL) might be due to its acute sleep-promoting or circadian-related entraining effects. Differentiating between these two potential mechanisms of melatonin action will be a subject of further investigation.
Based on studies in mice and Drosophila, it has been suggested that the core circadian genes, especially Bmal and Per, might play an important role in the aging process [for review, 18]. This is supported by the premature aging phenotype in mutant mice lacking the core circadian genes, Bmal1 or Per1,2 [17]. Similarly, male Drosophila mutants for the CYCLE protein, a product of the gene homologous to Bmal1 in vertebrates, show a reduced life span [10].
We find that the expression of Bmal1 and Per1 is altered in aged zebrafish. The zBmal1 expression in their eyes undergoes the most pronounced changes, involving both reduced amplitude and a phase delay, relative to young fish. The observed phase delay in zBmal1 expression, however, does not appear to correlate with the entrained patterns of activity, sleep or melatonin production in aged zebrafish, since none of these parameters display a phase delay in aged fish studied in LD. The pattern of zClock1 expression does not significantly change with age and maintains the same circadian phase as in young zebrafish. zPer1, the expression of which is promoted by the combined action of the CLOCK/BMAL1 heterodimer, shows no sign of a phase shift but its late night and morning surge is significantly attenuated in aged zebrafish. Considering that not only central oscillators but also peripheral tissues express the clock genes and the latter may play an important role in aging [30], it remains to be seen whether age-related changes in the circadian gene expression in one of the principal clock structures in zebrafish, the eyes, correlate with those in other zebrafish tissues and organs.
Based on our findings, we submit that, in zebrafish, age-dependent changes in the circadian system and down-stream processes, such as sleep or cognitive performance, have important similarities with those in mammals. Understanding the circadian aging in a diurnal and genetically well-characterized vertebrate provides new opportunities to address the role of the circadian factors in human aging and to design adequate prophylactic or treatment strategies.
Acknowledgments
The authors are grateful to the members of our lab, Alex Kubyshkovsky, Christina Quasarano and Jason Best for excellent technical assistance; Patrick Mabray for critical reading of the manuscript. This work was supported by NIMH grant (MH 065528 to IZ).
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of references
- 1.Ancoli-Israel S. Sleep and aging: prevalence of disturbed sleep and treatment considerations in older adults. J Clin Psychiatry. 2005;66:24–30. [PubMed] [Google Scholar]
- 2.Asai M, Yoshinobu Y, Kaneko S, Mori A, Nikaido T, Moriya T, Akiyama M, Shibata S. Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J Neurosci Res. 2001;66:1133–1139. doi: 10.1002/jnr.10010. [DOI] [PubMed] [Google Scholar]
- 3.Benloucif S, Green K, L’Hermite-Baleriaux M, Weintraub S, Wolfe LF, Zee PC. Responsiveness of the aging circadian clock to light. Neurobiol Aging. 2006;27:1870–1879. doi: 10.1016/j.neurobiolaging.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahill GM. Clock mechanisms in zebrafish. Cell Tissue Res. 2002;309:27–34. doi: 10.1007/s00441-002-0570-7. [DOI] [PubMed] [Google Scholar]
- 5.Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-x. [DOI] [PubMed] [Google Scholar]
- 6.Carr AJ, Tamai TK, Young LC, Ferrer V, Dekens MP, Whitmore D. Light reaches the very heart of the zebrafish clock. Chronobiol Int. 2006;23:91–100. doi: 10.1080/07420520500464395. [DOI] [PubMed] [Google Scholar]
- 7.Driver C. The circadian clock in old Drosophila melanogaster. Biogerontology. 2000;1:157–162. doi: 10.1023/a:1010091829946. [DOI] [PubMed] [Google Scholar]
- 8.Gerhard GS. Comparative aspects of zebrafish (Danio rerio) as a model for aging research. Exp Gerontol. 2003;38:1333–1341. doi: 10.1016/j.exger.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Hastings MH, Herzog ED. Clock genes, oscillators, and cellular networks in the suprachiasmatic nuclei. J Biol Rhythms. 2004;19:400–413. doi: 10.1177/0748730404268786. [DOI] [PubMed] [Google Scholar]
- 10.Hendricks JC, Lu S, Kume K, Yin JC, Yang Z, Sehgal A. Gender dimorphism in the role of cycle (BMAL1) in rest, rest regulation, and longevity in Drosophila melanogaster. J Biol Rhythms. 2003;18:12–25. doi: 10.1177/0748730402239673. [DOI] [PubMed] [Google Scholar]
- 11.Hofman MA, Swaab DF. Living by the clock: the circadian pacemaker in older people. Ageing Res Rev. 2006;5:33–51. doi: 10.1016/j.arr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Johns CE, Newton JL, Westley BR, May FE. Human pancreatic polypeptide has a marked diurnal rhythm that is affected by ageing and is associated with the gastric TFF2 circadian rhythm. Peptides. 2006;27:1341–1348. doi: 10.1016/j.peptides.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Keller ET, Murtha JM. The use of mature zebrafish (Danio rerio) as a model for human aging and disease. Comp Biochem Physiol C Toxicol Pharmacol. 2004;138:335–341. doi: 10.1016/j.cca.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Kishi S. Functional aging and gradual senescence in zebrafish. Ann N Y Acad Sci. 2004;1019:521–526. doi: 10.1196/annals.1297.097. [DOI] [PubMed] [Google Scholar]
- 15.Kolker DE, Fukuyama H, Huang DS, Takahashi JS, Horton TH, Turek FW. Aging alters circadian and light-induced expression of clock genes in golden hamsters. J Biol Rhythms. 2003;18:159–169. doi: 10.1177/0748730403251802. [DOI] [PubMed] [Google Scholar]
- 16.Kolker DE, Vitaterna MH, Fruechte EM, Takahashi JS, Turek FW. Effects of age on circadian rhythms are similar in wild-type and heterozygous Clock mutant mice. Neurobiol Aging. 2004;25:517–523. doi: 10.1016/j.neurobiolaging.2003.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondratov RV. A role of the circadian system and circadian proteins in aging. Ageing Res Rev. 2007;6:12–27. doi: 10.1016/j.arr.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Martin C, Dutertre-Catella H, Radionoff M, Debray M, Benstaali C, Rat P, Thevenin M, Touitou Y, Warnet JM. Effect of age and photoperiodic conditions on metabolism and oxidative stress related markers at different circadian stages in rat liver and kidney. Life Sci. 2003;73:327–335. doi: 10.1016/s0024-3205(03)00271-6. [DOI] [PubMed] [Google Scholar]
- 20.Mintz EM, Phillips NH, Berger RJ. Daytime melatonin infusions induce sleep in pigeons without altering subsequent amounts of nocturnal sleep. Neurosci Lett. 1998;258:61–64. doi: 10.1016/s0304-3940(98)00849-0. [DOI] [PubMed] [Google Scholar]
- 21.Monk TH. Aging human circadian rhythms: conventional wisdom may not always be right. J Biol Rhythms. 2005;20:366–374. doi: 10.1177/0748730405277378. [DOI] [PubMed] [Google Scholar]
- 22.Munoz E, Baler R. The circadian E-box: when perfect is not good enough. Chronobiol Int. 2003;20:371–388. doi: 10.1081/cbi-120022525. [DOI] [PubMed] [Google Scholar]
- 23.Murakami N, Kawano T, Nakahara K, Nasu T, Shiota K. Effect of melatonin on circadian rhythm, locomotor activity and body temperature in the intact house sparrow, Japanese quail and owl. Brain Res. 2001;889:220–224. doi: 10.1016/s0006-8993(00)03205-4. [DOI] [PubMed] [Google Scholar]
- 24.Oster H, Baeriswyl S, Van Der Horst GT, Albrecht U. Loss of circadian rhythmicity in aging mPer1−/−mCry2−/− mutant mice. Genes Dev. 2003;17:1366–1379. doi: 10.1101/gad.256103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pando MP, Sassone-Corsi P. Unraveling the mechanisms of the vertebrate circadian clock: zebrafish may light the way. Bioessays. 2002;24:419–426. doi: 10.1002/bies.10091. [DOI] [PubMed] [Google Scholar]
- 26.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 27.Tsai SB, Tucci V, Uchiyama J, Fabian NJ, Lin MC, Zhdanova IV, Kishi S. Differential effects of genotoxic stress on both concurrent body growth and gradual senescence in the adult zebrafish. Aging Cell. 2007;6:209–224. doi: 10.1111/j.1474-9726.2007.00278.x. [DOI] [PubMed] [Google Scholar]
- 28.Weinert D, Waterhouse J. The circadian rhythm of core temperature: effects of physical activity and aging. Physiol Behav. 2007;90:246–256. doi: 10.1016/j.physbeh.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Weinert H, Weinert D, Schurov I, Maywood ES, Hastings MH. Impaired expression of the mPer2 circadian clock gene in the suprachiasmatic nuclei of aging mice. Chronobiol Int. 2001;18:559–565. doi: 10.1081/cbi-100103976. [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci U S A. 2002;99:10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu L, Tucci V, Kishi S, Zhdanova IV. Cognitive Aging in Zebrafish. PLoS ONE. 2006;1 doi: 10.1371/journal.pone.0000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhdanova IV, Cantor ML, Leclair OU, Kartashov AI, Wurtman RJ. Behavioral effects of melatonin treatment in non-human primates. Sleep Res Online. 1998;1:114–118. [PubMed] [Google Scholar]
- 33.Zhdanova IV. Melatonin as a hypnotic: Pro. Sleep Med Rev. 2005;9:51–65. doi: 10.1016/j.smrv.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Zhdanova IV. Sleep in Zebrafish. Zebrafish. 2006;3:215–226. doi: 10.1089/zeb.2006.3.215. [DOI] [PubMed] [Google Scholar]
- 35.Zhdanova IV, Geiger DA, Schwagerl AL, Leclair OU, Killiany R, Taylor JA, Rosene DL, Moss MB, Madras BK. Melatonin promotes sleep in three species of diurnal nonhuman primates. Physiol Behav. 2002;75:523–529. doi: 10.1016/s0031-9384(02)00654-6. [DOI] [PubMed] [Google Scholar]
- 36.Zhdanova IV, Wang S, Leclair OU, Danilova N. Melatonin promotes sleep-like behavior in zebrafish. Brain Res. 2001;903:263–268. doi: 10.1016/s0006-8993(01)02444-1. [DOI] [PubMed] [Google Scholar]