Abstract
Background
The incidence of carcinoma of the distal esophagus and GE junction is rapidly increasing. A large single-center experience was reviewed to determine the impact of lymph node positivity and ratio on survival.
Methods
All patients undergoing esophagogastrectomy at Thomas Jefferson University Hospital between January 1994 and December 2004 were reviewed. Univariate and multivariate analyses were performed using log-rank and Cox proportional hazard models, and survival curves estimated using the Kaplan-Meier method.
Results
Of 173 patients with invasive cancer, 123 (71%) underwent preoperative chemoradiation therapy (CRT). The largest number of patients (45%) had adenocarcinoma of the GE junction; 29% of patients had esophageal adenocarcinoma while 14% had squamous cell cancer of the esophagus. Perioperative mortality was 5.7%. Median overall survival (OS) of the entire group was 22 months and 5-year OS was 27%. The most significant prognostic factor for overall survival was the presence of positive LN (p=0.01). Additionally, patients with zero involved LN had a 5-year survival of 34%, while patients with 1–3 positive LN and >3 positive LN had 5-year survival of 27% and 9%, respectively (p=0.01). Finally, an increasing ratio of positive to examined LN was linearly associated with a worsening 5-year survival, (p=0.153).
Conclusions
Increasing number of positive LN in patients with esophageal cancer and increasing ratio of metastatic to examined LN portend a poor prognosis. These factors should play an important role in determining which patients receive adjuvant therapy.
Keywords: esophageal cancer, lymph nodes, lymph node ratio, prognosis
INTRODUCTION
While uncommon in the United States, cancer of the esophagus is one of the most deadly malignancies in humans and is increasing in frequency. In 2005, it was estimated that 14,520 new cases were diagnosed with 13,570 deaths from the disease. The incidence of esophageal cancer is higher among Caucasians than among all other ethnic groups and higher among men than women. The overall five-year survival, as determined between 1995 and 2000, is 14%.1
Surgical resection is the preferred approach for definitive treatment of this type of cancer, but increasingly physicians are utilizing preoperative regimens combining chemotherapy and radiation to downstage primary tumors both to increase the likelihood of achieving complete resection and to prolong survival.2 Randomized trials examining this treatment have yielded mixed results; only one phase III trial has shown increased survival using neoadjuvant therapy, while several others failed to show an increased survival when compared to surgical resection alone.3
Current research trends include examining treatment outcomes and prognostic factors for survival. Hofstetter et al have reported that patient survival has increased over time, likely due to improvements in pre-operative staging and advances in surgical technique; the use of pre-op chemoradiation was found to be the most significant factor in achieving a complete resection of the tumor.4 Berger et al examined response to neoadjuvant therapy in relation to survival, and found that a complete response to the therapy was associated with a 5-year survival of 48% compared to 15% for patients whose tumor was not responsive to therapy; even down-staging the primary tumor to Stage I increases overall survival to 34%.2
The presence of lymph node metastasis (LNM) has been demonstrated to be one of the most significant prognostic factors. Earlier studies have reported significant differences amongst patients with different numbers of LNM, all following a general trend of increasing positive nodes leading towards a worse prognosis.5–7 Lymph node ratio has also been investigated as a prognostic indicator but its significance is less clear. Previous authors have shown that increasing LNR was associated with increased mortality, however there is disagreement of what ratios are significant.6, 7 Eloubeidi reported that an overall increasing ratio was inversely related to outcome.8
The goal of this study was to examine a large database of patients undergoing esophagectomy to determine the impact of increasing numbers of pathologically confirmed positive LN and LN ratio on survival in patients undergoing esophagectomy for cancer of the esophagus, GE junction and gastric cardia.
MATERIALS AND METHODS
All patients undergoing esophagogastrectomy for esophageal cancer or proximal gastric cancer at Thomas Jefferson University Hospital (TJUH) between January 1994 and December 2005 were retrospectively reviewed. The total number of patents identified was 173; patients with high grade dysplasia were eliminated from the survival analysis. Although 173 patients with invasive cancer met the criteria to be included in the study sample, the multivariate analyses include a subset of 144 patients with complete records with respect to all known risk factors and complete lymph node information.
Patient medical records were examined and the information entered into a database with the approval of the TJUH Institutional Review Board. Data collected included: Preoperative factors (age, co-morbidities, extent of symptoms, presence of Barrett’s esophagus, and imaging studies), treatment factors (chemotherapy drugs, radiation dosage, weight loss, and need for pre-operative nutritional support), tumor factors (histology, clinical and pathologic staging, and completeness of resection – R0, R1, or R2), and operative and hospital factors (type of operation, blood loss, type of conduit, type of anastomosis, and complications), tumor recurrence (date and location), and long term survival.
All patients were staged both pre- and postoperatively according to the classification scheme of the American Joint Committee for Cancer Staging 6th edition. Patients were clinically staged utilizing CT scan and endoscopic ultrasound (EUS), when available. Patients without residual, viable, tumor cells present in the surgical specimens (T0N0M0, stage 0-Rx) were regarded as having pathologic complete response (pCR). Patients with minimal residual tumor (T1N0M0) were classified as down-staged (stage 1-Rx). Follow-up data was obtained from patient charts, tumor registry, and referring physicians. Numerous patients were treated at outside institutions and only underwent surgery at TJUH. For these patients, initial clinical stage information was often missing.
Most patients receiving induction chemoradiation received a combination of 5-FU and a platinum based agent, such as cisplatin or carboplatin. The standard dose of radiation, given concurrently with chemotherapy, was 45Gy. Thirty-six patients were treated under a phase I/II protocol at TJU combining 5FU, carboplatin, paclitaxel, and 45Gy of radiation.9 The patients were given a 4–6 week rest period before definitive resection. During this period the patients were often restaged using CT and EUS.
The overall survival (OS) was evaluated using the Kaplan-Meier estimator and the log-rank test for univariate analyses and Cox proportional hazards model for multivariate analyses. The Cox models controlled for all known risk factors including age, pathological stage, length of stay, induction chemotherapy, type of surgery, and tumor location (stratification variable), the total number of lymph nodes and N stage. Separate Cox models were fitted with adding either an indicator of more than 3 positive lymph nodes or indicators of >25% and >50% positive lymph nodes. The primary analysis of lymph node positivity and lymph node ratio was based on pathologic staging information and not preoperative staging information. Proportional hazards assumptions were based on the corresponding tests10. Statistical analyses were performed in SAS (SAS Institute, Cary, NC) and S-Plus (Insightful, Seattle, WA).
RESULTS
There were 93 men and 30 women, ranging in age from 37 to 86 (mean 62 years). Eighty-nine percent of patients were Caucasian (n=110). Seventy-two percent of patients had a smoking history (n=88). The majority of patients (n=123, 71%) underwent induction chemoradiation. Of these patients, 26 (21.1%) had a pathologic complete response and 42 (34.1%) were down-staged.
The operative technique was dictated by the location of the tumor as well as surgeon preference. Table 1 illustrates that the majority of patients (46%) underwent a transhiatal esophagectomy. However, approximately 54% underwent some type of transthoracic approach. There did appear to be a difference in survival based on the type of resection employed. Patients who underwent 3-hole esophagectomy had significantly better survival than those undergoing transhiatal (p=0.044) and also better survival than those undergoing Ivor-Lewis esophagectomy (p=0.052). This however, did not appear to be a function of lymph node harvest. The average number of lymph nodes harvested in the 3-hole group was 9.6 which was higher than those undergoing transhiatal approaches (mean=6.4) but lower than those undergoing Ivor-Lewis esophagectomies (mean=12.0). There was no difference in the number of lymph nodes examined depending on whether patients did or did not receive preoperative CRT (median equals 6 versus 7 LN, p=0.99).
Table 1.
Operative Techniques
| Procedure | No. of Patients (n=173) | Overall Percentage, % |
|---|---|---|
| Ivor-Lewis | 53 | 30.6 |
| Transhiatal | 80 | 46.2 |
| 3-Hole | 34 | 19.7 |
| Thoracoabdominal | 1 | 0.6 |
| Thoracoscopic 3-Hole | 5 | 2.9 |
Most patients presented with advanced disease, stage II-IV, (n=117, 67.6%), half of whom had Stage III disease (n=59) (Table 2). Most patients were clinically staged using CT and 56 (28.9%) patients also underwent staging with EUS. Of the patients treated with induction CRT, 98 had Stage II or III disease (77.2%). A large portion (n=25, 37.3%) of the patients not treated with neoadjuvant therapy had Stage 0 or I disease.
Table 2.
Initial Clinical Diagnosis
| Stage, TNM | No. of Patients (n=173) | Percentage, % |
|---|---|---|
| 0, Cis | 8 | 4.6 |
| I, T1N0M0 | 17 | 9.8 |
| IIa, T2–3, N0M0 | 42 | 24.3 |
| IIb, T1–2, N1M0 | 5 | 2.9 |
| III, T3N1M0 | 59 | 34.1 |
| IV, M1a | 11 | 6.4 |
| Unknown | 31 | 17.9 |
The vast majority of patients had adenocarcinomas (80.3%) which occurred in the distal esophagus (n=45, 32.4%), GE junction (n=62, 44.6%) and gastric cardia (n=26, 15%) (Table 3). Tumor location was more varied than histologic type. The majority of the tumors arose in the distal portions of the esophagus and into the proximal stomach. Specifically, 126 tumors (73%) were found in either the distal third or at the GE junction (Table 4).
Table 3.
Histology
| Histologic Diagnosis | No. of Patients (n=173) | Overall Percentage, % |
|---|---|---|
| Adenocarcinoma | 139 | 80.3 |
| Squamous Cell Carcinoma | 31 | 17.9 |
| High Grade Dysplasia | 1 | 0.5 |
| Other/Unknown | 2 | 1.2 |
Table 4.
Tumor Location
| Location | No. of Patients (n=173) | Overall Percentage, % |
|---|---|---|
| Proxmial | 3 | 1.7 |
| Middle | 17 | 9.8 |
| Distal | 58 | 33.5 |
| GE-Junction | 68 | 39.3 |
| Cardia | 27 | 15.6 |
Follow-up ranged from 1 to 128 months (median=18 months). Overall, the median survival for the group was 22 months and the 5-year overall survival rate was 27.1%. There was not a significant difference in survival for patients receiving induction chemoradiation versus surgery alone (p=.244) however, tumor response to chemoradiation was an important prognostic factor. The overall survival rate for patients with a pathologic complete response (pCR) was 38.8% with a median survival of 27.6 months compared to 27.2% and 18.4 months for non-pCR patients (p=0.118). Pathologic tumor stage was a significant predictor of survival in the univariate analysis (p=0.037). However, this factor was not significant in the multivariate model because of the dramatic significance of lymph node positivity (a major component of pathologic stage grouping).
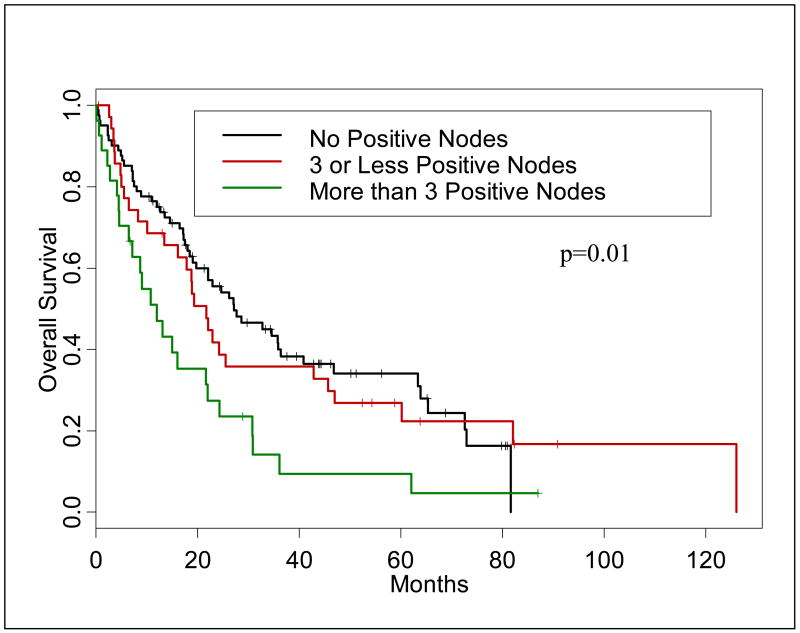
Nodal metastasis were found in 38.2 % of the patients (n=66) and played a role in overall survival; patients without nodal involvement had a median survival 27 months versus 16.1 months for node positive patients (p=0.010). In the initial analysis of lymph node positivity, a dichotomized cutoff of < or ≥ 3 was determined to be a statistically significant predictor of OS by univariate and multivariate analysis (p=0.021, HR=2.29). We then analyzed the lymph nodes in a manner similar to that used by the AJCC for colon cancer—that is LNM=0, LNM=1–3, and LNM=4 or more. In this manner, patients with zero involved LN had a 5-year survival of 34.1%, while patients with 1–3 positive LN and >3 positive LN had 5-year survival of 27% and 9%, respectively (p=0.010 for this group). Figure 1 presents the Kaplan-Meier survival curves for the groups of patients with zero, 1–3 and more than 3 positive lymph nodes. The difference in survival among these three groups was significant (p=0.010).
Figure 1.
Kaplan-Meier curve demonstrating overall survival for patients by lymph node category.
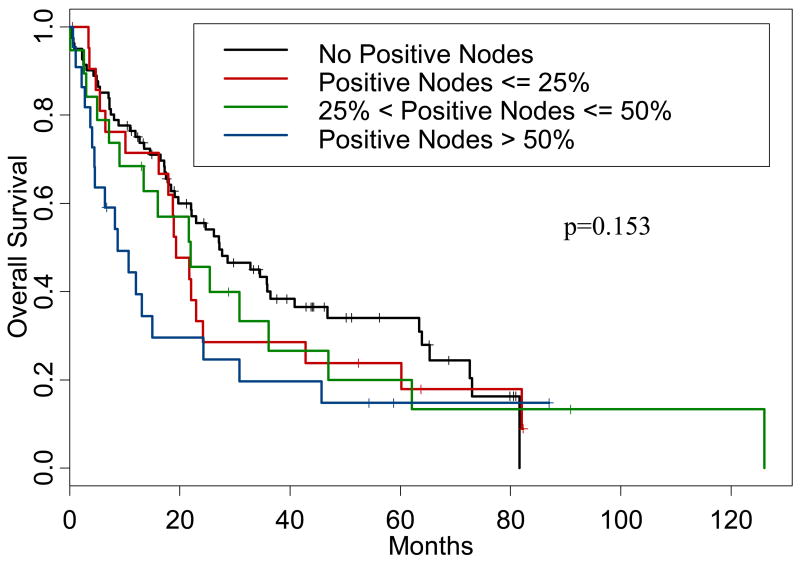
Finally, we examined the impact of an increasing LNR. The proportion of positive lymph nodes was analyzed by the following categories: zero, ≤25%, >25% but ≤50%, and >50%. The difference in survival among these four groups was not significant (p=0.153), but there were some important differences between the groups as outlined in Table 5. Corresponding Kaplan-Meier survival curves are shown in Figure 2.
Table 5.
Survival Data
| Median Survival Time (months) | ||||||
|---|---|---|---|---|---|---|
| n | Kaplan-Meier estimate | 95% confidence limits | p-value | 5-year Survival | ||
| Lower | Upper | |||||
| LN + Category | ||||||
| 0 LN + | 81 | 27 | 20 | 36 | 0.01 | 34.1% |
| 1–3 LN+ | 36 | 22 | 13 | 43 | 26.8% | |
| >3 LN + | 27 | 12 | 6 | 22 | 9.4% | |
| % LN + Category | ||||||
| 0 LN + | 81 | 27 | 20 | 36 | 0.153 | 34.1% |
| 0–25% | 22 | 19 | 16 | 24 | 24% | |
| 25–50% | 19 | 22 | 9 | 36 | 20% | |
| >50% | 22 | 9 | 4 | 15 | 15% | |
Figure 2.
Kaplan-Meier curve demonstrating overall survival for patients by lymph node ratio category.
DISCUSSION
Surgical resection remains the treatment of choice for esophageal cancer. Despite improvements in operative technique and advances in tumor staging, overall survival is still poor.12 Current attempts are being made to improve survival by using combinations of treatments in a multi-modality approach. Chemotherapy, radiation, and surgical resection are often used in conjunction in order to diminish recurrence. Most often neoadjuvant therapy is prescribed, when chemoradiation is given prior to surgery it tends to be better tolerated than when given post-operatively. In addition, administration prior to surgery allows chemotherapeutics and radiation access to the tumor with its blood supply intact. Despite the promise shown in many phase II trials, for the most part neoadjuvant therapy has not held up under phase III trial conditions.
The main goal of this study was to examine to utility of the number of lymph node metastasis and the ratio of positive lymph nodes to excised lymph nodes as prognostic factors in survival of patients with tumors of the esophagus, GE junction and gastric cardia.
Consistent with previously reported data, the number of LN metastasis proved to be an important indicator of survival. According to our findings, patients with more than 3 LNM fared worse than patients with 3 or fewer LNM. Other authors examining LNM have found that less than 5 LNM compared to 5 or more LNM to be a significant cutoff.7, 8 However, those studies included mostly squamous cell carcinoma patients, whereas our study concerned predominantly (81.4%) adenocarcinoma patients, the predominant type in the western hemisphere.13 This discrepancy may reflect a difference in the behavior of the histologic types. Nigro et al reported less than 4 vs. 4 or more LNM as significant in a study including only adenocarcinoma patients, but that data was collected on a much smaller patient population (n=44) and dealt only with transmural tumors.6
Lymph node ratio examination did not reach significance in our study. However, Table 5 demonstrates that increasing LNR may associated with worsening survival. This observation has been previously reported for other malignancies such as colon cancer, but not in esophageal cancer.14 We were able to elucidate differences in survival of patients based upon the categorization of their LNR as zero, less than 0.25, 0.26–0.50, and greater than 0.51, an observation not previously reported. Elboubeidi reported a similar finding; that increasing LNR was associated with a poorer prognosis.8 Other authors have had differing cutoff points for LNR. Bollschweiler et al reported that LNR only became significant if it exceeded 0.20 (p<0.01) and Nigro et al showed patients with a LNR <0.1 fared significantly better than those who had a LNR ≥0.1.6, 7 These two studies, when compared to the current study, removed a large number of nodes (avg 26.4 and 51 respectively vs. 8.6) which may have artificially lowered the observed ratio. We feel that using these cutoff points will be easier to remember and utilize since they are based on 25%, 50%, and 75% points. Although this factor did not reach statistical significance in our study, this is most likely due to the small number of patients per group with <20 patients per positive lymph node grouping—larger studies will need to be done to confirm this.
One concern is that it may be difficult to assess the lymph node ratio after chemoradiation because the number of lymph nodes examined is often decreased. However, when we evaluated the number of lymph nodes examined in the patients who received neoadjuvant chemoradiation, there was no difference between these patients and those who did not receive neoadjuvant therapy (n=6 vs. 7, p=0.99). Further investigation should examine the locations of the nodes prior to removal and compare that data to the LNR to see if a positive node in at one anatomic site differs from a positive node at another site in terms of survival relative to the tumor location. Unfortunately, we were unable to examine the exact locations of lymph node metastases in this study due to its retrospective nature. We chose to include gastric cardia tumors with GE junction and esophageal tumors because they were all treated with esophagogastrectomy with similar lymph node stations being removed.
Our experience shows that the number of positive lymph nodes and the ratio of positive lymph nodes to examined lymph nodes are significant prognostic factors for esophageal carcinoma. When the AJCC next revises the TNM staging scheme these two factors should be incorporated, thereby giving a more complete picture of a particular patient’s disease state in order to guide clinical decision making.
Acknowledgments
Matthew Wilson’s research was supported by the following NIH grant: Short-Term Training Program in Translational Cancer Research—CA069277
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jamel A, Murray T, Ward E. Cancer statistics 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated wth significantly improved survival. J Clin Oncol. 2005;23:4330–4337. doi: 10.1200/JCO.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–467. doi: 10.1056/NEJM199608153350702. [see comment] [erratum appears in N engl J med 1999 jul 29;341(5):384] [DOI] [PubMed] [Google Scholar]
- 4.Hofstetter W, Swisher SG, Correa AM, et al. Treatment outcomes of resected esophageal cancer. Ann Surg. 2002;236:376–384. doi: 10.1097/00000658-200209000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korst RJ, Rusch VW, Venkatraman E, et al. Proposed review of the staging classification for esophageal cancer. J Thorac Cardiovasc Surg. 1998;115:660–670. doi: 10.1016/S0022-5223(98)70332-0. [DOI] [PubMed] [Google Scholar]
- 6.Nigro JJ, DeMeester SR, Hagen JA, et al. Node status in transmural esophageal adenocarcinoma and outcome after en bloc esophagectomy. J Thorac Cardiovasc Surg. 1999;117:960–968. doi: 10.1016/S0022-5223(99)70377-6. [DOI] [PubMed] [Google Scholar]
- 7.Bollschweiler E, Baldus SE, Schroeder W, Schneider PM, Hoelscher AH. Staging of esophageal carcinoma: Length of tumor and number of involved regional lymph nodes. are these independent prognostic factors? J Surg Oncol. 2006;94:355–363. doi: 10.1002/jso.20569. [DOI] [PubMed] [Google Scholar]
- 8.Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Milcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the US; the importance of tumor length and lymph node status. Cancer. 2002;95:1434–43. doi: 10.1002/cncr.10868. [DOI] [PubMed] [Google Scholar]
- 9.Anne P, Axelrod R, Rosato F, et al. A phase I trial of preoperative paclitaxel, carboplatin, 5-FU, and radiation in patients with resectable esophageal or gastric cancer. J Clin Oncol. 2004;22:4031. [Google Scholar]
- 11.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 12.Refaely Y, Krasna MJ. Multimodality therapy for esophageal cancer. Surg Clin North Am. 2002;82:729–746. doi: 10.1016/s0039-6109(02)00029-4. [DOI] [PubMed] [Google Scholar]
- 13.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [see comment] [DOI] [PubMed] [Google Scholar]
- 14.Berger AC, Sigurdson ER, LeVoyer T, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. Journal of Clinical Oncology. 2005;23:8706–8712. doi: 10.1200/JCO.2005.02.8852. [DOI] [PubMed] [Google Scholar]