Surgery is defined as the branch of medicine concerned with the treatment of disease, injury, and deformity by operation or manipulation. (Stedman’s Medical Dictionary, 28th edition).
Surgery, like medicine in general, is a dynamic entity that changes and evolves with time. Surgeons themselves are a product of the society in which they live, and thus, the operations they perform are also a reflection of the times. What is interesting about the history of surgical practice is how it has managed to mold and change while still maintaining its core principles.
Classically, surgical procedures can be divided into four main categories: incisional, exemplified by the drainage of an abscess or the release of a carpel tunnel compression; excisional, utilized in most major cancer operations, from colectomies to lobectomies and gastrectomies; reparational, seen in the repair of an inguinal hernia or the re-implantation of a digit; and finally replacement, a technique used in transplantation and joint surgery. Despite our vast advances, these categories hold true to this day.
Another area of surgery that remains stable is that every operation has both risks and benefits. The benefits, which have not changed significantly with time, arise from the durable or permanent treatment of a condition and or the avoidance of the need to take chronic medication that may have potential side effects. The results of surgery are usually immediately obvious to both the patient and the treating surgeon. The risks on the other hand, are where the creative surgeon has the most power to affect change.
Until recently, a major risk or downside to surgery was related to the incision. The incision needs to be sufficiently large for the surgeon to palpate the tissues with his hands or instruments as well as to obtain adequate illumination of the internal structures. The deeper the target structure, the more shadowing there is, and generally the larger the incision has to be for adequate exposure. The cost of a large incision may range from immediate pain to a prolonged period of recovery before the tissues may be fully functional. Surgery thus may result in deformity or cosmetic scarring and there is often a substantial period before one is able to return to usual activities. Finally, another consideration regarding surgery is that surgery requires considerable technical skill, and there is a potential for large variation between outcomes of the identical procedure performed by different treating surgeons. Thus surgical outcomes may be less predictable than those related to pharmaceutical treatments.
Fortunately, surgery has evolved at an incredible rate, particularly over the past 30 or 40 years. Advances in imaging, electronics, and optics combined with an innovative spirit have led to vast changes. With the introduction of open-heart surgery, total parenteral nutrition, transplantation, biomaterials, minimally invasive and robotic surgery, surgery as a discipline has evolved in leaps and bounds. James C. Thompson, M.D., one of the giants of surgery over the past 25 years once said, “Without research, the surgery of today would be the surgery of yesterday, and the surgery of tomorrow would be the surgery of today” (personal communication). This spirit sums up the determination of academic surgeons to provide the benefits of durable and effective surgical care without the costs associated with the incision. As Yogi Berra has often been quoted as saying, “The future ain’t what it used to be”.
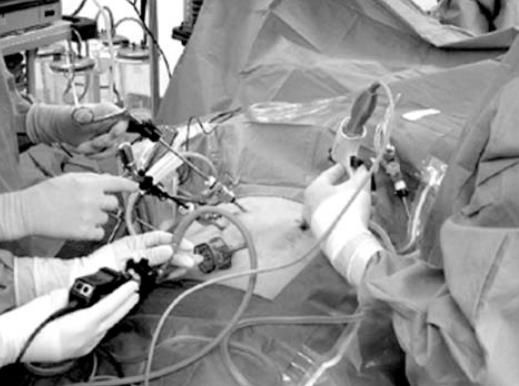
One of the most dramatic advances in surgical care is the development of minimally invasive surgery (MIS), also known as laparoscopy, arthroscopy, thoracoscopy, or minimal access surgery. MIS involves image-guided surgery. This utilizes small diameter telescopes connected to a miniature video camera to provide magnified, bright and shadow free visualization of internal structures. Instruments placed through small puncture-size incisions access the target organ without the need for a large incision (Figure 1). In order to create a working space, carbon dioxide is pumped into the cavity of choice using an electronic insufflator to a specified pressure, usually between 12 and 15 mmHg. In most relaxed and compliant abdominal cavities this allows an space of between 3 and 6 liters to be created, and allows one to visualize the abdomen quite clearly. The benefit of MIS is less pain, nearly invisible scars, and a markedly accelerated recovery. This is a dramatic improvement on the previous risks associated with most surgical operations. Many operations that required 7 to 14 days of hospitalization and several weeks of post hospital recovery in the past are now done as outpatient procedures, i.e. the patient is discharged within a few hours of surgery and is able to resume normal activities often within a week. MIS techniques have been applied in a large number of procedures in general surgical care.
Figure 1.
Minimally Invasive Surgery: The surgeon is manipulating instruments placed through ports (trocars) in the abdominal wall. The internal organs and instrument tips cannot be seen directly, but can be viewed on a monitor that displays the video image created by an endoscopic camera.
The most common is its use in gallbladder surgery. Today ~ 90% or more of elective cholecystectomies are carried out using laparoscopic techniques (1,2). MIS has also been applied to hernia surgery, colorectal surgery, repair of hiatus hernias, anti reflux surgery as well as radical prostatectomies, donor nephrectomies and a large number of other procedures (3).
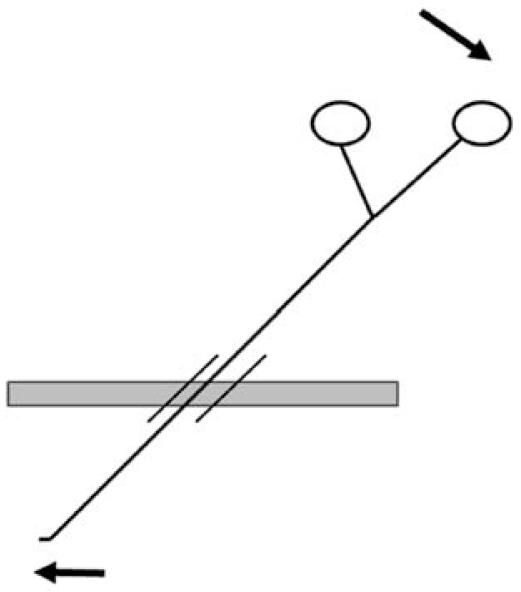
However, MIS poses some very real challenges to the surgeon. First, internal structures are usually visualized through a two dimensional optical system which diminishes depth perception. The surgeon must acquire new cues to know where he or she is in three-dimensional space. As well, the tissues being operated on are accessed by long instruments interposed between the surgeon’s hands and the target tissue, thus resulting in decreased tactile sensation and amplification of any tremor. Furthermore, trocars must be placed through the body wall to allow passage of instruments toward the target tissue. Because the instruments must be passed through these trocars, there is a limited range of motion of the surgeon’s instruments and there is a lever like relationship whereby the surgeon’s hands move in a direction 180 degrees opposite to the motion of the tip of the instrument (Figure 2). A fourth difference arises in the way a tissue appears during MIS. The endoscope provides a markedly sharper and magnified view of the target compared to what could be seen with the naked eye. When visualizing internal structures through an endoscope, a certain focal distance must be present to separate the tip of the scope from the target organs. Without this minimal focal distance red out occurs, and the lens may become coated with blood or mucous impairing the view. As evidenced by all these differences, MIS requires very unique skills that are not necessarily transferable from those skills acquired during traditional or open surgery.
Figure 2.
The Fulcrum Effect: The MIS instrument is inserted into the abdomen through a trocar. The trocar allows instruments to be inserted and withdrawn through a valve so that the positive intra-abdominal pressure can be maintained. To make the instrument tip go in one direction the handle of the instrument must be directed in the mirror-image direction.
A second congruent and equally important change in surgical practice is that simulators have been developed to teach surgeons in training, and surgeons wishing to learn minimal access techniques, how to use their instruments and a monocular optical system effectively. The McGill Inanimate System for Training and Evaluation of Laparoscopic Skills (MISTELS) is one of the most widely used such simulators internationally (4–7). Students are taken through a series of exercises in a trainer box under monocular optical guidance and their performance is measured according to efficiency and precision. Standards have been developed such that proficiency in the MISTELS system is highly predictive of a surgeon’s skill in the operating room.
This concept of proficiency-based training is an important new paradigm in surgical education. It is based on the premise that surgeons learn better once they develop the fundamental skills outside of the stressful operating room. They can then apply their knowledge and skill to the surgical care of their patients in a much more productive and less stressful situation once they have the opportunity to assist in the operating room.
While advancement is important, it needs to be done in a controlled manner and the ultimate goal of surgical innovation should be to apply new technology to benefit the patient, to introduce innovation safely, and to ensure the competence of those adopting innovative techniques in their surgical practice. The use of simulation-based training is a better process than that of the traditional model of graded responsibility, and simulation-based training can be thought of as complimentary to on the job learning. A battery of physical and virtual reality simulators are available to McGill medical students and residents to acquire that skill set necessary to perform this complex new type of surgery.
Another recent achievement is the concomitant use of other imaging modalities to further enhance the surgeon’s capabilities, without requiring larger incisions or increasing the risks. At the surgeon’s disposal are technologies such as ultrasound, fluoroscopy, and views via flexible endoscopes passed through natural orifices such as the mouth or rectum. By combining traditional laparoscopic visualization with these other modalities surgeons have an opportunity to see more than the surface of their target. In exchange for the loss of direct touch and the sensation of texture, a tissue’s density and its characteristics can be evaluated by three-dimensional imaging such as ultrasound in conjunction with the surface view provided by the scopes.
Along with advancement in surgical technique, evolution in the surrounding conditions have helped to push this revolution even further. We have learned a great deal about creating an enabling environment for MIS. A surgeon requires multiple images of the surgical field. The use of multiple flat panel monitors and plasma displays allows a surgeon to have access to laparoscopic images, flexible endoscopic images as well as radiologic images. In addition, preoperative imaging studies can be viewed as required, and the vital signs of the patient can be seen without the surgeon’s eyes ever leaving the surgical field. Further, operating rooms designed with ergonomics in mind have resulted in enhanced technical capabilities, less fatigue, and safer procedures. We have recently designed integrated platforms for performing MIS procedures that are optimal both from the perspective of the surgeon as well as the rest of the medical team. The operating room can now be a source of rich educational material. Whatever is displayed on the monitors can be recorded on digital media, which can then be used for teaching purposes, to document surgical findings, or provide feedback to the radiologist who provided preoperative diagnoses. A complete video-editing suite is available at McGill to provide enduring educational materials based on images acquired in the operating rooms. Additionally, a videoconferencing link between the MIS suites and the outside world provides an opportunity for two-way communication, intra operative consultation, and a wonderful opportunity for teaching.
In conclusion, MIS is an innovative application of surgical therapeutics that was developed to minimize patients’ pain and suffering while improving surgical precision. Performing minimally invasive surgical procedures requires a new set of skills in addition to those required for traditional open surgery. The simulation center provides an environment to hone these skills and to verify the technical proficiency of surgeons before they apply MIS techniques to the care of their patients. Due to progress in engineering capabilities and optics, and as a result of surgical creativity, MIS principles are being applied to an increasing number of health care problems. The technology required to optimally incorporate MIS into clinical care requires a creative surgical environment that addresses issues of ergonomics and patient safety while providing the entire health care team access to a variety of digital data.
Looking to the future, an extension of MIS is robotic assisted surgery. This takes MIS a step further and the surgeon is physically detached from the patient. The surgeon sits at a console, which may be in the same room as the patient, or quite a distance away. By using his hands and feet the surgeon controls instruments that are being manipulated by a robot. Robotic surgery provides the capability of scaling, whereby the movement of the surgeon can be set to a particular ratio with respect to movement of the instruments inside the patient. Scaling allows a surgeon to produce very small and precise movements such as those required for microsurgery. The robot can also filter out human tremor, providing the potential to further improve the accuracy of very fine movements. Robotic technology is addressing the universal nursing shortage as well. A robotic scrub nurse, called Penelope (website: http://www.roboticsystech.com/), has been developed at Columbia University in New York and responds to voice commands from the surgeon with remarkable accuracy.
Surgery has dramatically evolved in the recent past as a result of breakthroughs in engineering, optics, and the information age combined with human creativity and ingenuity. The benefits of durable and effective care with minimal pain and loss of productive time is the goal of every surgeon, and thus we continue to push surgical care further and further forward.
REFERENCES:
- 1.Fried GM, Ferri LE. Laparoscopic cholecystectomy. In: Soper NJ, Swanstrom LEL, Eubanks WS, editors. Mastery of Endoscopic and Laparoscopic Surgery. 2. Philadelphia: Lippincott, Williams & Wilkins; 2005. [Google Scholar]
- 2.Fried GM, Klassen DR, Feldman LS. Cholecystectomy and Common Bile Duct Exploration. In: Souba WW, Fink MP, Jurkovich GJ, et al., editors. ACS Surgery Online. New York: WebMD Inc; 2005. Website: http://www.acssurgery.com/ [Google Scholar]
- 3.Bergman S, Feldman LS, et al. “First do no harm.”-Monitoring outcomes during the transition from open to laparoscopic live-donor nephrectomy. Canadian Journal of Surgery. 2005;48:S19–20. [PMC free article] [PubMed] [Google Scholar]
- 4.Fried GM. Lessons from the Surgical Experience with Simulators: Incorporation into Training and Utilization in Determining Competency. Gastrointestinal Endoscopy Clinics of North America. 2006;16:425–434. doi: 10.1016/j.giec.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Swanstrom LL, Fried GM, Hoffman KI, et al. Beta test results of a new system assessing competence in laparoscopic surgery. Journal of the American College of Surgeons. 2006;202:62–9. doi: 10.1016/j.jamcollsurg.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Fraser SA, Feldman LS, Stanbridge D, Fried GM. Characterizing the learning curve for a basic laparoscopic drill. Surgical Endoscopy. 2005;19(12):1572–8. doi: 10.1007/s00464-005-0150-5. [DOI] [PubMed] [Google Scholar]
- 7.Fried GM, Feldman LS, Vassiliou MC, et al. Proving the value of simulation in laparoscopic surgery. Annals of Surgery. 2004;240:518–528. doi: 10.1097/01.sla.0000136941.46529.56. [DOI] [PMC free article] [PubMed] [Google Scholar]