Abstract
Hydrolytically more stable analogues of (–)-epicatechin gallate (ECg) have been synthesised from ECg where an amine or amide function has been substituted for the ester linkage that joins the C-ring with the galloyl D-ring. Sub-inhibitory concentrations (25 mg/L) of the amide analogue 7, possessing the natural C-3 stereochemistry, were able to reduce the resistance to oxacillin of three strains of methicillin resistant Staphylococcus aureus (BB 568, EMRSA-15 and EMRSA-16) comparable to levels achieved with ECg.
Keywords: MRSA, (–)-Epicatechingallate analogues, β-Lactam antibiotics
1. Introduction
The emergence of multi-drug resistance in pathogenic bacteria has created an urgent need for new antibiotics and new approaches to the treatment of bacterial infections.1 Staphylococcus aureus is one of the major causes of hospital- and community-acquired infections worldwide; serious infections with this pathogen include wound infections, bacteraemia and sepsis and are associated with a high mortality rate.2 Staphylococcal resistance to wide spectrum β-lactam antibiotics, such as methicillin, oxacillin and flucloxacillin, emerged soon after the introduction of the first drug in this class and there has been a steady rise in the incidence of methicillin resistant S. aureus (MRSA) clinical isolates.3 Staphylococci show a strong tendency to accumulate antibiotic resistant genes and the majority of MRSA isolates are now resistant to a range of antibiotics.4
Agents that suppress the expression of β-lactam resistance in MRSA would facilitate the treatment of serious MRSA infections with generic, cost-effective agents that are currently of little use against these multi-resistant bacteria. The polyphenolic compounds (–)-epicatechin gallate (ECg) and (–)-epigallocatechin gallate (EGCg) are major constituents of green tea (Camellia sinensis) and have been shown to sensitise MRSA isolates to a wide spectrum of β-lactam antibiotics.5,6
In particular, ECg, without exception, reduced the in vitro oxacillin resistance of around forty MRSA clinical isolates to levels compatible with therapeutic doses.6 Unfortunately, naturally occurring catechin gallates such as ECg and EGCg are not suitable for in vivo therapeutic use as they are poorly absorbed from the intestine and are susceptible to hydrolysis by bacterial and possibly host esterases,7 properties that would almost certainly abrogate their β-lactam-modifying capacity in human subjects. In order to prevent the esterase-mediated removal of the galloyl moiety from catechin gallates, the initial step in in vivo metabolism of catechin gallates, we have designed, synthesised and evaluated modified ECg derivatives in which the hydrolytically susceptible ester bond has been replaced with an inherently more stable amide linkage.
2. Chemical synthesis
Our strategy for the synthesis of potentially hydrolytically stable ECg analogues relied upon the substitution of the C-3 oxygen with an amino function. This was achieved firstly by global protection of the seven hydroxyl groups as their tert-butyldimethylsilyl ethers to give 2 and cleavage of the ester linkage with LiALH4 to give 3 and 4 (Scheme 1). Sufficiently mild conditions for hydrolysis of the ester function could not be found due to the lability of the protecting groups and sensitivity of the ACB segment to strong acid or base. The stereochemical integrity at C-2 of the ACB fragment 3 was verified by analysis of pertinent coupling constants in the 1H NMR spectrum with those of ECg and (–)-epicatechin (EC).8 This position is prone to epimerisation in basic systems9 through a quinone methide intermediate.10 Fragment 3 is a pivotal compound to us in the synthesis of other C-3 analogues where we have explored ester,6 ether, sulfonate ester and carbamate analogues at this position. Synthesis of the selectively protected 3 from EC would be very difficult in terms of selectivity and the use of synthetically useful protecting groups11 and this route from 1 represents the most efficient to date.
Scheme 1.
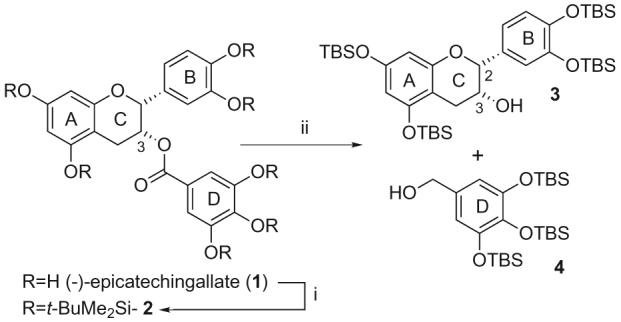
Reagents and conditions: (i) TBSCl, imidazole, DMF, rt, 14 h, 91%; (ii) LiAlH4, THF, 0 °C, 30 min, 3 80%, 4 67%.
Substitution of the naked C-3 hydroxyl group of 3 for an amino function was performed by reductive amination of the corresponding ketone with the amine 512 and sodium cyanoborohydride (Scheme 2). Global deprotection led to the amino analogue of ECg 6.13
Scheme 2.
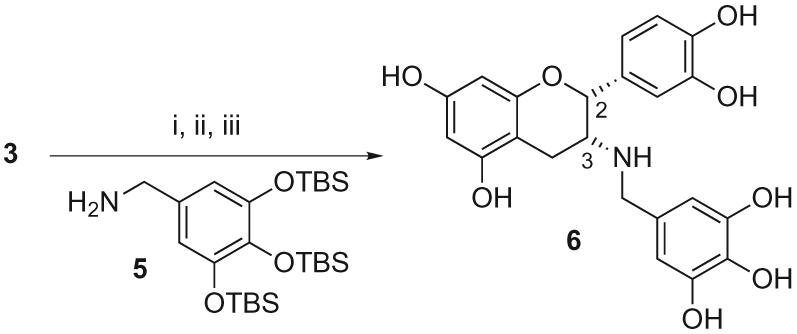
Reagents and conditions: (i) DMP, CH2Cl2, 0 °C, 9 h, 91%; (ii) 5, AcOH, NaCNBH3, THF, rt 14 h, 30%; (iii) HF·Py, THF/Py (4:1), 0 °C, 2 h, 51%.
With conditions found for reductive amination that did not affect the C-1 stereocentre this methodology was then used to synthesise the epimerically pure amide analogues 7 and 8 (Scheme 3). Treatment of ketone 9, formed from the Dess-Martin periodinane14 oxidation of 3 in 91% yield (Scheme 2), with benzylamine and sodium cyanoborohydride gave a 2:1 mixture of separable amines 10 and 11 in 45% and 26% yield, respectively, favouring the natural C-3 (3R) stereochemistry of EC and ECg.15 These could each individually be taken through to the desired amides 7 and 8 by hydrogenolysis of the N-benzyl group, DCC coupling with the carboxylic acid derived from 416 and global cleavage of the silyl ethers with HF·pyridine complex in good overall yield.
Scheme 3.
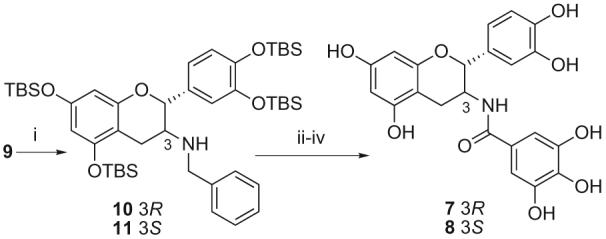
Reagents and conditions: (i) BnNNH2, AcOH, NaCNBH3, THF, rt 14 h, 10 45% and 11 26%; (ii) H2, 5% Pd-C, EtOH, rt, 16 h, 3R 100% and 3S 91%; (iii) tri-O-TBS protected gallic acid, DCC, DMAP, CH2Cl2, rt, 16 h, 3R 83% and 3S 59%; (iv) HF·Py, THF/Py (4:1), 0 °C, 2h, 7 41% and 8 69%.
3. Microbiological evaluation
With the amide derivatives (7 and 8) in hand, their efficacy as modulators for β-lactam resistance in S. aureus was evaluated by determining their capacity to reduce the minimum inhibitory concentration (MIC) of oxacillin against MRSA strains BB 568, EMRSA-15 and EMRSA-16 (Table 1).17
Table 1.
Antibacterial activity of ECg, 6-8 and in combination with oxacillin against methicillin resistant Staphylococcus aureus (MRSA) strains
| MRSA strain | MIC (mg/L)a |
Oxacillin MIC (mg/L)a |
|||||
|---|---|---|---|---|---|---|---|
| ECg | 7 | 8 | — b | ECg | 7b,c | 8b,c | |
| BB 568 | 256 | 256 | 256 | 256/256 | ≤0.5 | 4/8 | 64/64 |
| EMRSA 15 | 256 | 256 | 256 | 32/32 | ≤0.5 | ≤0.5/≤0.5 | 2/4 |
| EMRSA 16 | 128 | 128 | 128 | 512/512 | ≤0.5 | 1/1 | 256/512 |
MIC’s were determined in Mueller-Hinton broth + 2% salt at 35 °C after 24 h incubation.
Data for two separate experiments are shown.
Fixed concentration of 25 mg/L.
The galloyl amides 7 and 8 possessed extremely weak intrinsic antibacterial activity against three MRSA strains: BB 568 and the two epidemic strains EMRSA-15 and EMRSA-16 (MIC mg/L, Table 1). This level of activity was comparable to that found with ECg (this study and Ref. 6). Sub-inhibitory concentrations (25 mg/L) of 7 were able to reduce the resistance to oxacillin of all three strains examined (oxacillin MIC mg/L, Table 1). In particular, oxacillin MICs for EMRSA-15 and EMRSA-16 were comparable to those obtained when these strains were cultured in the presence of ECg. The MIC of oxacillin against BB 568 by 7 (from 256 mg/L to 4-8 mg/L) was less than that observed with ECg, but represented a very large diminution of sensitivity. Compound 8 was less effective that 7 with regard to its capacity to modify the sensitivity to oxacillin of BB 568 and EMRSA-16, although a significant degree of sensitisation was observed with MRSA-15 (Table 1).
4. Conclusion
The results show that sub-inhibitory concentrations (25 mg/L) of the amide analogue 7, possessing the natural C-3 stereochemistry, was able to reduce the resistance of three strains of methicillin resistant S. aureus (BB 568, EMRSA-15 and EMRSA-16) to oxacillin comparable to levels achieved with ECg. The higher activity of amide 7, compared to amide 8 indicates that carbonyl derived linkers demonstrating the natural 3R stereochemistry may provide other compounds for improved sensitisation of MRSA isolates to a wide spectrum of β-lactam antibiotics.
Acknowledgements
This work was supported by MRC Strategic Grant G0000996. We thank S. Shah for undertaking the antibacterial assays, Dr. S. Pih for preliminary studies, Dr. S. Brocchini and Professor J. M. T. Hamilton-Miller for helpful discussions, Mr. T. Hollingworth and Mr. D. Hooper for providing mass spectra and Mr. T. J. Spencer for micro analytical data.
References and notes
- 1.Taylor PW, Stapleton PD, Luzio JP. Drug Discov. Today. 2002;7:1086. doi: 10.1016/s1359-6446(02)02498-4. [DOI] [PubMed] [Google Scholar]
- 2.Romero-Vivas J, Rubio M, Fernandez C, Picazo JJ. Staphylococcus aureus. Clin. Infect. Dis. 1995;21:1417. doi: 10.1093/clinids/21.6.1417. [DOI] [PubMed] [Google Scholar]
- 3.In PHLS . Antimicrobial Resistance in 2002: England and Wales. London: Public Health Laboratory Service; 2002. [Google Scholar]
- 4.Firth N. In: Multiple Drug Resistant Bacteria. Ambile-Cuevas CF, editor. Wymondham: Horizon Scientific; 2003. pp. 33–60. [Google Scholar]
- 5.Yam TS, Hamilton-Miller JMT, Shah S. J. Antimicrob. Chemother. 1998;42:211. doi: 10.1093/jac/42.2.211. [DOI] [PubMed] [Google Scholar]
- 6.Stapleton PD, Shah S, Anderson JC, Hara Y, Hamilton-Miller JMT, Taylor PW. Int. J. Antimicrob. Agents. 2004;23:462. doi: 10.1016/j.ijantimicag.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 7.Kohri T, Matsumoto N, Yamakawa M, Suzuki M, Nanjo F, Hara Y, Oku N. J. Agric. Food Chem. 2001;49:4102. doi: 10.1021/jf001491+. [DOI] [PubMed] [Google Scholar]
- 8.The 1H NMR signal of C-2H (δ 4.90, s) compares favourably with the analogous signal in 5,7,3′,4′-tetra-O-benzyl-(—)-epicatechin (δ 4.91, s) and is in stark contrast to the same signal in 5,7,3′,4′-tetra-O-benzyl-(+)-catechin (δ 4.63, d, J = 8.5 Hz) in ; Tuckmantel W, Kozikowski AP, Romanczyk LJ. J. Am. Chem. Soc. 1999;121:12073. [Google Scholar]
- 9.(a) Freudenberg K, Böhme O, Purrman L. Ber. Dutsch. Chem. Ges. B. 1922;55:1734. [Google Scholar]; (b) Freudenberg K, Purrman L. Justus Liebigs Ann. Chem. 1924;437:274. doi: 10.1002/jlac.19677030126. [DOI] [PubMed] [Google Scholar]
- 10.Mehta PP, Whalley WE. J. Chem. Soc. 1963:5327. [Google Scholar]
- 11.Selective protection of the hydroxyl groups of catechins involves multistep procedures. See Ref. 8 and ; Cren-Olive C, Lebrun S, Rolando C. J. Chem. Soc., Perkin Trans. 1. 2002:821. [Google Scholar]; and references therein.
- 12.Derived from 4 by treatment with PPh3, phthalimide, DEAD, rt, 1 h then NH2NH2·H2O, EtOH, rt, 14 h, in 60% yield over two-steps
- 13.The natural C-3 stereochemistry (R) was confirmed based on the small coupling constant for the C-2H (δ 5.11, d, J = 2.6 Hz) indicating the relative cis-stereochemistry between C-2 and C-3 as before (Ref. 8)
- 14.(a) Dess DB, Martin JC. J. Org. Chem. 1983;48:4155. [Google Scholar]; (b) Dess DB, Martin JC. J. Am. Chem. Soc. 1991;113:7277. [Google Scholar]
- 15.These were assigned based on the coupling constants their C-2H protons as before (Ref. 8), 10 (natural 3R stereochemistry) C-2H (δ 5.09, d, J = 2.2 Hz) and 11 (3S) C-2H (δ 4.70, m, J = 7.6 Hz)
- 16.Prepared by Dess-Martin oxidation (Ref. 14) of 4 to give the corresponding aldehyde which was oxidised with NaO2Cl in the presence of 2-methyl-2-butene in t-BuOH and pH 4 buffer in 58% yield over two-steps
- 17.MIC determinations: the capacity of the various compounds to modulate β-lactam resistance was evaluated by determination of the MIC at a fixed concentration in combination with oxacillin. Assays were performed in 96-well microtitre trays with an inoculum of about 104 colony-forming units (cfu) in 100 μL of Mueller-Hinton broth (Oxoid, Basingstoke, UK) supplemented with 2% w/v NaCl. Doubling dilutions of oxacillin were employed. MIC values were recorded after incubation of the trays at 35 °C for 24 h; S. aureus ATCC29213 was used as the standard. The intrinsic anti-staphylococcal activity of compounds was also evaluated using these methods


