Abstract
Myofibril formation was visualized in cultured live cardiomyocytes that were transfected with plasmids expressing green fluorescent protein (GFP) linked to the Z-band protein, α-actinin. The expression of this fluorescent protein provided an in vivo label for structures containing α-actinin. The GFP–α-actinin fusion protein was incorporated into Z-bands, intercalated discs, and attachment plaques, as well as into the punctate aggregates, or Z-bodies, that are thought to be the precursors of Z-bands. Observations of live cells over several days in culture permitted us to test aspects of several theories of myofibril assembly that had been proposed previously based on the study of fixed cells. Fine fibrils, called premyofibrils, that formed de novo at the spreading edges of cardiomyocytes, contained punctate concentrations of α-actinin, termed Z-bodies. The punctate Z-bodies grew and aligned with Z-bodies in adjacent fibrils. With increasing time, adjacent fibrils and Z-bodies appeared to fuse and form mature myofibrils and Z-bands in cytoplasmic regions where the linear arrays of Z-bodies had been. These new myofibrils became aligned with existing myofibrils at their Z-bands to form myofibrils that spanned the length of the spread cell. These results are consistent with a model that postulates that the fibrils that form de novo near the cell membrane are premyofibrils—i.e., the precursors of mature myofibrils.
Keywords: transfections, α-actinin, green fluorescent protein, video microscopy
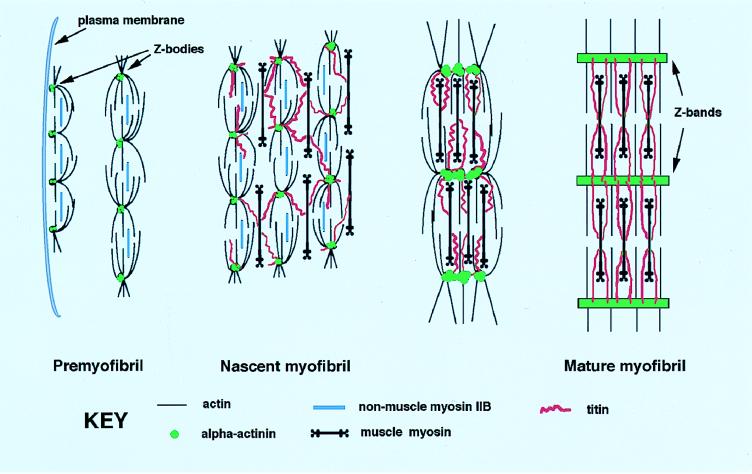
The formation of a myofibril involves the precise ordering of multiple subunits into a linear array of sarcomeres. One of the challenges in muscle research is to delineate the sequence of steps occurring in a cell during the assembly of the thick and thin filaments and Z-bands to form sarcomeres and myofibrils. We have recently proposed a model for the assembly of myofibrils based on antibody staining of chicken cardiomyocytes fixed at different times after spreading in culture (1). This model postulates that premyofibrils, characterized by banded patterns of α-actinin-rich Z-bodies and nonmuscle myosin IIB, form at the edges of spreading cardiomyocytes and develop into mature myofibrils (Fig. 1). During the transition from premyofibril to myofibril, it is postulated that there is an exchange of nonmuscle myosin IIB filaments for muscle myosin II filaments and a growth and fusion of Z-bodies into Z-bands (1, 2). The Z-bodies appear initially as discrete aggregates of α-actinin along the premyofibrils. As the myofibrils increase in width, the Z-bands appear to be composed of laterally aligned Z-bodies and finally continuous bands of α-actinin (see Fig. 1). A second model of myofibrillogenesis proposes that the first fibrils that form at the periphery of spreading cardiomyocytes are temporary scaffolds along which myofibrils assemble (3). Finally, there is a third hypothesis that spatially separate complexes of actin filaments and Z-bands, I-Z-I brushes, and groups of myosin thick filaments assemble independently of one another and become spliced together by titin filaments and then inserted at the ends of fully formed myofibrils (4, 5). Time-lapse observations of sarcomeric proteins in single cells undergoing myofibrillogenesis allows aspects of these models to be tested.
Figure 1.
Model for the assembly of a mature myofibril based on the data presented in this and previous papers (1, 2). The position of α-actinin (shaded circles) relative to actin, muscle myosin II, nonmuscle myosin IIB, and titin is shown for each stage of myofibril development. Near the spreading edge of the cell (far left), α-actinin is found in mini-sarcomeric arrays in the premyofibrils. In the nascent myofibril, the α-actinin-rich Z-bodies have begun to laterally associate, possibly through interaction with titin. Overlapping muscle myosin II filaments bound to titin are present at this stage. In the mature myofibril the α-actinin containing Z-bodies fuse to form the wide lateral arrays of mature Z-bands; nonmuscle myosin IIB is lost whereas muscle myosin filaments are now aligned into A-bands. The Z-bodies grow in size in the progression from premyofibril to mature myofibril.
The development of plasmids coding for green fluorescent protein (GFP) (6) has permitted fluorescent cytoskeletal and muscle proteins to be expressed and observed in living cells for extended periods of time (7–9). We have coupled GFP to muscle α-actinin and transfected spreading embryonic chicken cardiomyocytes to follow myofibrillogenesis in spreading cardiomyocytes. α-Actinin, a 100-kDa F-actin binding protein, is concentrated in the dense bodies of stress fibers and in the Z-bodies and Z-bands of muscles and marks the boundaries of the sarcomeric units in nonmuscle stress fibers and in muscle myofibrils. In this paper, we show that premyofibrils precede in space and time mature myofibrils. As predicted from previous studies, the premyofibrils first appeared at the spreading edges of living cardiomyocytes. They were not, as some have suggested, breakdown products of mature myofibrils. The Z-bodies of the premyofibrils appeared to fuse laterally with one another to form the Z-bands of mature myofibrils. With increasing time, mature myofibrils and Z-bands emerged in regions where linear arrays of Z-bodies had been.
METHODS AND MATERIALS
The sarcomeric α-actinin cDNA (10) was amplified by PCR with a 5′ primer (5′-CCCAAGCTTATGAACAGCATG-3′) and a 3′ primer (5′-CCCAAGCTTGAGATCGCTCT-3′) to obtain a clone starting at the ATG initiation codon and eliminating the stop codon and the 3′ untranslated region. The PCR product was subcloned into the HindIII site of the pEGFP-N1 vector (CLONTECH), at the 5′ end of the GFP cDNA sequence. The resultant plasmid was purified with a Qiagen (Chatsworth, CA) column and used for transfection of cardiac myocytes. We found that it was essential for GFP to be fused to the carboxyl terminus of α-actinin for the fusion protein to become specifically localized in transfected cells. A GFP-fusion to the amino terminus of α-actinin resulted in a diffuse fluorescence with no discernible localization in transfected cells, possibly due to interference by the GFP with the amino-terminal actin-binding site.
Chicken cardiac myocytes were isolated from 7-day-old embryos as described (11, 12) and plated onto 35-mm glass-bottomed dishes at a density of 1 × 106 cells per dish. The next day, the cells were transfected with 1 μg of plasmid mixed with 5 μl of Lipofectamine in 1 ml OptiMEM medium (GIBCO/BRL) and overlaid on cells for 5 h before addition of 1 ml of medium with serum. The next day, the cells were washed with normal culture medium.
The cells were observed at various times after 24 h of transfection using a Nikon Diaphot 200 microscope with a ×63 or ×100 phase-contrast objective lens. The temperature of cells was maintained at 36°C with a heat curtain (ASI 400 Air Stream Incubator; NevTek, Burnsville, VA) and the culture dish was covered by a small homemade chamber, through which 5% CO2 flowed. Humidity was provided with a reservoir of distilled water in the chamber, and the culture medium was replaced with fresh medium every 6–12 h. Images were acquired with a liquid-cooled charge-coupled device (CCD) camera (Photometrics, Tuscon, AZ) and processed with metamorph image processing software (Universal Imaging, West Chester, PA). In the time-lapse images of the spreading live cells sequential focal planes were added digitally for each time point to include all of the α-actinin containing structures.
Immunostaining of α-actinin–GFP-transfected cells was performed as described (1) using anti-nonmuscle myosin IIB antibody (Chemicon) and a rhodamine-labeled secondary antibody. Some transfected cultures were also fixed and stained with rhodamine labeled phalloidin (Fluka).
RESULTS
The α-actinin–GFP construct, when introduced into embryonic cardiomyocytes, produced fluorescently labeled protein within 24 h after transfection. This fusion protein incorporated into all the normal cellular structures that are rich in endogenous α-actinin. Most prominently, in contracting myofibrils, α-actinin–GFP colocalized in a banded pattern with the phase-dense Z-bands (Fig. 2). Intercalated disks that were most clearly seen at junctions between transfected and untransfected cardiomyocytes also incorporated α-actinin–GFP (Fig. 3). Transfections of muscle cells with plasmids coding for GFP alone did not lead to any specific localization of GFP fluorescence (9).
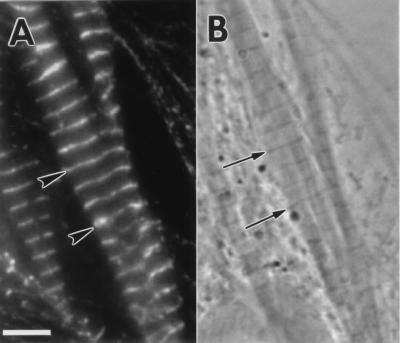
Figure 2.
(A) Fluorescent image of an embyronic chicken cardio-myocyte transfected with the α-actinin–GFP plasmid showing the colocalization of α-actinin–GFP (arrowheads) with the phase-dense Z-bands (arrows) (B). (Bar = 5 μm.)
Figure 3.
Fluorescent image of an actively contracting embryonic chicken cardiomyocyte transfected with α-actinin–GFP. The fusion protein localizes to the Z-bands of the mature contracting myofibrils, the Z-bodies of the premyofibrils (arrowheads), and an intercalated disc (arrow) that is shown in phase-contrast in the inset (arrow). (Bar = 5 μm.)
In peripheral areas of transfected myocytes, α-actinin–GFP was localized in small punctate aggregates (Z-bodies) (Figs. 3 and 4) that were localized along phalloidin-positive fibrils (not shown) that we call premyofibrils (Fig. 1). Antibody to nonmuscle IIB stained the premyofibrils (Fig. 4), between the α-actinin–GFP positive Z-bodies, but was absent from the more central region of the cell where mature myofibrils were located (Fig. 4). No contractions were detected in premyofibrils; however, when they were linked to contracting myofibrils, as in the cell in Fig. 3, movements initiated in the centrally located myofibrils were transmitted to the peripheral premyofibrils.
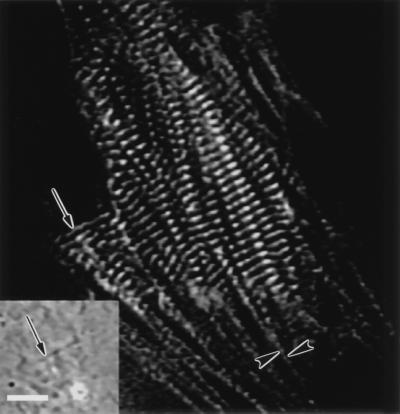
Figure 4.
Fluorescent images of an embryonic chicken cardiomyocyte (A) transfected with α-actinin and (B) fixed and stained with a nonmuscle myosin IIB antibody. Nonmuscle myosin IIB is in the premyofibrils at the edge of the cardiomyocyte (B, arrowheads) but not in the mature myofibrils in the central region of the cell. (Bar = 5 μm.)
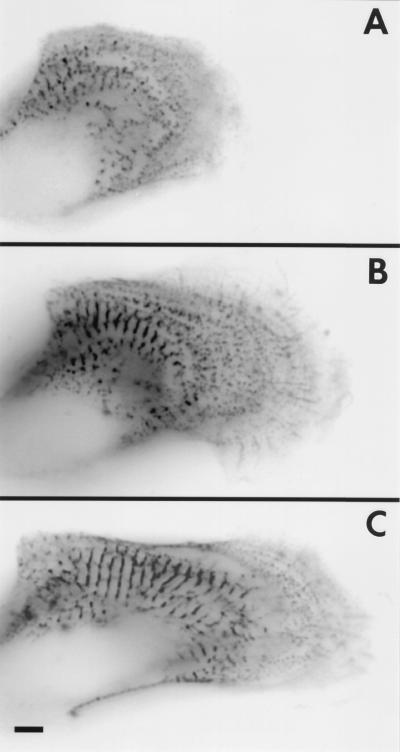
To observe myofibril dynamics as the embryonic chicken cardiomyocytes spread in culture, images of transfected cells were acquired over a period spanning from several hours to several days. The intervals between time points were usually 2 h or more to reduce possible adverse effects from the excitation light used to detect the α-actinin–GFP. Typically, isolated cardiomyocytes in culture initially spread out and then develop new myofibrils. The active spreading of the cells, however, diminishes after several days. After this point there were no significant changes in the distribution of Z-bands and Z-bodies. Therefore, we only recorded cells in the first few days of culture and focused on the spreading margins of the cells where myofibril assembly has been suggested to occur from previous studies of fixed cells (1, 2, 11–13). The cells shown in Figs. 5 and 6 are representative of 10 cells that were recorded while spreading and forming myofibrils.
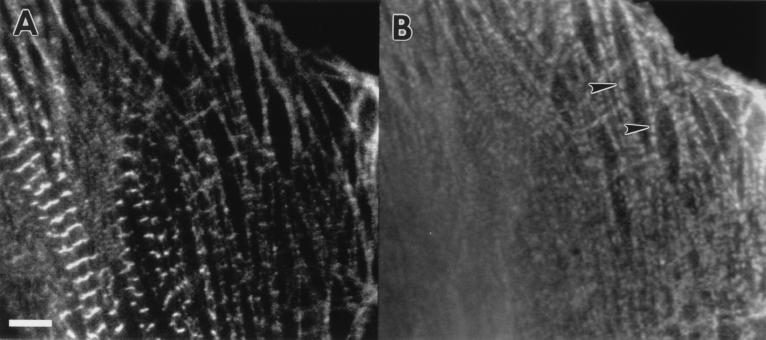
Figure 5.
Time-lapse fluorescent images of a spreading and beating embyronic chicken cardiomyocyte transfected with α-actinin–GFP are shown as reverse contrast at the initial time point (A) 0 h, (B) 3 h later, and (C) 10 h. Four focal planes were digitally added for each time point to identify all the Z-bodies. (Bar = 5 μm.)
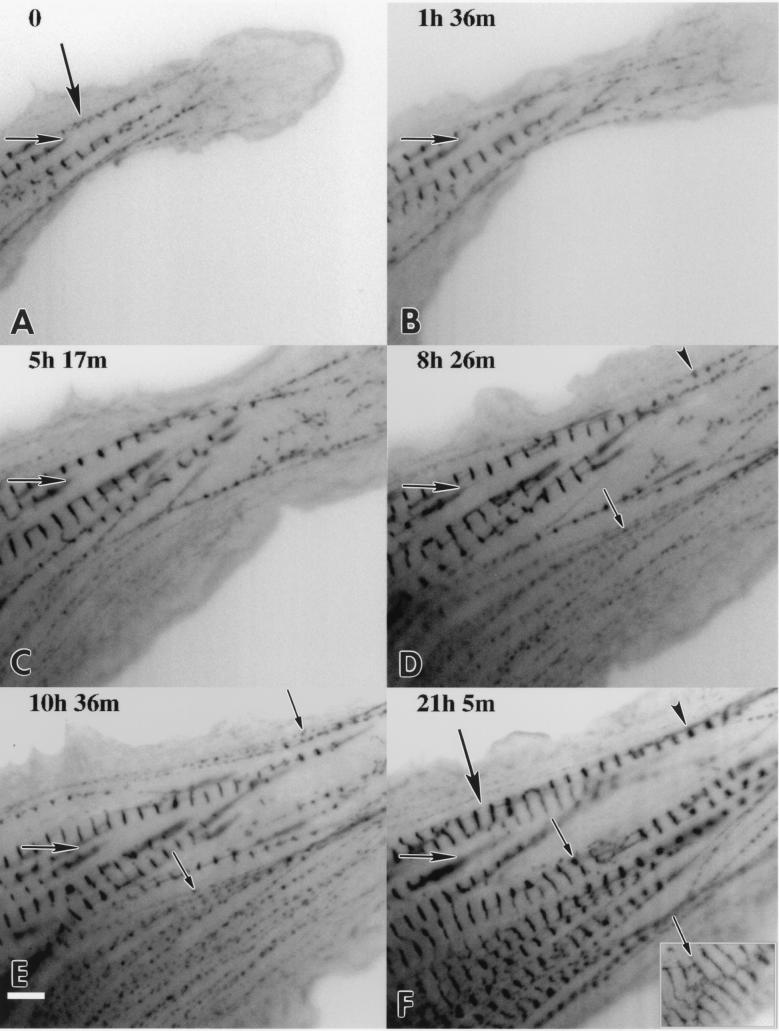
Figure 6.
Time-lapse fluorescent image observation of a spreading and beating embyronic chicken cardiomyocyte transfected with α-actinin–GFP shown in reverse contrast with the time of acquisition relative to A indicated on each micrograph (B–F). The same prominent adhesion plaque is marked in each panel as a reference point (A–F, horizontal arrows). Note the extent of spreading with respect to this plaque. As the cell spreads toward the lower part of the image, punctate Z-bodies assemble into linear arrays that mature into Z-bands of myofibrils. One myofibril indicated by a large arrow (A) appears to double in length over 21 h. This appears to occur by lateral coalescence of myofibrils (D–F, arrowheads). Adjacent Z-bodies (D, small arrow) increase in diameter (E, small arrow) and fuse into a mature Z-band (F, small arrow). At 28 h the Z-bands became thinner and less punctate (F Inset, small arrow). (Bar = 5 μm.)
The spreading edge of a cardiomyocyte expressing α-actinin–GFP was observed at six time points over a 10-h period, three of which are shown in Fig. 5. At the first recorded time point, the cell contained small aggregates of α-actinin (Z-bodies) at the leading edge of the cell and a few mature Z-bands, separated by spacings of 2 microns, in the central region of the cell (Fig. 5A). Three hours later (Fig. 5B) the cell had spread about 15 microns at its leading edge with the area occupied by Z-bodies expanding 62%, and the population of well-ordered Z-bands increasing in the central region of the cell. Ten hours later (Fig. 5C) the leading edge had spread an additional 10 microns and the Z-bands had become aligned. Thus, the area in which premyofibrils initially formed became the site of new mature myofibrils, characterized by well defined A-bands.
Another cardiomyocyte expressing α-actinin–GFP was followed at seven time points over a 28-h period (Fig. 6). The beating cardiomyocyte was actively ruffling and spreading, primarily toward the lower portion of the image. As the cell spread, Z-bodies formed in the new expanse of cytoplasm. The size and intensity of fluorescence of the Z-bodies increased with time, and 21 h after the first time point, fluorescent Z-bands were present where the punctate Z-bodies had been. The formation of Z-bands often was preceded by the lateral alignment of Z-bodies that increased in size (small arrow in Fig. 6 D and E) and then fused to form a Z-band (small arrow in Fig. 6F). The Z-bands became less punctate and thinner as they continued to align with neighboring Z-bands over the next 7 h (small arrow, Fig. 6F Inset). Newly formed myofibrils appeared to overlap with existing ones to form longer myofibrils, or bundles of myofibrils. For example, a myofibril in Fig. 6A (large arrow) extended 11 microns from the edge of the field. Twenty-one hours later the myofibril in the same location extended 28 microns from one edge of the field to the other edge (large arrow in Fig. 6F). The increase in length did not seem to occur by sequential addition of new sarcomeres to the ends of the myofibrils, but seemed to involve a lateral coalescing of adjacent, shorter myofibrils. This is particularly striking in the region of Fig. 6 D–F indicated by the arrowheads. Myofibrils terminating in well-defined adhesion plaques did not appear to lengthen (horizontal arrow on left side of Fig. 6 A–F).
DISCUSSION
In this study, we linked GFP to the carboxyl-terminal end of α-actinin. This 28-kDa GFP did not appear to interfere with the incorporation of the α-actinin into Z-bands, Z-bodies, intercalated discs, and adhesion plaques of embryonic cardiomyocytes where endogenous α-actinin is located; nor did it interfere with the beating of transfected embryonic cardiomyocytes. Therefore, with this construct we were able to follow the same transfected cardiomyocytes for several days in culture, thus gaining the opportunity for the direct observation of myofibrillogenesis in culture. We detected the formation of premyofibrils in previously vacant areas of cytoplasm in spreading cardiomyocytes. Subsequently, mature myofibrils were seen in the same areas previously occupied by premyofibrils.
Our results are consistent with the hypothesis that myofibril formation begins at the spreading edges of the cardiomyocytes with the formation of premyofibrils that subsequently fuse at the level of the Z-bodies to form mature myofibrils (1, 2, 11–13). Fig. 1 shows a model for the assembly of myofibrils in cardiomyocytes that was initially proposed on the basis of studying embryonic chicken cardiomyocytes fixed at different stages of spreading and then stained with a variety of nonmuscle and muscle antibodies (1, 2, 11–13). The model is consistent with our current dynamic observations on live cells. We find that Z-bodies first appear in phalloidin positive fibrils at the spreading edges of the cardiomyocytes and grow bigger before they eventually fuse to form the Z-bands of mature myofibrils. Nonmuscle myosin IIB is located between the Z-bodies in the transfected cells, but not between the Z-bands of the mature myofibrils. Thus, premyofibrils with their Z-bodies and type IIB myosin are distinct from mature myofibrils, with Z-bands and muscle specific myosin II. The sequential appearance of premyofibrils followed by mature myofibrils in the same cytoplasmic location, and the intermediate stages in the transition between Z-bodies and Z-bands are consistent with the hypothesis that premyofibrils are the precursors of myofibrils in living cardiomyocytes. Further support for this premyofibril model derives from the immunofluorescent staining of cardiomyocytes with muscle specific sarcomeric antibodies (14, 15). These studies have revealed the presence of the same muscle-specific isoforms of α-actinin, tropomyosin, and troponin in premyofibrils, nascent, and mature myofibrils.
A second theory of myofibril formation proposes that stress fiber-like structures (SFLS), composed of nonmuscle proteins, serve as scaffolds or templates—i.e., one SFLS per myofibril—on which recruited sarcomeric proteins are assembled into myofibrils (3). This would predict that one Z-body in a premyofibril is replaced by a Z-band, whereas we observed, on the contrary, that several small Z-bodies fuse into a single Z-band. These results are consistent with the premyofibril model of Fig. 1, but inconsistent with the SFLS substitution model. Further support for the premyofibril model was demonstrated when living cardiomyocytes and nonmuscle cells were injected with monomer actin binding proteins (vitamin D binding protein; DNase I) and it was discovered that while stress fibers in the nonmuscle cells were induced to disassemble, premyofibrils, nascent myofibrils, and mature myofibrils were unaffected (16). If the premyofibrils and nascent myofibrils were stress fibers, they would have been disassembled by the injection of the monomer actin binding proteins.
A third theory of myofibril formation postulates the independent self-assembly of thick filaments of muscle myosin and isolated, linearly aligned I-Z-I brushes (i.e., thin filaments attached to Z-bands) with no nonmuscle myosin II. Titin molecules are thought to attach subsequently to the Z-bands to capture and align the thick muscle myosin filaments into A-bands and form myofibrils. The zippering together of the isolated I-Z-I brushes and the isolated thick filaments is suggested to take place at the ends of the existing myofibrils, thus ensuring their continued growth. It appeared from our observations that the formation of longer myofibrils occurred through lateral coalescing of neighboring myofibrils (see Fig. 6). Furthermore nonmuscle myosin IIB is present in premyofibrils in both spreading embryonic and adult cardiomyocytes (1, 2, 14), and its previously reported absence in embryonic cardiomyocytes (17) was due to the fact that antibodies used were directed against the IIA isoform that is lacking in cardiomyocytes (1, 2, 18). In this study, we found that nonmuscle myosin IIB present, and muscle myosin II absent, in all premyofibrils of the cardiomyocytes expressing α-actinin–GFP. Thus, both the premyofibril model (Fig. 1) and this third model of myofibril formation concur that muscle thick filaments and thin filaments are assembled in different cellular locations.
Further study of living cells transfected with GFP-linked probes for intact and truncated sarcomeric proteins should lead to the delineation of the steps necessary for the assembly of complex structures like myofibrils. It will also be of interest to see how this premyofibril model applies to the formation of myofibrils in other systems (19, 20).
Acknowledgments
This work was supported by grants from Muscular Dystrophy of America, the National Institutes of Health (HL 15835 and HL48954), and the National Science Foundation (MCB 93-19041). G.A.D. was supported by a postdoctoral fellowship from the American Cancer Society.
ABBREVIATION
- GFP
green fluorescent protein
References
- 1.Rhee D, Sanger J M, Sanger J W. Cell Motil Cytoskeleton. 1994;28:1–24. doi: 10.1002/cm.970280102. [DOI] [PubMed] [Google Scholar]
- 2.Turnacioglu K K, Mittal B, Dabiri G A, Sanger J M, Sanger J W. Cell Struct Funct. 1997;22:73–82. doi: 10.1247/csf.22.73. [DOI] [PubMed] [Google Scholar]
- 3.Dlugosz A A, Antin P B, Nachmias V T, Holtzer H. J Cell Biol. 1984;99:2268–2278. doi: 10.1083/jcb.99.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultheiss T, Lin Z, Lu M-H, Murray J, Fischman D A, Weber K, Masaki T, Imamura M, Holtzer H. J Cell Biol. 1990;110:1159–1172. doi: 10.1083/jcb.110.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein H F, Fischman D A. Science. 1991;251:1039–1044. doi: 10.1126/science.1998120. [DOI] [PubMed] [Google Scholar]
- 6.Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 7.Gerisch G, Albrecht R, Heizer C, Hodgkinson S, Maniak M. Curr Biol. 1995;5:1280–1285. doi: 10.1016/s0960-9822(95)00254-5. [DOI] [PubMed] [Google Scholar]
- 8.Moores S L, Sabry J H, Spudich J A. Proc Natl Acad Sci USA. 1996;93:443–446. doi: 10.1073/pnas.93.1.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turnacioglu K, Mittal B, Dabiri G A, Sanger J M, Sanger J W. Mol Biol Cell. 1997;8:705–717. doi: 10.1091/mbc.8.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imamura M, Endo T, Kuroda M, Tanaka T, Masaki T. J Biol Chem. 1988;263:7800–7805. [PubMed] [Google Scholar]
- 11.Sanger J W, Mittal B, Sanger J M. Cell Motil. 1984;4:405–416. doi: 10.1002/cm.970040602. [DOI] [PubMed] [Google Scholar]
- 12.Sanger J M, Mittal B, Pochapin M B, Sanger J W. J Cell Biol. 1986;102:2053–2066. doi: 10.1083/jcb.102.6.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanger J M, Mittal B, Pochapin M B, Sanger J W. J Cell Sci Suppl. 1986;5:17–44. doi: 10.1242/jcs.1986.supplement_5.2. [DOI] [PubMed] [Google Scholar]
- 14.LoRusso S M, Rhee D, Sanger J M, Sanger J W. Cell Motil Cytoskeleton. 1997;37:183–198. doi: 10.1002/(SICI)1097-0169(1997)37:3<183::AID-CM1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Sanger J M, Rhee D, Leonard M, Price M, Zhukarev V, Shuman H, Sanger J W. Mol Biol Cell. 1994;5:165a. (abstr.). [Google Scholar]
- 16.Sanger J M, Dabiri G A, Mittal B, Kowalski M A, Haddad J G, Sanger J W. Proc Natl Acad Sci USA. 1990;87:5474–5478. doi: 10.1073/pnas.87.14.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu M-H, DiLullo C, Schultheiss T, Holtzer S, Murray J M, Choi J, Fischman D A, Holtzer H. J Cell Biol. 1992;117:1007–1022. doi: 10.1083/jcb.117.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conrad A H, Clark W A, Conrad G W. Cell Motil Cytoskeleton. 1991;19:189–206. doi: 10.1002/cm.970190307. [DOI] [PubMed] [Google Scholar]
- 19.Beall C, Sepanski M, Fyrberg E. Genes Dev. 1989;3:131–140. doi: 10.1101/gad.3.2.131. [DOI] [PubMed] [Google Scholar]
- 20.Epstein H F, Casey D L, Ortiz I. J Cell Biol. 1993;122:845–858. doi: 10.1083/jcb.122.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]