Abstract
The discovery of the Th17 lineage of T helper cells and the realization that this subset was implicated in the pathogenesis of a variety of inflammatory conditions has lead to an intense effort devoted to identifying the cytokines and transcription factors that promote their development. In contrast, less attention has been paid to understanding the cytokines that temper Th17 activity. Recent studies, however, have provided insights into the cytokines that limit these T cells. The aim of this article is to review our current understanding of the regulatory networks that limit T helper subsets and how they relate to the Th17 lineage.
Keywords: Th17, cytokine, chronic inflammation, STAT
Cross-regulation of T helper cell subsets
In 1986 Mosmann and Coffman described the presence of two types of CD4+ T-helper (Th) cell clones, Th1 and Th2, that had distinct profiles of cytokine production[1]. The signature cytokine of the Th1 subset is IFNγ, whereas Th2 cells secrete a variety of soluble factors that are now recognized as IL-4, IL-5 and IL-13. At that time, the authors predicted that additional Th cell subsets would exist that are important for driving immune effector functions. This prediction has been exemplified most recently with the identification of the Th17 subset characterized by the production of IL-17A, IL-17F, IL-6, IL-21 and IL-22[2–7]. While IL-17 may be protective against certain extracellular pathogens that bind to mucosal surfaces[8, 9] it is now recognized that this specialized subset of T cells are important contributors to pathology in mouse models of arthritis and multiple sclerosis[2, 10, 11], and they have been directly implicated in other inflammatory conditions, including Crohn’s disease and psoriasis[12, 13]. As a consequence, it has been proposed that strategies that specifically target Th17 cells, while sparing other Th subsets, may represent one way to manage a variety of chronic inflammatory diseases[14]. Therefore, there has been considerable effort devoted to defining the factors that promote and sustain this particular Th subset. While the factors that govern Th17 generation is a topic covered in more detail elsewhere in this issue, the focus of this article is to review the emerging studies on the natural antagonists of Th17 activity with the expectation that these inhibitors may also be of use from a therapeutic perspective.
The initial studies on naïve CD4+ T-helper cell differentiation into polarized Th1 and Th2 cells revealed that these subsets were capable of considerable cross-regulation. Thus, Th1 and Th2 cells inhibit the development of one another through the action of their lineage-specific cytokines IFNγ and IL-4 respectively[15, 16]. This principle also held true for Th17 cells with evidence that IFNγ and IL-4 could antagonize initial Th17 development[17, 18] (Figure 1). Additionally, once fully matured, Th17 cells are resistant to the suppressive effects of IFNγ and IL-4 in vitro[17, 18] providing evidence that this subset of Th cells represents a committed phenotype. However, to date there has been no evidence that Th17 cells can antagonize the development of Th1 or Th2 responses in the same fashion.
Figure 1.
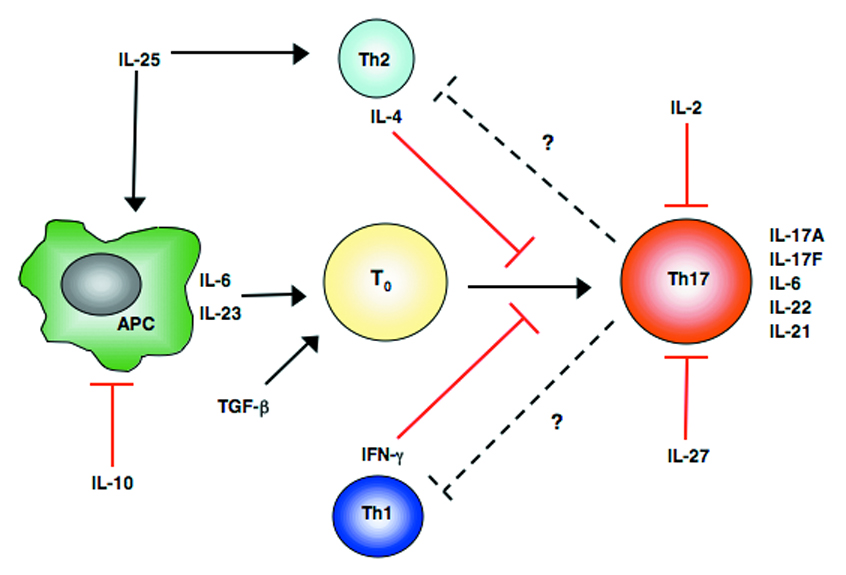
Antagonists of Th17 cells. Commitment of T cells to a particular lineage is highly dependent on the cytokine milieu surrounding the activated T cells. In the case of Th17 cell development the presence of TGF-β, IL-6, IL-23 and IL-21 serve to positively regulate Th17 differentiation. However, the presence of additional cytokines in the milieu can inhibit Th17 commitment. The production of IL-10 by a number of cell types serves as a key regulator of all adaptive immune responses as it impedes accessory cell functions necessary for optimal T cell responses. IL-27, a product of antigen presenting cells can have a direct effect on activated T cells preventing Th17 differentiation. Th17 development can also be cross-regulated by the signature cytokines IFN-γ and IL-4 of Th1 and Th2 cells respectfully. Additional products of T cells such as IL-2 and IL-25 (IL-17E) can have direct and indirect effects on the commitment of Th17 cells.
While IFNγ and IL-4 provide examples of lineage-specific products that serve the dual purpose of promoting effector activities as well as counter-regulating the development of other Th subsets, numerous studies on the biology of Th cells has led to the identification of multiple cytokines and cell-associated factors that limit their expansion, irrespective of polarity. This list has steadily increased to include, among others, CTLA-4, BTLA-4 and PD1; while there is a general assumption that they will also modulate Th17 activities, there are few studies that specifically address their function in the biology of this particular subset. The aim of this article however is to highlight some of the recent advances in understanding the cytokine networks that antagonize Th17 responses.
Enhanced Th17 responses in the absence of IL-10
One of the most prominent cytokines involved in the regulation of T-cell-mediated inflammatory responses is IL-10. This cytokine, first described as a product of Th2 cells[19] that limits Th1 cell production of IFNγ, was subsequently recognized as a product of many cell types that limits both Th1 and Th2 responses. Indeed, the absence of IL-10 can lead to exacerbated Th cell activity in a number of experimental settings including infection and autoimmunity[20–23]. This was largely attributed to the ability of IL-10 to inhibit accessory cell functions required for optimal T cell responses[24–26]. This includes the ability to suppress the production of IL-12p40 required to polarize and expand Th1 cells as well as IL-1β and IL-6 that are now recognized as being important in Th17 development. Consistent with this activity, deletion of Il-10 results in the development of an enterocolitis, characterized by enhanced production of IL-12 and IFNγ[27–29]. Treatment of Il-10-/- mice with anti-IL-12 (p40) or anti-IFNγ before disease onset reduces pathology and implicates Th1 cells in the development of this condition. Paradoxically, at later timepoints only anti-IL-12 (p40), not anti-IFNγ, can ameliorate established disease[30] suggesting that perhaps Th1 effector cells were not contributing to the ongoing inflammation. Once it was recognized that the p40 subunit of IL-12 was also a component of IL-23[31], which in turn can support Th17 activity[32], it then became apparent that the absence of IL-10 would not only result in enhanced Th1 responses but also lead to increased levels of IL-23 and increased Th17 activity. Indeed, Il-10-/- mice that also lack IL-23 fail to develop colitis; this is associated with normal IFNγ responses but reduced IL-17[33]. This finding suggests a model in which the ability of IL-10 to limit the production of multiple cytokines (especially IL-6 and IL-23) is required to limit the pathological consequences of Th17 cells, particularly at mucosal sites.
Regulation of Th17 cells by the IL-17 family member IL-25 (IL-17E)
While the production of IL-17 served at one point to define Th17 cells, there are actually six members, of this gene family IL-17A-F[34], of which only IL-17A (IL-17) and IL-17F are associated with Th17 cells[11]. In contrast to the pro-inflammatory effects ascribed to IL-17A/F, recent studies have implicated another member of this family, IL-25 (IL-17E), in limiting chronic inflammation in the gastrointestinal tract[35]. IL-25, which is produced by activated Th2 cells and mast cells, has been shown to induce the expression of the Th2 signature cytokines IL-5 and IL-13 [36]. In the absence of IL-25, mice infected with the gastrointestinal parasite Trichuris muris fail to develop an optimal Th2 response required to expel this nematode, which consequently establishes a persistent infection. Normally, in immunocompetent mice that are unable to expel Trichuris the parasites do not cause excessive inflammation. However, in the absence of IL-25 the establishment of a chronic infection results in severe intestinal inflammation characterized by heightened production of IFNγ and IL-17, but normal levels of IL-10 at this site. More recently in a mouse model of experimental autoimmune encephalitis (EAE) the absence of IL-25 was associated with more severe disease, elevated levels of IL-23 in the periphery and increased numbers of T cells producing IL-17, TNF-α and IFNγ in the central nervous system (CNS)[37]. Administration of recombinant murine IL-25 to the IL-25-/- mice led to decreased IL-17 production and subsequently induced a Th2 response. Thus, it appears that by promoting the development of Th2 cells, IL-25 is able to antagonize the development of Th17 cells (Figure 1).
IL-2 antagonizes Th17 activity
In part because IL-2 was identified based on its ability to promote T- cell proliferation as well as its ability to produce cytokines associated with Th1 and Th2 responses, it has been assumed that this growth factor is a general activator of T-cell responses[38]. This biology was complicated by the recognition that IL-2 is also a growth factor for regulatory T (Treg) cells and these cells can limit T-cell responses, either through consumption of IL-2 or other contact-dependent or -independent inhibitory effects. Thus, the absence of IL-2 results in diminished Treg cell numbers and this is associated with the development of a T-cell-mediated colitis and wasting disease reminiscent of the Il-10-/- mice[39]. Further insights into this phenotype were provided by O’Shea and colleagues who revealed that IL-2 was a potent inhibitor of Th17 cells[40] (Figure 1). This observation was correlated in Il-2-/- mice that had elevated serum levels of IL-17 and increased numbers of IL-17-producing T cells in secondary lymphoid organs. Further evidence of a role for IL-2 in inhibiting IL-17 production was found in a mouse model of systemic autoimmune disease in which the absence of IL-2 resulted in increased infiltration of IL-17–producing T cells to the skin[41]. While the complex biology of IL-2 means these data have to be interpreted with care it appears that, similar to IL-10, IL-2 may play a critical role in the homeostasis of these potentially pathogenic T cells at barrier sites.
IL-27 as an inhibitor of Th17-responses
With the recent discovery of the combination of cytokines (TGF-β, IL-6, IL-23, IL-21) required to promote development of Th17 cells, it seemed intuitive that the ability of IL-10 to antagonize the production of IL-12/23 p40 and IL-6 would indirectly influence these cells. In contrast, based on early reports it would have been difficult to predict that IL-27, a close relative of IL-6, would be an inhibitor of this T-helper response. Initial studies on IL-27 focused on its pro-inflammatory activities[42, 43], but subsequent work with a range of pathogens provided unexpected insights into the role of this cytokine in limiting inflammation[44, 45]. For example, during toxoplasmosis the ability of IL-12 to drive the development of a parasite-specific T-cell response dominated by the production of IFNγ, is essential for the control of this intracellular infection[46]. However, in contrast to initial predictions, infected Il-27ra-/- mice generated normal CD4+ and CD8+ T cell IFNγ responses that allowed the control of parasite replication, but developed a lethal CD4+ T-cell-dependent inflammatory disease[47]. This pathological response was intrinsic to the T cells and was characterized by enhanced T-cell proliferation, increased production of IFNγ and IL-2, and the maintenance of a population of highly activated (CD62Llow, CD25+) CD4+ and CD8+ T cells. A similar phenotype has also been observed following challenge with Trypanosoma cruzi[48], Leishmania donovani[49] and Mycobacterium Tuberculosis[50]. Furthermore, several non-infectious models including concanavalin A (ConA)-induced hepatitis and experimental allergic asthma in Il-27ra-/- mice have resulted in an analogous pheontype[51, 52].
While the studies described above established the credentials of IL-27 as a bona fide suppressor of T-cell-activity, more recent publications illustrated the ability of IL-27 to antagonize the development and/or function of Th17 responses. Two of these reports focused on the role of IL-27 in controlling inflammation in the brain in infectious and autoimmune systems. Thus, Il-27ra-/- mice infected with T. gondii or immunized with MOG peptide to induce EAE[53–55] developed enhanced CNS pathology that was associated with local increases in Th17 activity. These findings implied that IL-27 antagonizes the production of IL-17, and in vitro studies revealed that IL-27 was able to directly inhibit the development of Th17 cells as well as the ability of pathogen-specific cells isolated from the brain to produce IL-17 (Figure 1). In addition to these studies, IL-27 was also implicated in the inhibition of IL-17 in a murine model of uveitis [56] in which the expansion of activated Th17 cells was inhibited by primary retinal cells by a mechanism dependent on endogenous IL-27. Further insight into the mechanism of action of IL-27 on Th17 development has revealed that this cytokine inhibits the expression of RORγt, a transcription factor involved in the differentiation of Th17 cells (Figure 2 and [5]).
Figure 2.
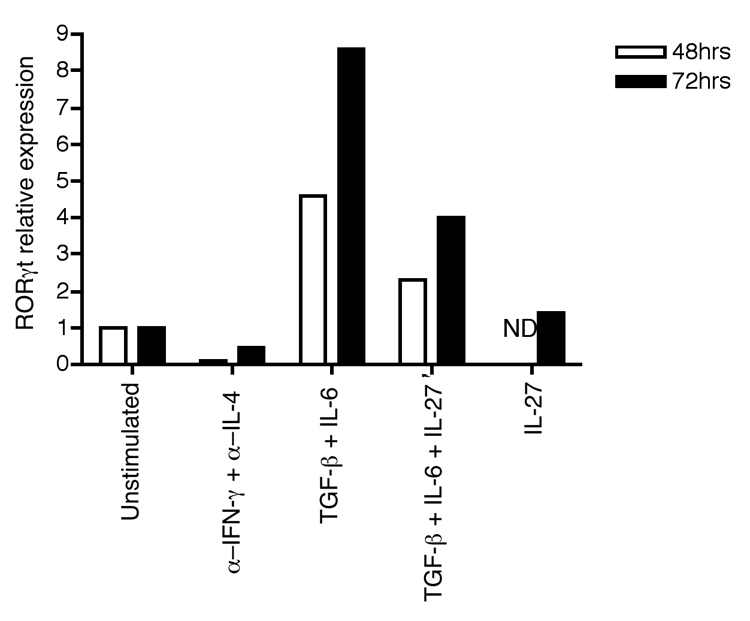
Inhibition of RORγt expression by IL-27. The expression of RORγt mRNA was measured by real-time quantitative PCR using magnetic bead (MACS)-purified CD4+ T cells from C57BL/6 mice. T cells were activated for 48 or 72 h with plate bound anti-CD3 and anti-CD28 in the presence of anti–IFN-γ and anti–IL-4 as well as the indicated cytokines. Results were normalized against HPRT mRNA expression levels. ND, not detected.
Studies to better understand the effects of IL-27 on T cells included an in vitro screen that led to the recognition that while IL-27 could suppress expression of multiple cytokines associated with Th1, Th2 and Th17 activities, it also promoted IL-10[57]. A finding that was corroborated by other groups[58, 59]. While IL-27 was able to inhibit Th17 differentiation in vitro independently of IL-10[57] its ability to inhibit EAE was shown to be dependent on IL-10[59]. However, IL-10 was not required for additional anti-inflammatory activities of IL-27[59](J. Stumhofer and C. Hunter unpublished findings). Thus, these results provided an additional mechanism that allowed IL-27 to directly downregulate effector functions and at the same time promote a T-cell phenotype that could further contribute to the termination of an inflammatory response. Additional studies also demonstrated that Th17 cells polarized in the presence of IL-6 and TGF-β resulted in the induction of IL-10[57, 60]. Together these studies have identified new regulatory pathways that lead to T-cell production of IL-10, an event important in several systems to prevent pathology[61, 62].
One of the surprising elements of the studies on IL-27 is that while IL-6 promotes the generation of Th17 cells, IL-27, which is structurally related to IL-6 and which also signals through the same gp130 receptor subunit utilized by IL-6, is able to antagonize Th17 activity yet both can promote T cell production of IL-10. The molecular basis for T cells to distinguish and integrate these pro- and anti-inflammatory signals from similar cytokines has not been explored extensively. How these signals are integrated in the setting of inflammation is also poorly understood but may relate to the spatial and temporal production of these two cytokines. Indeed, there remain several questions regarding the factors that regulate the production of IL-27 and its tissue-specific effects. For example, while recent studies have begun to define the microbial stimuli and the signaling pathways that lead to IL-27 production[63] it is still unclear when and where IL-27 is produced during an active immune response. Additionally, there is a possibility that the two components of IL-27, p28 and EBI3, may bind to other proteins or are secreted as individual subunits that bind to WSX-1 or gp130 and signal on their own.
STAT-mediated inhibition of T-cell responses
One of the common themes that has emerged from the studies on Th17 cells is the central role that the STAT family members have in promoting the development of this lineage as well as their ability to negatively regulate these cells. Since the mid-1990s it was recognized that STAT4 and STAT6 were closely associated with the ability of IL-12 and IL-4 to drive Th1 and Th2 responses, respectively. However, while these activators of transcription can promote T-cell activity they can also have profound inhibitory activities. Perhaps the best example is the ability of IL-10 to induce STAT3, which is essential to its ability to inhibit accessory cell functions required to activate T cells. In addition, the ability of IL-6, IL-23 and IL-21 to activate STAT3 promotes the differentiation of Th17 cells [5–7, 53, 64, 65]. Activation of STAT3 results in the up-regulation of the inhibitory protein SOCS3, which has been shown to limit IL-23 and IL-6–induced IL-17 by attenuating phosphorylation of STAT3[53, 64] providing an autocrine feedback loop for limiting IL-17 production.
In the context of Th17 cells, the ability of IL-27 or IFNγ to activate STAT1 leads to the suppression of Th17 activity[17, 18, 53, 54] (Figure 3). Additionally, the type I interferons IFNα and IFNβ, which also recruit STAT1, can inhibit Th17 development[18]. The ability of IL-2 to inhibit IL-17 production has been linked to its activation of STAT5 and subsequent recruitment to the IL-17 promoter. It has been suggested that STAT5 may have a direct inhibitory effect on IL-17 transcription, either through competition with other STAT proteins required for Th17 activity (STAT3)[66] or through the recruitment of inhibitory complexes to directly mediate repression of the IL-17 locus.
Figure 3.
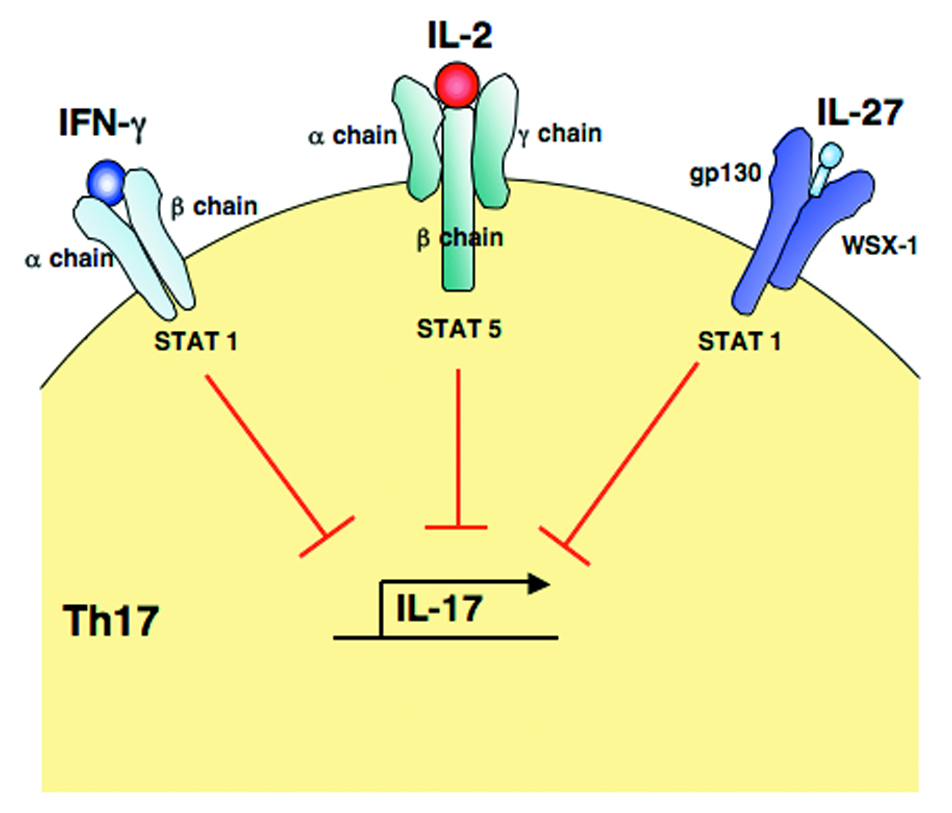
STAT mediated inhibition of IL-17 production. The binding of IFN-γ, IL-2 and IL-27 to their individual receptor complexes results in the activation of selective STAT proteins (STAT5 for IL-2, and STAT1 for IFN-γ and IL-27) that in turn regulate the differentiation of Th17 cells by inhibiting IL-17 production.
Conclusions
Since the initial description that linked Th17 cells to experimental arthritis and EAE, there have been tremendous advances in the understanding of how these cells are generated and their biological significance in vivo. It is notable that there are large numbers of RORγt+ Th17 cells that reside in the gut[67] and that in multiple models there is a strong link between IL-10 and IL-17 that seems most prominent at this mucosal site[68, 69]. Parallel to those studies are data that have identified novel pathways that can serve to limit Th17 responses. Some of these might have been predicted (IL-10), but others have been less obvious. Thus, with the realization that IL-2, IL-25 and IL-27 are involved in the control of the activities of Th17 cells, new questions arise about the molecular basis for their antagonistic activities. These inhibitory pathways may not just provide new therapies (i.e., whether treatment with IL-25, IL-2 or IL-27 will ameliorate inflammation) to manage chronic inflammation, but an understanding of how they actually function may provide novel ways of targeting inappropriate T-cell activities and lead to the development of small molecules that also target these pathways.
Acknowledgments
We thank our colleagues Christiaan J.M. Saris, Nico Ghilardi, Hiroki Yoshida, Matthias Ernst, John J. O’Shea and Lothar Heninghausen as well as the faculty and students in the Pathobiology Department. This work is supported by grants from the State of Pennsylvania and NIH (AI42334). Jason Stumhofer is supported by 1-T32-AI-055428 and Jonathan Silver is supported by AI-07532.
Abbreviations
- STAT
Signal Transduced Activator of Transcription
- Th
T helper
- EAE
experimental autoimmune encephalitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mosmann TT, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone I. Definition according to profiles of lymphokine activities and secreted proteins. Journal Immunology. 1986;136:2348–2353. [PubMed] [Google Scholar]
- 2.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL- 12 in joint autoimmune inflammation. J Exp Med. 2003;198(12):1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung Y, et al. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006;16(11):902–907. doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- 4.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203(10):2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007 doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007 doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 7.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007 doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 8.Happel KI, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202(6):761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006 doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 10.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 11.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med, 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujino S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52(1):65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Y, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445(7128):648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 14.Lubberts E, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50(2):650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 15.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 16.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nature Reviews Immunology. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 17.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 19.Moore KW, et al. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990;248:1230–1233. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- 20.Grunig G, et al. Interleukin-10 is a natural suppressor of cytokine production and inflammation in a murine model of allergic bronchopulmonary aspergillosis. Journal Experimental Medicine. 1997;185:1089–1099. doi: 10.1084/jem.185.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wynn TA, et al. Analysis of granuloma formation in double cytokine-deficient mice reveals a central role for IL-10 in polarizing both T helper cell 1- and T helper cell 2-type cytokine responses in vivo. Journal Immunology. 1997;159:5014–5023. [PubMed] [Google Scholar]
- 22.Gazzinelli RT, et al. In the absence of endogenous IL-10 mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ and TNF-α. Journal Immunology. 1996;157:798–805. [PubMed] [Google Scholar]
- 23.Hunter CA, et al. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. Journal Immunology. 1997;158:3311–3316. [PubMed] [Google Scholar]
- 24.Ding L, Shevach EM. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. Journal Immunology. 1992;148:3133–3139. [PubMed] [Google Scholar]
- 25.Waal Malefyt Rd, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. Journal Experimental Medicine. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding L, et al. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J. Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 27.Kuhn R, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 28.Davidson NJ, et al. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interelukin 10-deficient mice. Journal Experimental Medicine. 1996;184:241–251. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berg DJ, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. Journal Clinical Investigation. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson NJ, et al. IL-12, but not IFN-γ, plays a major role in sustaining the chronic phase of colitis in IL-10-deficient mice. Journal Immunology. 1998;161:3143–3149. [PubMed] [Google Scholar]
- 31.Oppmann B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL- 23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal S, et al. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. Journal Biological Chemistry. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 33.Yen D, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116(5):1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Owyang AM, et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203(4):843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fort MM, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15(6):985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 37.Kleinschek MA, et al. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204(1):161–170. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seder RA, et al. CD28-mediated costimulation of interleukin 2 production plays a critical role in T cell priming for IL-4 and interferon γ production. Journal Experimental Medicine. 1994;179:299–304. doi: 10.1084/jem.179.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sadlack B, et al. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 40.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Lohr J, et al. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203(13):2785–2791. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Q, et al. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida H, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 44.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 45.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5(7):521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 46.Denkers EY, Gazzinelli RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clinical Microbiology Reviews. 1998;11:569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villarino A, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19(5):645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 48.Hamano S, et al. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19:657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 49.Rosas LE, et al. Interleukin-27R (WSX-1/T-Cell Cytokine Receptor) gene-deficient mice display enhanced resistance to Leishmania donovani infection but develop severe liver immunopathology. Am J Pathol. 2006;168(1):158–169. doi: 10.2353/ajpath.2006.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holscher C, et al. The IL-27 Receptor Chain WSX-1 Differentially Regulates Antibacterial Immunity and Survival during Experimental Tuberculosis. J Immunol. 2005;174(6):3534–3544. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 51.Miyazaki Y, et al. Exacerbation of experimental allergic asthma by augmented Th2 responses in WSX-1-deficient mice. J Immunol. 2005;175(4):2401–2407. doi: 10.4049/jimmunol.175.4.2401. [DOI] [PubMed] [Google Scholar]
- 52.Yamanaka A, et al. Hyperproduction of proinflammatory cytokines by WSX-1-deficient NKT cells in Concanavalin A-induced hepatitis. Journal Immunology. 2004;172:3590–3596. doi: 10.4049/jimmunol.172.6.3590. [DOI] [PubMed] [Google Scholar]
- 53.Stumhofer JS, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7(9):937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 54.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7(9):929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 55.Fitzgerald DC, et al. Suppressive Effect of IL-27 on Encephalitogenic Th17 Cells and the Effector Phase of Experimental Autoimmune Encephalomyelitis. J Immunol. 2007;179(5):3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 56.Amadi-Obi A, et al. T(H)17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007 doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 57.Stumhofer JS, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007 doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 58.Awasthi A, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007 doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 59.Fitzgerald DC, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007 doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 60.McGeachy MJ, et al. TGF-beta and IL-6 drive the production of IL-17 and IL- 10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8(12):1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 61.Anderson CF, et al. CD4+CD25-Foxp3- Th1 cells are the source of IL-10- mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204(2):285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jankovicx D, et al. Conventional T-bet+Foxp3- Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204(2):273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molle C, et al. IL-27 synthesis induced by TLR ligation critically depends on IFN regulatory factor 3. J Immunol. 2007;178(12):7607–7615. doi: 10.4049/jimmunol.178.12.7607. [DOI] [PubMed] [Google Scholar]
- 64.Chen Z, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103(21):8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mathur AN, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178(8):4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 66.Chen Z, et al. Selective regulatory function of Socs3 in the formation of IL-17- secreting T cells. Proc Natl Acad Sci U S A. 2006;103(21):8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 68.Kullberg MC, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203(11):2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hue S, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203(11):2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]


