Abstract
Background/Aims
Despite the well recognized association between diabetes (DM) and pancreatic cancer (PaC), little is known about DM associated with PaC (PaCDM). We compared the prevalence and clinical characteristics of DM in subjects with and without PaC.
Methods
We prospectively recruited 512 newly-diagnosed PaC cases and 933 controls of similar age, who completed detailed demographic and clinical questionnaires, and had fasting blood glucose (FBG) measured at recruitment and after pancreaticoduodenectomy (n=105). Subjects with FBG≥ 126 mg/dl or on anti-diabetic treatment were classified as having DM.
Results
DM was more prevalent (47% vs. 7%, p<0.001) and predominantly new-onset (<2 yr. duration) (74% vs. 52%, p=0.002)among cases compared to controls. Among PaC cases, those with DM (n=243) were older (68±10 vs. 64±12 yr., p<0.001), reported higher usual adult BMI (30±6 vs. 27±5 kg/m2, p<0.001), and a greater frequency of family history of DM (47% vs. 31 %, p<0.001)compared to those without DM (n=269). Prevalence of DM in PaC did not differ by tumor stage or site (head vs. body/tail). Median survival was similar in PaC with and without DM. Among diabetic PaC patients who underwent pancreaticoduodenectomy, DM resolved in 17/30 (57%) new-onset diabetics, while DM prevalence was unchanged in long-standing diabetics (n=11) (p=0.009).
Conclusions
PaC is a powerful diabetogenic state nearly half the patients have DM, which is frequently new-onset and often resolves with tumor resection. PaCDM is associated with conventional risk factors for DM such as obesity and family history. New-onset DM in PaC is likely induced by the tumor.
Introduction
It is estimated that approximately 37,000 individuals were diagnosed with pancreatic cancer (PaC) in the United States (US) in 2007 and it remains the fourth leading cause of cancer death. Since development of disease-specific symptoms in PaC usually signifies advanced disease, most newly-diagnosed PaC patients have unresectable disease. Further insight into clinical conditions such as diabetes mellitus (DM) that have been reported to be associated with PaC may offer avenues for developing screening strategies for the early detection of this cancer.
The complex relationship between DM and PaC has long been recognized. A recent meta-analysis of epidemiologic studies of the association between DM and PaC reported a combined age- and sex-adjusted odds ratio (OR) of 1.82[95% confidence interval (95% CI) 1.66,1.89]for PaC among patients with DM. When stratified according to duration, DM of ≤4 years duration was associated with a 50% greater risk of PaC, compared to DM of ≥5 years duration(OR 2.1 vs. 1.5 p= 0.005). We and others have reported that subjects with new-onset DM have a significantly increased likelihood of diagnosis of PaC within 2-3 years of meeting criteria for DM 4–6.
The prevalence of DM in PaC varies considerably in the published literature, from 4% to 64%, depending on the methodology used to identify patients with DM and criteria used to diagnose DM 7. In most studies, patients were identified as having DM if the diagnosis was documented or coded in the medical record or if the patient or proxy reported previous diagnosis of DM. Very few studies have directly screened PaC patients for DM with either oral glucose tolerance tests using World Health Organization criteria or fasting blood glucose (FBG) using American Diabetes Association criteria. In the absence of accurate estimates of the prevalence of DM in PaC, less is known about the prevalence of new-onset DM in PaC. In a previous study we noted that in 88% of patients with PaC and DM, the DM was <2 years in duration. Based on this observation, we defined new-onset DM in this study as DM reported to be of <2 years duration. In the present study we utilized a large, prospectively collected series of subjects with and without PaC, who not only self-reported previous treatment and duration of DM but also had FBG measured at recruitment. This enabled us to accurately estimate the prevalence of previously diagnosed or treated DM as well as identify those with previously undiagnosed and/or untreated DM.
There is growing evidence that PaC causes diabetes. This notion is supported by the fact that not only is there a very high prevalence of DM in PaC1,2 but also a close temporal relationship between the onset of DM and the diagnosis of PaC,11, 12. In a recent study we showed that onset of DM in PaC may be up to 18–24 months before diagnosis of cancer, suggesting that new-onset DM may be the only clue to the presence of asymptomatic sporadic PaC. However, the success of the strategy to use hyperglycemia as a screening tool to identify subjects with a high likelihood of having asymptomatic PaC will depend largely on our ability to differentiate PaC-associated DM (PaCDM) from the more common type 2 DM. Some authors have suggested that PaC should be suspected in patients with new-onset DM who are lean and do not have family history of DM, i.e., in patients with new-onset DM without the conventional risk factors for type 2 DM 1. In our study we compared cases and controls to determine if there were any clinical clues that distinguish PaCDM from type 2 DM.
The pathogenesis of new-onset PaCDM remains unknown. One possible explanation for the high prevalence of DM in PaC is that the DM is simply a consequence of glandular destruction by the tumor and therefore, a late manifestation of the cancer. If so, one would expect that compared to PaC subjects without diabetes, PaC subjects with diabetes would have larger tumors, have more advanced stage PaC and be more likely to be in the islet-rich body and tail of the pancreas. We therefore compared mean tumor size among patients with and without DM in the subset of patients who underwent pancreaticoduodenectomy, compared the prevalence of DM in early stage (stage I and II) versus late stage PaC (Stage III/IV), and compared the prevalence of DM in tumors located in head of the gland versus those in the body and tail.
Another hypothesis to explain the development of new-onset DM in PaC is that DM is induced by PaC through tumor-secreted products. The resolution of diabetes and improvement in glucose tolerance after resection of the tumor3,4 in two small studies supports this hypothesis. However, it is unclear if the reported improvement in post-operative glucose tolerance in PaCDM patients3,4 could be attributed largely to post-operative weight loss due to sub-total pancreatectomy, which, on average, is ~8% of body weight 5. In our series, 105 PaC patients had pancreaticoduodenectomy and had pre- and post-operative FBG measurements. In this subset, we determined if FBG values and DM prevalence improved after cancer resection. In order to evaluate the effect of weight loss on glycemic status, we compared reported pre-and post- operative weight loss in diabetic PaC patients who underwent pancreaticoduodenectomy with the premise that if the degree of weight loss is similar, changes in glycemic status are likely attributable to resection of the tumor.
Patients and Methods
The study was approved by the Mayo Foundation Institutional Review Board (IRB). The analyses in this study were performed on data entered into a prospectively collected database of PaC cases and controls of similar age maintained by the Mayo Clinic Pancreas Cancer Specialized Program of Research Excellence (SPORE) (P50 CA102701).
Mayo Clinic Pancreas Cancer SPORE Patient Registry
In an ongoing study, PaC patients seen at Mayo Clinics in Rochester, Minnesota and Jacksonville, Florida are recruited to the Registry at the time of their first visit or later by mail if clinic contact was not possible (approximately 20%). Control subjects without a prior history of cancer (except non-melanoma skin cancer) visiting Mayo Clinic for a general medical exam in the Department of Internal Medicine are approached for participation in the Registry. Recruitment of controls is frequency- matched to cases by five-year age groups. In addition, cases and controls are required to speak English, be 18 years or older, and be able to give informed consent. At the time of this analysis, the majority of the PaC cases (60%) and all controls were residents of the five-state region surrounding Mayo Clinic (Minnesota, Iowa, Wisconsin, North Dakota, and South Dakota). Participation rates were 68% among cases and 70% for controls.
Questionnaire
Cases and controls were requested to self-complete a detailed questionnaire that collected information on known and suspected pancreatic cancer risk factors and demographic data including race/ethnicity, usual adult height and weight, use of tobacco, alcohol intake, education level, and history of DM in first-degree relatives. We also inquired about existing medical conditions including DM, duration of these medical problems, and current medications. The database also includes details of weight measured at the time of recruitment and the body mass index (BMI), calculated as weight (kg)/height 2 (m2). In subjects with PaC, additional details about the PaC are entered, including histology, stage, and treatment received. Patients’ vital status is periodically updated by the Registry.
Cases and controls also had FBG measured on the first day of the visit as part of routine clinical care. Patients were given instructions to remain fasting from midnight before the day of FBG measurement. FBG values were retrieved electronically from the Mayo Clinic Laboratory Information System database. Among PaC patients who underwent surgical resection, the first post-operative FBG value and weight after discharge from hospital were abstracted from the electronic medical record. To assess long-term DM status, we reviewed the medical records of this subset of patients and noted DM status and the length of follow up that was available.
For this study, only cases and controls who consented to be enrolled in the Registry, filled out the questionnaire, and had a FBG value measured were included. PaC cases included in the study had histologically confirmed pancreatic ductal adenocarcinoma and were recruited before they had surgery or received chemotherapy. PaC was staged according to the staging system proposed by the American Joint Committee on Cancer (AJCC).
Patients receiving prescription anti-diabetic medications for previously diagnosed DM were classified as having DM regardless of their FBG value. Among patients not reporting treatment for DM, classification of DM status was based on the American Diabetes Association criteria patients were classified as having DM if the FBG was ≥126 mg/dl (7 mmol/L), as impaired fasting glucose (IFG) if their FBG value was between 100–125 mg/dl (5.6–6.9 mmol/L) and as normal fasting glucose (NFG) if their FBG value was ≤99 mg/dl (5.5 mmol/L).
Statistical Analyses
Cases and controls who completed the questionnaire and had FBG measurement within 30 days of diagnosis (cases) or recruitment (controls) were included in the final analyses. Data are presented as mean± SD and as percentages. Univariate analyses were conducted using the chi-squared test. Paired t-test was used to compare pre-and post-operative mean FBG values in the various glycemic groups and the Fisher exact test was used to evaluate long-term DM status. We used multivariate logistic regression modeling to assess the relationship between DM and PaC and results are presented as odds ratios and 95% confidence intervals. A p-value of 0.05 was considered statistically significant. Statistical analyses were performed using SAS V9.1 (SAS Institute, Inc. Cary, NC).
Results
Among patients recruited prospectively to the registry, 512 PaC patients and 933 controls who gave consent for study, completed a demographic and clinical questionnaire, and had FBG measurement within 30 days of diagnosis (cases) or recruitment (controls) were included in the study.
Comparison of PaC Cases and Controls
Mean age was similar in cases and controls (66±11 vs. 66±11 yr, p=0.6) (table 1) and the racial/ethnic composition of both groups was predominantly Caucasian. Compared to controls, PaC subjects were more likely to be smokers (59% vs. 46%, p<0.001) and have a family history of DM (39% vs. 33%, p=0.04). Usual adult BMI was greater among cases than controls (28±5 vs. 27±5 kg/m2, p<0.001) but BMI at the time of recruitment into the study was significantly lower in cases (25±5 vs. 28±5 kg/m2, p<.001). Nearly two-thirds of PaC cases had lost more than 4.5 kg (10 lbs) preceding the diagnosis of PaC as compared to 7% of controls (p<0.001).
Table 1.
Demographic characteristics and features of diabetes among pancreatic cancer cases and controls.
| Cases | Controls | ||
|---|---|---|---|
| Variable | (N=512) | (N=933) | p-value |
| Demographic Characteristics | |||
| Age (y), mean* | 66±11 | 66±11 | 0.60 |
| Male gender, n (%) | 294 (57%) | 436 (43%) | <.001 |
| Present BMI* | 25±5 | 28±5 | <.001 |
| Usual adult BMI* | 28±5 | 27±5 | <.001 |
| Weight loss > 4.5 kg (10 lbs), n (%) | 317 (64%) | 58 (7%) | <.001 |
| Smoking, n (%) | 302 (59%) | 413 (46%) | <.001 |
| Diabetes Features | |||
| Family history of DM#, n (%) | 198 (39%) | 297 (33%) | 0.04 |
| Current DM treatment, n (%) | 125 (24%) | 43 (5%) | <.001 |
| DM treatment or FBG≥126, n(%) | <.001 | ||
| Yes | 243 (47%) | 67 (7%) | |
| No | 269 (53%) | 866 (93%) | |
| Proportion of new-onset DM (<2y) | 177/243 (74%) | 33/67 (53%) | 0.002 |
± values represent SD.
DM in first-degree relative DM Diabetes mellitus, FBG fasting blood glucose, BMI body mass index (kg/m2)
Tumor characteristics
The mean tumor diameter in the entire PaC sample was 35.3±22.5 mm majority of the tumors were moderately-differentiated (grade 2, 12%) or poorly-differentiated (grade 3, 44%). PaC was stage I/II in 232 patients (45%), stage III/IV in 278 patients (54%), and information on stage was missing in 2 patients. In the subset of patients who underwent pancreaticoduodenectomy (n=105), the mean tumor diameter was 32.8±12.6 mm and 71% of tumors were poorly differentiated (grade 3). Among diabetic PaC subjects who underwent pancreaticoduodenectomy, the mean tumor diameter was 33.5±10.0 mm and tumors were also predominantly grade 3 (76%).
Comparison of DM in PaC Cases and Controls
Patients were classified as diabetic if they had FBG≥126 mg/dl or reported being on anti-diabetic treatment 213 of 512 PaC cases (41.6%) had FBG≥126 mg/dl as compared to 53/933 (5.7%) of controls (p<0.001) (Table 1). An additional 30 PaC cases (5.9%) and 14 controls (1.5%) were classified as diabetic as they were on antidiabetic treatment even though their FBG was ≤126 mg/dl. Thus the overall prevalence of DM (reported treatment for DM and/or FBG≥126) was higher in cases compared to controls (47.4% vs. 7.2%, p<0.001) (Figure 1). Impaired fasting glucose (FBG 100–125 mg/dl) was present in 197 (38%) cases and 314 (34%) of controls (p=0.06). Overall, only 14% of PaC patients had normal FBG as compared to 59% of controls (p<0.001).
Figure 1.
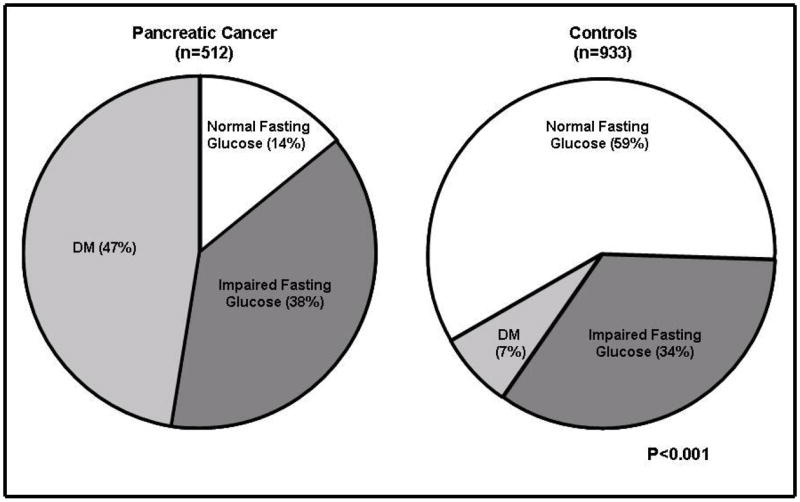
Distribution of fasting blood glucose among pancreatic cancer cases (n=512) and controls (n=933), p<.001
FBG Fasting blood glucose
Normal fasting glucose (NFG) [≤99mg/dl (5.5 mmol/L)], Impaired fasting glucose (IFG) [100–125 mg/dl (5.6–6.9)], Diabetes mellitus (DM) [≥126mg/dl (7 mmol/L)]
To convert FBG values from mg/dl to mmol/L, multiply by 0.055.
Among diabetic subjects, the DM was new-onset (< 2 yr duration) in a higher proportion of cases compared to controls (177/240 (74%)vs. 33/62 (53%), p<0.001). PaC cases with DM, as compared to controls with DM, were of similar age (68±10 vs. 69±8 yr., p=0.5) and gender distribution (% male: 61% vs. 63%, p=0.8), and did not differ significantly in terms of self-reported usual adult BMI (30±6 vs. 31±4 kg/m2) and family history of DM (47% vs. 60%, p=0.1). PaCDM patients tended to have higher FBG values compared to controls with DM 47% of PaCDM subjects had FBG≥160 mg/dl compared to 26% of diabetic controls (p<0.001).
In univariate analysis, PaC cases were five times more likely to have impaired fasting glucose (95% confidence interval [CI] 3.4, 6.0) and 26 times more likely to be diabetic compared to controls (95% CI 17.6, 37.1). In multivariate analyses, diabetes was 14 times more likely in PaC patients as compared to controls (95% CI 8.7, 21.5), after controlling for age, gender, BMI, family history of DM, smoking history, and weight loss of 2.3 kg (5lbs) or greater.
Comparison of PaC cases with and without DM
PaC cases with DM (n=243) were slightly older (68±10 vs. 64±12 yr, p<.001) and more likely to be male as compared to PaC cases without DM (n=269) (table 2). Diabetic PaC cases were also more likely to have a family history of DM compared to PaC patients without DM (47% vs. 31 %, p<0.001). The mean usual adult BMI was higher in PaC with DM vs. PaC without DM (30±6 vs. 27±5 kg/m2, p<.001) and diabetic PaC cases were more likely to have weight loss of >4.5 kg (10 lbs) compared to PaC patients without DM (72% vs. 57%, p<.001). There was no difference in the smoking status between the two groups. The prevalence of DM was not significantly associated with tumor location (% head: 72% vs. 66%, p=0.2) or tumor stage (stage I/II: 47% vs. Stage III/IV 43%, p=0.2). Median survival was also not significantly different in PaC with and without DM (259 vs. 270 days, p=0.9).
Table 2.
Characteristics of pancreatic cancer (PaC) patients with and without DM
| Pancreatic cancer
|
|||
|---|---|---|---|
| Characteristic | DM* (n=243) | No DM (n=269) | p-value |
| Age (y), mean** | 68±10 | 64±12 | <.001 |
| Male sex n (%) | 148 (61%) | 146 (54%) | 0.13 |
| Family history of DM# n (%) | 115 (47%) | 83 (31%) | <.001 |
| Weight loss (kg), mean | 13±10 | 10±8 | <.001 |
| Premorbid BMI | 30±6 | 27±5 | <.001 |
| BMI at diagnosis | 26±5 | 25±5 | 0.005 |
DM Diabetes Mellitus
± signs represent SD
DM in first-degree relative
BMI, body mass index (kg/m2)
Effect of pancreaticoduodenectomy on glycemic status in resectable PaC
Of 512 PaC cases in our series, 105 (21%) patients underwent pancreaticoduodenectomy, and 14 (3%) patients had a distal pancreatectomy for resectable PaC. Forty two of 105 (40%) patients who underwent pancreaticoduodenectomy were diabetic, 48 (46%) had IFG, and 15 (14%) had NFG. Among 41 diabetic PaC patients undergoing pancreaticoduodenectomy on whom data on duration of DM was available, 30 (73%) had new-onset DM and 11 (27%) had long-standing DM. Reported pre-operative weight loss preceding PaC diagnosis was similar to post-operative weight loss among patients with DM (8.8±6.1% vs. 7.9±5.2%, p=0.7), IFG (7.3±16.1% vs. 7.4±6.9%, p=0.05), and NFG (5.4±4.7 vs. 5.2±8.2%, p=0.9). Overall, among diabetic PaC patients who underwent pancreaticoduodenectomy, mean FBG decreased from 175±48 mg/dl pre-operatively to 120±32 mg/dl post-operatively (p<0.001) at a median duration of 48 days (range 30–676 days) after surgery. Among new-onset diabetics (n=30), mean FBG decreased from 174.2±49.0 mg/dl to 110.5±19.1 mg/dl (p<0.001). There was no significant difference in the pre-and post-operative mean FBG values among those with long-standing DM (175.5±49.3 vs. 145.8±44.3 mg/dl, p=0.1), IFG (111.2±7.3 vs. 108.0±15.6 mg/dl, p=0.15), and NFG (94.3±5.1 vs. 98.7±10.2 mg/dl, p=0.2). Seventeen of 30 (57%) PaC patients with new-onset diabetes who underwent pancreaticoduodenectomy no longer met criteria for DM postoperatively in contrast all 11 long-standing diabetics remained diabetic postoperatively (p=0.009) (figure 2). In long-term follow up (median duration of follow up: 237 days, range 30–1798 days), 16 of 30 (53%) subjects in the new-onset DM group continued to remain non-diabetic while among long-standing diabetics only 1 of 11 patients (9%) became non-diabetic (p=0.01).
Figure 2.
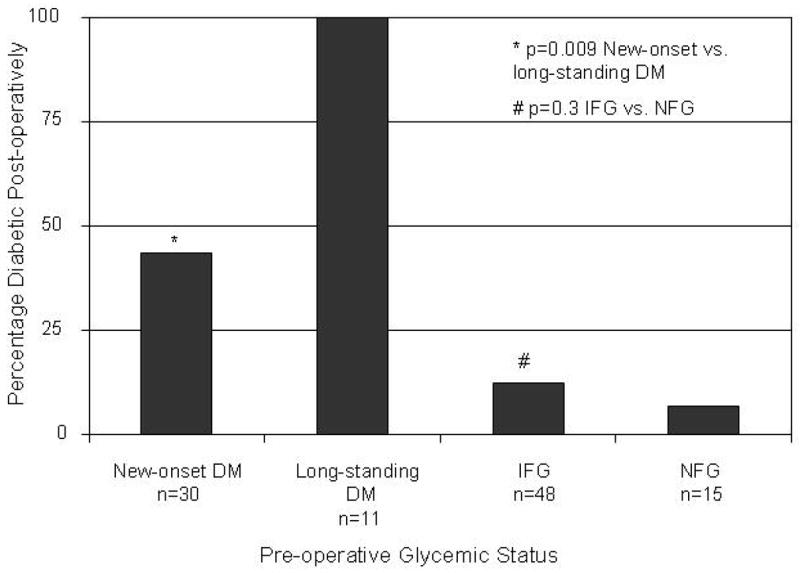
Diabetes mellitus (DM) prevalence after pancreaticoduodenectomy for pancreatic cancer (PaC)
New-onset DM (<2 yr duration) Long-standing DM (>2 yr duration)
Normal fasting glucose (NFG) [≤99mg/dl (5.5 mmol/L)], Impaired fasting glucose (IFG) [100–125 mg/dl (5.6–6.9)], Diabetes mellitus (DM) [≥126mg/dl (7 mmol/L)]
To convert FBG values from mg/dl to mmol/L, multiply by 0.055.
Discussion
Despite the well known association between DM and PaC, the DM associated with PaC (PaCDM) has received little attention in literature. In a prospective study of 512 cases and 933 controls, we found that nearly half of PaC patients met criteria for DM, which was frequently new-onset (<2 yr. duration). Further, DM in PaC was associated with conventional risk factors for type 2 DM such as age, BMI, and family history of DM but not with tumor stage (Stage I/II vs. III/IV) or location (head vs. body/tail). Strikingly, nearly 60% of PaC patients with new- onset DM who underwent pancreaticoduodenectomy had resolution of their DM following tumor resection. These data suggest that new-onset DM in PaC is likely induced by the tumor which is not likely to be explained simply by tumor-induced gland destruction.
The reported prevalence of DM in PaC varies from 4%–64% depending on the methods used to identify subjects with DM and the criteria used for the diagnosis of DM 7, 13, 17, 18. Epidemiologic studies have generally used self-report or review of medical records or death certificates to identify physician-diagnosed DM. The prevalence of DM in PaC in these studies is in the range of 4–23% 19–22. On the other hand, a few small studies that have screened PaC subjects for DM using oral glucose tolerance tests and used the World Health Organization (WHO) criteria for the diagnosis of DM report 45%–65% prevalence of DM in PaC6,7.
In the present study we used the American Diabetes Association criterion of FBG≥126 mg/dl to diagnose DM. In addition, a small minority of subjects were also classified as having DM with a FBG<126 if they reported being on treatment for DM at the time of recruitment. Using these criteria, we found that 47% of PaC subjects met criteria for DM this figure is remarkably similar to the 46% prevalence of DM in PaC reported by us in a previous study of 130 patients where we used identical criteria to define DM in a different cohort of newly diagnosed PaC patients 2. Thus, when screened for DM using FBG measurements or OGTT, nearly half the patients with PaC have DM. Remarkably the prevalence of DM in PaC is much higher than in other well known diabetogenic states such as severe obesity 8, polycystic ovarian syndrome 24, and pregnancy 9.
In our study, majority (75%) of DM in PaC was new-onset (<2 yr duration). It is well recognized that PaC is associated with recent diagnosis of DM. Also, epidemiologic studies that have examined the relationship between DM and PaC report a stronger association with new-onset DM compared to long-standing DM. However, the magnitude of this association is likely underestimated in these studies as most new-onset DM remains undiagnosed. As DM is a chronic disease, the longer it is present, the more likely it is to be clinically recognized. If the duration of DM in PaC patients is short, the proportion with previously undiagnosed DM is likely much higher in PaC than in the general population. This probably accounts for the lower reported prevalence of DM in PaC in studies relying on information in the medical record.
We have previously noted that approximately 1% of diabetic subjects aged ≥ 50 years will be diagnosed with PaC within 3 years of first meeting criteria for DM 10. Identifying clinical characteristics that point to DM associated with PaC rather than type 2 DM would enable us to differentiate subjects with new-onset DM who are more likely to have PaC. Others have suggested that lean, new-onset DM subjects who lack a family history of DM may be candidates for PaC screening 1. However, in our study, PaC patients with DM had a significantly higher BMI prior to onset of symptoms compared to the usual adult BMI in controls, findings which are in concert with our previous report 2. In our experience, a PaC patient with DM who is lean has generally lost considerable weight in the months preceding cancer diagnosis. We also noted that approximately a third of PaCDM patients had a family history of DM, which is similar to that reported by Gullo et al previously 11. Thus, prior to the onset of symptoms, the clinical profile of the PaCDM is not very different from that of the type 2 DM patient and does not help distinguish between the two forms of DM.
The remarkably high prevalence of DM in PaC and its close temporal association with the diagnosis of cancer noted in the present study and previous reports”6,7 suggests that DM is likely a consequence of the cancer. This is unlikely to be simply due to destruction of the gland by the tumor or due to obstructive chronic pancreatitis induced by the tumor. This is supported by the findings of our study that prevalence of DM in PaC was not affected by tumor stage or location and the observation that the mean tumor diameter in PaC patients with DM was similar to PaC patients without DM. Furthermore, insulin and C-peptide levels in PaC, especially in those with DM have been reported to be higher than in healthy controls26, 27, which reflect the presence of insulin resistance in PaC. If pancreatic destruction and resultant decrease in beta cell mass was the cause of PaCDM, one would have expected low levels of C-peptide and insulin, as are seen in diabetes associated with chronic pancreatitis28, 29.
Further support for the hypothesis that DM in PaC is caused not so much by local effects of tumor infiltration, as by remote effects impairing glucose metabolism is provided by the findings of our study that there was a significant decrease in FBG levels and resolution of DM after resection of PaC in patients with new-onset DM. Various factors, including post-operative weight loss and extent of pancreatic resection, likely influence the eventual glycemic status of the patient following major surgery such as pancreaticoduodenectomy. In our study, diabetic PaC patients lost an equal amount of weight in the months preceding the diagnosis of PaC as they did after the operation. Prior to surgery, patients became newly diabetic or their pre-existing DM worsened, even as they lost nearly 8% of their body weight. Following cancer resection the DM improved or resolved in those with new-onset DM despite a similar amount of weight loss to what they had experienced pre-operatively. In our study, FBG measurement at a median of 48 days (range 30–676 days) post-operatively was used to assess DM status. In addition, the resolution of DM in the majority of patients with new-onset DM persisted in long-term follow up while nearly all patients with long-standing DM continued to remain diabetic. Our findings are in concert with those of Permert et al3 and Fogar et al4 and confirm their reports in a larger sample of patients.
Experimental observations that PaC cell line supernatants are metabolically active provide further support for the hypothesis that PaCDM is caused by tumor-secreted products. Supernatants from PaC cell lines have been shown to induce glucose intolerance in SCID mice 12, alter glucose metabolism in the liver 13 and skeletal muscle 32, 33, and cause inhibition of insulin from beta cells in vitro34, 35. The report by Basso et al. 14 that a peptide (MW2030) may be a putative diabetogenic factor in PaC suggests that a serologic marker of diabetes induced by pancreatic cancer may be identified in the near future.
One of the limitations of our study is that certain DM characteristics such as duration and treatment, family history, and risk factors such as usual adult BMI, weight loss, and smoking status were self-reported. However, information was collected from cases and controls in a similar manner and the prevalence of DM was objectively assessed. Differential misclassification of DM duration in the present study is unlikely to be substantial given that the vast majority of DM in PaC was new-onset and DM of recent onset resolved in a majority of patients following surgery. In addition, the results of the present study are similar to our previous report where we used similar criteria in a different sample of patients.
In conclusion, our study reveals that nearly half of newly-diagnosed PaC patients have DM 74% of DM in PaC is new-onset (<2 yr.). We have also shown that tumor characteristics do not affect the prevalence of DM. Resection of cancer leads to amelio ratio nor resolution of DM in a majority of diabetic PaC patients with new-onset DM. Our results suggest that DM in PaC is likely a consequence of the cancer. Future identification of a specific mediator of this DM may facilitate screening in new-onset DM.
Acknowledgments
Grant Support: Dr Chari’s research was funded by grants from NIH (R01 CA 100685) and the Mayo Clinic Pancreas Cancer SPORE (P50 CA 10270). Dr Petersen’s research was funded by grants from NIH (R01 CA 100685 and P50 CA 10270)
Abbreviations used
- PaC
Pancreatic cancer
- PaCDM
Pancreatic cancer-associated diabetes
- FBG
Fasting blood glucose
- IFG
Impaired fasting glucose
- NFG
normal fasting glucose
Footnotes
Financial Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Seigel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Wang F, Herrington M, Larsson J, Permert J. The relationship between diabetes and pancreatic cancer. Mol Cancer. 2003;2:4. doi: 10.1186/1476-4598-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–83. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chari S, Leibson CL, de Andrade M, Rabe KG, Ransom JE, Petersen GM. Probability of pancreatic cancer following diabetes: A population-based study. Gastroenterology. 2005;129:504–511. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S, Vittinghoff E, Bertenthal D, Corley D, Shen H, Walter LC, McQuaid K. New- onset diabetes and pancreatic cancer. Clin Gastroenterol Hepatol. 2006;4:1366–72. doi: 10.1016/j.cgh.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Gupta S, Holly EA. Diabetes mellitus and pancreatic cancer in a population- based case control study in the San Francisco Bay Area, California. Cancer Epidemiology, Biomarkers & Prevention. 2006;15:1458–63. doi: 10.1158/1055-9965.EPI-06-0188. [DOI] [PubMed] [Google Scholar]
- 7.Noy A, Bilezikian JP. Clinical review 63: Diabetes and pancreatic cancer: clues to the early diagnosis of pancreatic malignancy. J Clin Endocrinol Metab. 1994;79:1223–31. doi: 10.1210/jcem.79.5.7962312. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation; Geneva. 2006. [Google Scholar]
- 9.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30:S42–47. doi: 10.2337/dc07-S042. [DOI] [PubMed] [Google Scholar]
- 10.Chari ST, Klee GG, Miller LJ, Raimondo M, DiMagno EP. Islet amyloid polypeptide is not a satisfactory marker for detecting pancreatic cancer. Gastroenterology. 2001;121:640–5. doi: 10.1053/gast.2001.27210. [DOI] [PubMed] [Google Scholar]
- 11.Chari ST, Leibson CL, Rabe KG, Timmons LJ, Ransom J, de Andrade M, Petersen GM. Pancreatic Cancer-associated Diabetes Mellitus: Prevalence and Temporal Association with Diagnosis of Cancer. Gastroenterology. 2007;134(1):95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gullo L, Pezzilli R, Morselli-Labate AM. Diabetes and the risk of pancreatic cancer. Italian Pancreatic Cancer Study Group. N Engl J Med. 1994;331:81–4. doi: 10.1056/NEJM199407143310203. [DOI] [PubMed] [Google Scholar]
- 13.Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnquist HJ, Larsson J. Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br J Surg. 1993;80:1047–50. doi: 10.1002/bjs.1800800841. [DOI] [PubMed] [Google Scholar]
- 14.Fogar P, Pasquali C, Basso D, Sperti C, Panozzo MP, Tessari G, D'Angeli F, Del Favero G, Plebani M. Diabetes mellitus in pancreatic cancer follow-up. Anticancer Res. 1994;14:2827–30. [PubMed] [Google Scholar]
- 15.Seiler CA, Wagner M, Bachmann T, Redaelli CA, Schmied B, Uhl W, Friess H, Buchler MW. Randomized clinical trial of pylorus-preserving duodenopancreatectomy versus classical Whipple resection-long term results. Br J Surg. 2005;92:547–56. doi: 10.1002/bjs.4881. [DOI] [PubMed] [Google Scholar]
- 16.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6. New York, NY: Springer; 2002. Exocrine pancreas; pp. 157–164. [Google Scholar]
- 17.Cersosimo E, Pisters PW, Pesola G, McDermott K, Bajorunas D, Brennan MF. Insulin secretion and action in patients with pancreatic cancer. Cancer. 1991;67:486–93. doi: 10.1002/1097-0142(19910115)67:2<486::aid-cncr2820670228>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnqvist HJ, Larsson J. Pancreatic cancer is associated with impaired glucose metabolism. Eur J Surg. 1993;159:101–7. [PubMed] [Google Scholar]
- 19.Cuzick J, Babiker AG. Pancreatic cancer, alcohol, diabetes mellitus and gall-bladder disease. Int J Cancer. 1989;43:415–21. doi: 10.1002/ijc.2910430312. [DOI] [PubMed] [Google Scholar]
- 20.Hiatt RA, Klatsky AL, Armstrong MA. Pancreatic cancer, blood glucose and beverage consumption. Int J Cancer. 1988;41:794–7. doi: 10.1002/ijc.2910410603. [DOI] [PubMed] [Google Scholar]
- 21.Bell E. Carcinoma of the pancreas. I. A clinical and pathologic study of 609 necropsied cases. II The relation of carcinoma of the pancreas to diabetes mellitus. Am J Pathol. 1957;33:499. [PMC free article] [PubMed] [Google Scholar]
- 22.Green RC, Baggenross AH, Sprague RG. Diabetes mellitus in association with primary carcinoma of the pancreas. Diabetes. 1958;7:308. doi: 10.2337/diab.7.4.308. [DOI] [PubMed] [Google Scholar]
- 23.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. Jama. 1999;282:1523–9. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 24.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–9. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 25.Coustan DR, Nelson C, Carpenter MW, Carr SR, Rotondo L, Widness JA. Maternal age and screening for gestational diabetes: a population-based study. Obstet Gynecol. 1989;73:557–61. [PubMed] [Google Scholar]
- 26.Permert J, Larsson J, Westermark GT, Herrington MK, Christmanson L, Pour PM, Westermark P, Adrian TE. Islet amyloid polypeptide in patients with pancreatic cancer and diabetes. N Engl J Med. 1994;330:313–8. doi: 10.1056/NEJM199402033300503. [DOI] [PubMed] [Google Scholar]
- 27.Permert J, Larsson J, Fruin AB, Tatemoto K, Herrington MK, von Schenck H, Adrian TE. Islet hormone secretion in pancreatic cancer patients with diabetes. Pancreas. 1997;15:60–8. doi: 10.1097/00006676-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T, Imamura K, Takebe K, Terada A, Arai Y, Tandoh Y, Yamada N, Ishii M, Machida K, Suda T. Correlation between pancreatic endocrine and exocrine function and characteristics of pancreatic endocrine function in patients with diabetes mellitus owing to chronic pancreatitis. Int J Pancreatol. 1996;20:169–75. doi: 10.1007/BF02803765. [DOI] [PubMed] [Google Scholar]
- 29.Bank S, Marks IN, Vinik AI. Clinical and hormonal aspects of pancreatic diabetes. Am J Gastroenterol. 1975;64:13–22. [PubMed] [Google Scholar]
- 30.Basso D, Brigato L, Veronesi A, Panozzo MP, Amadori A, Plebani M. The pancreatic cancer cell line MIA PaCa2 produces one or more factors able to induce hyperglycemia in SCID mice. Anticancer Res. 1995;15:2585–8. [PubMed] [Google Scholar]
- 31.Basso D, Valerio A, Brigato L, Panozzo MP, Miola M, Lucca T, Ujka F, Zaninotto M, Avogaro A, Plebani M. An unidentified pancreatic cancer cell product alters some intracellular pathways of glucose metabolism in isolated rat hepatocytes. Pancreas. 1997;15:132–8. doi: 10.1097/00006676-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Basso D, Millino C, Greco E, Romualdi C, Fogar P, Valerio A, Bellin M, Zambon CF, Navaglia F, Dussini N, Avogaro A, Pedrazzoli S, Lanfranchi G, Plebani M. Altered glucose metabolism and proteolysis in pancreatic cancer cell conditioned myoblasts: searching for a gene expression pattern with a microarray analysis of 5000 skeletal muscle genes. Gut. 2004;53:1159–66. doi: 10.1136/gut.2003.024471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Adrian TE. A factor from pancreatic and colonic cancer cells stimulates glucose uptake and lactate production in myoblasts. Biochem Biophys Res Commun. 1999;260:626–33. doi: 10.1006/bbrc.1999.0955. [DOI] [PubMed] [Google Scholar]
- 34.Wang F, Larsson J, Abdiu A, Gasslander T, Westermark P, Adrian TE, Permert J. Dissociated secretion of islet amyloid polypeptide and insulin in serum- free culture media conditioned by human pancreatic adenocarcinoma cell lines. Int J Pancreatol. 1997;21:157–64. doi: 10.1007/BF02822387. [DOI] [PubMed] [Google Scholar]
- 35.Wang F, Larsson J, Adrian TE, Gasslander T, Permert J. In vitro influences between pancreatic adenocarcinoma cells and pancreatic islets. Journal of Surgical Research. 1998;79:13–9. doi: 10.1006/jsre.1998.5393. [DOI] [PubMed] [Google Scholar]
- 36.Basso D, Valerio A, Seraglia R, Mazza S, Piva MG, Greco E, Fogar P, Gallo N, Pedrazzoli S, Tiengo A, Plebani M. Putative pancreatic cancer-associated diabetogenic factor: 2030 MW peptide. Pancreas. 2002;24:8–14. doi: 10.1097/00006676-200201000-00002. [DOI] [PubMed] [Google Scholar]


