Abstract
Purpose of the study: Osteonecrosis of the hip mostly affects young individuals and often progresses to a debilitating disease. Several treatment modalities exist, but none are completely satisfactory. This study evaluates the clinical outcome of patients treated with core decompression and insertion of a porous tantalum implant in the femoral head. This procedure is similar to commonly performed procedures, but has the additional advantages of providing structural support to the necrotic femoral head while having no donor-site morbidity. Methods: We evaluated 15 patients with 18 osteonecrotic hips with Steinberg stage III (3 hips) and IV (15 hips) disease. The mean age of the patients was 42 years-old (eldest 66), and the mean time for follow-up was 23 months. The outcome measure was hip function, evaluated with the Harris hip score, and the end point was total hip arthroplasty, or referral for this procedure. Results: The success rate at twelve months postoperatively was 77.8%, and the overall success rate was 44.5%. Failures occurred at a mean time of 11.7 months, and one complication, a periprosthetic fracture, occurred 4 months postoperatively. On average, patients who did well improved their Harris hip scores by 21.7 points, and patients who eventually required arthroplasty decreased their scores by 14 points. Conlusions: Core decompression with porous tantalum implants showed encouraging success rates and early clinical results in patients with advanced stage osteonecrosis, but further larger scale studies are required to identify the population best suited for this procedure.
Keywords: osteonecrosis, femoral head, tantalum, core decompression
INTRODUCTION
Osteonecrosis of the hip, previously named avascular necrosis, is a naturally progressive disease typically affecting young individuals (the mean age approximately 35 years old) (1). It occurs when blood supply to the femoral head is disrupted, resulting in infarction and avascular necrosis of bone. Several etiologies have been identified, such as trauma (femoral neck fracture or posterior hip dislocation), blood disorders (e.g. sickle cell anemia), and radiation therapy (2, 3, 4). Other important risk factors identified are the use of alcohol, use of corticosteroids (e.g. in patients with systemic lupus erythematosus, or status-post renal transplant) and cigarette smoking, as well as pregnancy (5, 6). Having HIV has also been associated with an increased incidence of osteonecrosis of the femoral head, but it is unclear whether this is related to the virus or to the antiviral therapy (7). Much is still to be learned about the etiologies and the pathophysiology of this disease.
When left untreated, 80% of clinically diagnosed cases of femoral head osteonecrosis will progress, most often incapacitating the patient due to pain and decrease in hip mobility (8). The natural history of this condition is that it is usually initially confined to the superior weight-bearing portion of the femoral head, but progresses, rendering the area susceptible to collapse, and leading to subchondral fractures. Eventually, degenerative changes of the hip joint ensue (9). In the United States, the estimated incidence of this condition is 10,000–30,000 per year, and 5–12% of all total hip arthroplasties are performed to treat patients with osteonecrotic hips. This latter treatment modality was proven to be highly successful (3). However, it is not appealing to the young and active patient population often affected by osteonecrosis of the hip, as they will most likely outlive their prosthesis and require revision. Non-operative treatment modalities also exist. They are divided into external biophysical modalities (extracorporeal shock-wave therapy, hyperbaric oxygen, or pulsed electromagnetic field) and pharmacological therapies (lipid-lowering agents, anticoagulants, or bisphosphonates). Mont MA et al. did a complete review of the English literature from 1960 to 1993 and found 21 studies where clinical outcomes of osteonecrotic hips treated non-operatively (excluding electrical stimulation) were evaluated (3). Together, these studies yielded an overall clinical success rate 22.7%. Pulsed electromagnetic field has been proven to result in clinical improvement and stabilization of osteonecrosis on radiographs by two studies done in the late 1980s, but this modality has never gained popularity (10, 11). Also, new pharmacological measures as well as the use of growth and differentiation factors have shown potential in preventing and treating this disease, but clinical research projects are still awaiting long-term follow-up results to provide recommendations (4).
Joint-preserving surgical procedures are presently the most commonly used approach to treatment of osteonecrotic hips. They are described as temporizing measures, possibly preserving these femoral heads. Most have shown superiority over symptomatic treatment, but none are completely satisfactory. The two most common procedures are core decompression and fibular bone-grafting techniques. Core decompression alone has the disadvantage of lacking subchondral support, whereas fibular grafting techniques have increased morbidity associated with graft harvest, longer operative time and blood loss, as well as rehabilitative complications (3, 12, 13). The rationale for fibular bone-grafting is that it allows decompression of the femoral head as well as the removal of necrotic bone with its replacement by the graft. This graft plays the important role of providing structural support and scaffolding, facilitating repair and remodeling of subchondral bone (14, 15).
Since the main disadvantages of fibular grafting techniques are related to the bone-graft harvesting, the use of another material with characteristics similar to bone graft presents an interesting modality. Porous tantalum is an expanded (foam-like) metal currently being used in several orthopedic procedures, namely hip and knee arthroplasty, spine surgery, and as bone graft substitute. It was found to have an excellent biocompatibility and to be safe to use in vivo (16). It has a high volumetric porosity and is corrosion resistant. Its modular elasticity is similar to that of subchondral bone, yet its strength, fatigue properties, endurance limits, and initial stability against bone are all superior to natural bone grafts (17,18,19). In fact, a recent study done in animal models showed that it had rapid tissue ingrowth and fixation strength, which allows faster return to full weight-bearing compared to procedures using fibular bone graft (20). Furthermore, another study which mechanically tested porous tantalum implants in a model mimicking a necrotic femoral head, demonstrated that tantalum implants reduced subchondral plate deflection and that its strength was 9.3 times greater than the maximum force it sustains when placed in the femoral head (21). Finally, local foreign body infection is a possible complication of orthopedic implants, and in this regard, Schildhauer et al. have studied the adhesion of the two bacteria that are of most concern: Staphylococcus aureus and Staphyloccocus epidermis (22). The former was found to adhere significantly less (p<0.05) to pure tantalum compared to titanium alloy, polished stainless steel, and tantalum-coated stainless steel, and the latter bacteria adhered to pure tantalum to an extend similar to other materials used in orthopedics. All of these properties make tantalum implants good substitutes for fibular bone grafts in decompressing surgeries for osteonecrosis of the hip. Theoretically, porous tantalum implants have the advantages of fibular grafts, providing core decompression and structural support, and, in addition, they represent a minimally invasive procedure with no donor-site morbidity. They thus have the theoretical potential of limiting the progression of the disease, which could delay, and maybe even prevent, the need for a hip replacement. This clinical study was performed to verify this theoretical advantage of the tantalum plug relative to core decompression alone or to fibular bone-grafting techniques.
This study evaluates the early clinical outcomes of patients with advanced osteonecrotic femoral heads (presenting with subchondral collapse or femoral head flattening) treated with insertion of porous tantalum implants. We expected that this procedure would improve patients' clinical symptoms, most often being pain and limitations in hip function, and, in turn, delay the need for revision with total hip arthroplasty, which was the endpoint of our study.
METHODS
This prospective study was conducted at the Montreal General Hospital, from April 2002 to February 2004. Experimental subjects were taken from patients referred to a single surgeon for treatment of Steinberg Stage III and IV (Table 1) unilateral or bilateral femoral head osteonecrosis. Only patients with non-traumatic etiologies for the disease were considered for participation in the study. Exclusion criteria also included patients who were actively being treated with corticosteroids, had previous surgery to the affected hip, or were unwilling to have surgery at time of clinical presentation. Patients were offered the tantalum implant procedure when they were unwilling to have treatment with free vascularized fibular graft or total hip arthroplasty. 19 patients with 22 osteonecrotic hips treated with tantalum implants were initially entered in the study and signed an informed consent form prior to enrollment. Four patients were removed from the study as they were unavailable for follow-up at one year or later. One patient sustained trauma, more precisely a fall from her own height, resulting in a periprosthetic fracture one month postoperatively. This patient was not included in data analysis, but was rather included in the results as a complication. Thus, functional outcomes of 14 patients with 17 osteonecrotic hips were used for analysis. The average age of the patients was 42 years old (range 19 to 66). Table 2 outlines details on patient demographics.
Table 1.
Steinberg stages of osteonecrosis of the hip (30)
| Stage | Criteria |
|---|---|
| 0 | Normal or nondiagnostic radiograph, bone scan, MRI |
| I | Normal radiographs; abnormal bone scan and/or MRI
|
| II | Cystic and sclerotic changes in femoral head
|
| III | Subchondral collapse (crescent sign) without flattening
|
| IV | Flattening of femoral head
|
| V | Joint narrowing or acetabular changes |
| VI | Advanced degenerative changes |
Average femoral head and estimated acetabular involvement.
Table 2.
Patient Demographics
| Gender: | No of patients |
|---|---|
| Males | 6 |
| Females | 8 |
| Race: | |
| Caucasian | 11 |
| African-American | 2 |
| Asian | 1 |
| Etiology: | No of hips |
| Idiopathic | 4 |
| Corticosteroids | 3 |
| Alcohol induced | 4* |
| Sickle cell anemia | 1 |
| Chemotherapy | 1 |
| Radiation therapy | 1 |
| HIV | 2* |
| Pregancy induced | 1 |
| Extent & treatment of ON: | |
| Unilateral disease | 5 |
| Bilateral disease treated with: | |
| bilateral TI | 3 |
| TI and FVFG | 3 |
| TI and no surgical treatment | 3 |
| Steinberg Stage: | |
| III | 3 |
| IV | 14 |
Hips of patients with bilateral ON and TI
ON= Osteonecrosis; LTI= Tantalum implant; FVFG= Free vascularized fibular graft
All patients underwent the same operative procedure through a minimally invasive lateral approach (2–3 cm skin incision). First, a core decompression technique was done under c-arm fluoroscopy imaging. It consisted of inserting a guide pin from the lateral femoral cortex into the femoral head and using a core reamer over this guide pin to create a 10-mm diameter bone channel. A porous tantalum plug (Zimmer Trabecular Metal Technology, Trabecular Metal Osteonecrosis Intervention Implant System, Warsaw, Indiana; see Figure 1), was then inserted in this bony channel. This implant is fully made of pure porous tantalum, with an interconnected porosity of 75–80%. It has a cylindrical shape of 10 mm in diameter, and comes in lengths of 70 to 130 mm, available in 5 mm increments. The implant is threaded on a 25 mm length at one end, where the diameter is 14 mm, and a hemispherical tip at the other, for support of the subchondral plate (see Figure 2). Bilateral procedures were performed in the same operative period, whether they were both tantalum implants or one tantalum implant and one free vascularized fibular graft, except for one patient who had a tantalum implant inserted in each hip at 3-month interval. Postoperative care consisted of prophylactic intravenous antibiotic (Cefazolin 1–2 g IV q8h twice) and anticoagulation therapy (Low Molecular Weight Heparin 5000 IU SC qd until weight bearing). Patients were instructed to be non-weight-bearing for 3 weeks, to partial weight-bear for the next 3 weeks, and to weight bear as tolerated thereafter.
Figure 1.

Trabecular Metal Osteonecrosis Intervention Implant System; tantalum implant used in all patients of this study. Retrieved from http://www.zimmer.com
Figure 2.

Porous tantalum implant in a Steinberg stage IV osteonecrotic hip, 2 years post-operatively.
The primary outcome of this study was functional improvement, which was assessed with the Harris hip score. This 15-question scoring tool for rating hip function was formulated and published in 1969 by WH Harris, for evaluation of traumatic arthritis of the hip, and is now the scoring tool the most commonly used worldwide for assessment of hip function in general (23). It consists of a point scale of a maximum of 100 points subdivided into 4 subscales: pain (44 points), function (47 points), range of motion (5 points), absence of deformity (4 points). A total Harris hip score below 70 points is considered a poor result, 70 to 80 fair, 80 to 90 good, and 90 to 100 excellent (24). Söderman and Malchau performed a validity and reliability test for the Harris hip score (24). They found that test and retest reliability between two examinations by physicians had correlation coefficients of 0.94, and concluded that this scoring system had high content and construct validity. This score was obtained for each subject at their pre- and post-operative visits. Because functional improvement after orthopedic surgeries usually improve mostly throughout the first year following the procedure and plateaus thereafter, postoperative scores obtained at 12 months or later were used as data points for analysis. When more than one follow-up with Harris hip score recording had been done at one year post-operatively or later, the best score was taken, which was always the latest one. Paired T-tests were used to compare pre-operative and postoperative Harris hip scores among all patients, as well as within subgroups of patients who eventually failed and those who did not. Failure, defined as being referred for or undergoing a total hip arthroplasty was the end point of this study. Survival rate was calculated with the Kaplan-Meier Method and refers to patients' hips which did not progress to the point of requiring further surgical treatment. These analyses were performed with SSPS Manager (version 11.5; SSPS Inc, Chicago, Illinois). Significance was set at p<0.05.
RESULTS
Outcome at 12 months or more
Paired t-test analysis was done to compare pre-operative to post-operative Harris hip scores of patients' hips that had not failed at 12 months post-operatively (Refer to Table 3). There were 14 such hips in 12 patients. 2 patients with unilateral treatment were removed for analysis of hip function improvement since no pre-operative scores had been obtained. Of the 12 hips of 10 patients remaining, 8 hips (75%) in 7 patients improved their Harris hip scores, the mean improvement was of a magnitude of only 3.8 points. Standard deviations for the mean postoperative score and score improvement were very large, which reflects the discrepancy in individual postoperative values. When looking closely at these, it is noticed that they were very low in patients whose hips eventually failed, whereas they were greatly increased in patients whose tantalum implants succeeded, preventing the need for joint replacement surgery before the end of the study. Mean Harris hip score improvement was thus calculated separately for failed and non-failed tantalum implant procedures: a 21.7-point increase was obtained for patients who did well, compared with a mean decrease of 14.0 points for patients whose implants failed. This excludes three patients in whom three hips failed prior to the 12-month mark, and whose pre-operative Harris hip scores were particularly low (average 27.6 points).
Table 3.
Pre and post operative (?12 months) Harris hip scores
| Mean Harris hip scores (points) | |||||
|---|---|---|---|---|---|
| No of hips | Pre-op ± SD | Post-op ± SD | Improvement ± SD | P-value | |
| All hips | 12
14* |
56.1 ± 9.4 | 59.9 ± 25.6
63,9 ± 25.9 |
3.8 ± 23.8 | 0.590 |
| Non-failed hips | 6
8* |
59.5 ± 9.7 | 81.2 ± 10.0
82.9 ± 10.2 |
21.7 ± 15.0 | 0.017 |
| Failed hips: | |||||
| at any time | 9 | 44.3 ± 16.0 | |||
| >12 mo | 6 | 52.6 ± 8.6 | 38.6 ± 16.0 | (−)14.0 +/− 15.9 | 0.083 |
| <12mo | 3 | 27.6 ± 14.4 | |||
includes two hips for which no preoperative Harris hip score was recorded
Failures
The average time of final follow-up and recording of the 14 Harris hip scores used for analysis was 23.2 months (range 12 to 48). This excludes three patients, one of which had bilateral involvement, in whom one operated hip failed prior to the 12-month mark, more precisely at 7, 8 and 10 months post-tantalum insertion, as well as one patient who sustained mild trauma to her single operated hip (fall from own height) resulting in a periprosthetic fracture (see Figure 3). This represents a failure rate of 22.2% at one year postoperatively. 6 additional hips in 4 patients, including all 3 patients with bilateral implant intervention including one whose contralateral hip had failed earlier, had undergone total hip arthroplasty before the final time of follow-up. Therefore, 10 out of 18 (55.6%) tantalum implant procedures failed within the study period. The mean time for failure, excluding the patient with a periprosthetic hip fracture, was 11.7 months (SD=3.7, range 7 to 20 months), and the mean age at surgery of the patients who failed was 50.1 years old (SD=12.1, range 29 to 66), compared to a mean age of 36.8 years old (SD=12.2, range 19 to 55) for the patients whose tantalum implant did not fail. Refer to Figure 4 for the Kaplan-Meier survivorship curve.
Figure 3.
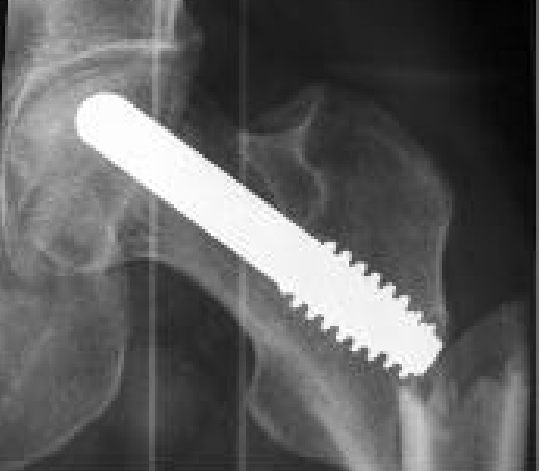
Periprosthetic fracture occurring 4 weeks post-porous tantalum implant insertion after a fall from her own height.
Figure 4.
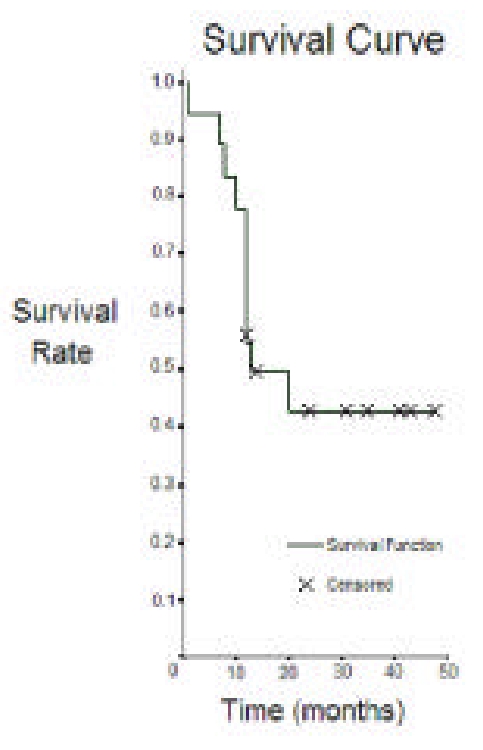
Kaplan-Meier survivorship curve, with conversion to total hip replacement or referral for this procedure, as the end point. Censored patients are ones who did not reach the end point before their final follow-up time.
DISCUSSION
Osteonecrosis of the femoral head is a debilitating disease that requires treatment. Since the late 1960s, several studies have evaluated effectiveness of potential techniques to treat this condition, such as core decompression, vascularized and nonvascularized fibular bone grafts, and angular or rotational osteotomies. Mont et al. performed a meta-analysis to look at the these studies, which were almost all performed on precollapse (Steinberg Stage I or II) osteonecrotic femoral heads and thus their results cannot be compared to ours (3). Furthermore, the conclusion of the meta-analysis was that early diagnosis and intervention prior to collapse of the femoral head, thus in earlier stages than in our patient population, is key to a successful outcome of joint-preserving procedures. However, 3 of the studies they looked at evaluated Steinberg Stage IV osteonecrotic hips treated with vascularized fibular bone grafts; they showed clinical success rates of 52, 48 and 71% at mean follow-up times of 12 months or more (25, 26, 27). Mont et al. concluded in their meta-analysis that for femoral heads that have already collapsed, such as in our patients' hips, the results of joint-preserving procedures are less satisfactory than the results of total hip arthroplasty (4).
In the present study, the success rate, defined as not requiring further hip treatment after core decompression tantalum implant insertion, was 77.8% at twelve months postoperatively. The overall success rate at final time of follow-up (mean of 23.2 months) was 44.5%. Failures (10 hips, 55.6%) occurred at a mean time of 11.7 months. One complication occurred: a periprosthetic fracture at four months postoperatively. This patient was included in the failure rate, as further surgical hip treatment was done, but was not included in the mean failure time, as this failure was due primarily to trauma, and thus does not reflect the time of failure of the tantalum implant per say. Patients who did not require further treatment within the follow-up time improved their Harris hip scores by 21.7 points, and patients who eventually underwent arthroplastic treatment decreased their score by 14 points, on average. The mean age at tantalum implant insertion in patients who failed was 50.1 years old, compared to 36.8 in patients who did well. This 13.4 years of age difference is statistically significant (p=0.040). The average preoperative Harris hip score of patients who failed was 44.3, compared with 59.5 for patients who did well. This represents a 15.2-point difference, which is also statistically significant (p=0.039). This suggests that age at surgery and preoperative hip function have prognostic implications for the porous tantalum implant insertion: the younger, less symptomatic, and less debilitated patients had the most favorable outcomes. Our patient population is not large enough to make an association between outcome and etiology or uni/bilateralism of the disease process. Our statistical findings are also limited in power because of the small number of subjects. The follow-ups, and thus postoperative score recording, were not done at the same time interval with respect to the surgery, making the results not as reproducible and precise as they could have been.
Tsao et al performed the only other published study to date evaluating the clinical outcome of porous tantalum implants in osteonecrotic human hips (28). They evaluated 113 hips treated with tantalum implants. Intraoperative classification yielded 7 and 12 hips of Steinberg Stage III and IV respectively. The mean Harris hip scores of hips improved by 26 and 9 points in patients with stage III and IV osteonecrotic hips respectively. This is comparable to our 21.7-point average improvement in our patients' hips. One (14%) stage III and three (25%) stage IV were revised, which is similar to our failure rate of 22.2% at one year. Their success rate for all stage II disease, which represented the bulk of their experimental data, was 85.3% at twelve months. They concluded that treatment of early stage osteonecrosis of the femoral head with core decompression and a porous tantalum implant show encouraging success rates, especially in association with early stage disease.
Veillette et al. have also recently done a study, with a publication in press, assessing clinical and radiographic outcomes of osteonecrotic hips treated with core decompression and porous tantalum implant (28). They evaluated 60 hips: 1 Steinberg stage I, 49 stage II, and 8 stage III. They used the same hip function evaluation tool and end point as we did. Their one-year survival rate was 91.8% at one year. This is higher than ours, but it is likely due to the fact that most of their hips had stage II osteonecrosis, compared to a majority of stage IVs in our study. Overall, 3 of their 8 hips with stage III disease were converted, representing a success rate of only 62.5%, which is more similar to our results. They concluded that treatment of early stage osteonecrosis of the femoral head with core decompression and a porous tantalum implant show encouraging success rates, especially in patients with early stage disease.
In conclusion, core decompression with porous tantalum implant insertion provides a minimally invasive surgical treatment option to treat advanced osteonecrotic hips, with clinical outcomes and success rates comparable to other commonly used surgical procedures. It has the advantage of providing structural support without having associated donor-site morbidity. It seems however that this treatment modality is more successful in younger patients with more functional and less symptomatic hips. This is fortunate as it is for younger patients that the only definitive treatment so far, total hip replacement, is least appealing, as they are likely to outlive a hip prosthesis, thus requiring one or more revisions. In further studies, it would be useful to perform larger studies, perhaps at a multicenter level, to clearly elucidate the association between pre-operative stage and etiology of the disease, as well as patients' age with success rate. This would enable us to make clear recommendations for choosing the best treatment modality for the individual patient.
REFERENCES
- 1.Collaborative Osteonecrosis Group. Symptomatic Multifocal Osteonecrosis: A Multicenter Study. Clinical Orthopaedics & Related Research. 1999;369:312–26. [PubMed] [Google Scholar]
- 2.Scully SP, Aaron RK, Urbaniak JR. Survival Analysis of Hips Treated with Core Decompression or Vascularized Fibular Grafting because of Avascular Necrosis. J Bone Joint Surg Am. 1998;80(9):1270–5. doi: 10.2106/00004623-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Mont MA, Carbone JJ, Fairbank AC. Core Decompression versus Nonoperative Management for Osteonecrosis of the Hip. Clin Orthop Relat Res. 1996;324:169–78. doi: 10.1097/00003086-199603000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Mont MA, Jones LC, Hungerford DS. Nontraumatic Osteonecrosis of the Femoral Head: Ten Years Later. J Bone Joint Surg Am. 2006;88(5):1117–32. doi: 10.2106/JBJS.E.01041. [DOI] [PubMed] [Google Scholar]
- 5.Chernetsky SG, Mont MA, LaPorte DM, et al. Pathologic Features in Steroid and Nonsteroid Associated Osteonecrosis. Clin Orthop Relat Res. 1999;368:149–61. [PubMed] [Google Scholar]
- 6.Inoue S, Horii M, Asano T, et al. Risk Ractors for Nontraumatic Osteonecrosis of the Femoral Head after Renal Transplantation. J Orthop Sci. 2003;8(6):751–6. doi: 10.1007/s00776-003-0716-9. [DOI] [PubMed] [Google Scholar]
- 7.Blacksin MF, Kloser PC, Simon J. Avascular Necrosis of Bone in Human Immunodeficiency virus Infected Patients. Clin Imaging. 1999;23(5):314–8. doi: 10.1016/s0899-7071(99)00151-5. [DOI] [PubMed] [Google Scholar]
- 8.Mont MA, Hungerford DS. Non-traumatic Avascular Necrosis of the Femoral Head. J Bone Joint Surg Am. 1995;77(3):459–74. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Ohzono K, Saito M, Takaoka K, et al. Natural History of Nontraumatic Avascular Necrosis of the Femoral Head. J Bone Joint Surg Br. 1991;73(1):68–72. doi: 10.1302/0301-620X.73B1.1991778. [DOI] [PubMed] [Google Scholar]
- 10.Aaron RK, et al. The conservative treatment of osteonecrosis of the femoral head. A Comparison of Core Decompression and Pulsing Electromagnetic Fields. Clin Orthop Relat Res. 1989;246:209–18. [PubMed] [Google Scholar]
- 11.Bassett CA, Schink-Ascani M, Lewis SM. Effects of Pulsed Electromagnetic Fields on Steinberg Ratings of Femoral Head Osteonecrosis. Clin Orthop Relat Res. 1989;246:172–85. [PubMed] [Google Scholar]
- 12.Tang CL, Mahoney JL, McKee MD, et al. Donor Site Morbidity Following Vascularized Fibular Grafting. Microsurgery. 1998;18(6):383–6. doi: 10.1002/(sici)1098-2752(1998)18:6<383::aid-micr8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg ME, Larcom PG, Strafford B, et al. Core Decompression with Bone Gafting for Osteonecrosis of the Femoral Head. Clin Orthop Relat Res. 2001;386:71–8. doi: 10.1097/00003086-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Kim SY, Kim YG, Kim PT, Ihn JC, Cho BC, Koo KH. Vascularized Compared with Nonvascularized Fibular Grafts for Large Osteonecrotic Lesions of the Femoral Head. J Bone Joint Surg Am. 2005;87(9):2012–8. doi: 10.2106/JBJS.D.02593. [DOI] [PubMed] [Google Scholar]
- 15.Mont MA, Etienne G, Ragland PS. Outcome of Nonvascularized Bone grafting for Osteonecrosis of the Femoral Head. Clin Orthop Relat Res. 2003;417:84–92. doi: 10.1097/01.blo.0000096826.67494.38. [DOI] [PubMed] [Google Scholar]
- 16.Kato H, Nakamura T, Nishiguchi S, Matsusue Y, Kobayashi M, Miyazaki T, et al. Bonding of Alkali- and Heat-Treated Tantalum Implants to Bone. J Biomed Mater Res. 2000;53(1):28–35. doi: 10.1002/(sici)1097-4636(2000)53:1<28::aid-jbm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 17.Bobyn JD, Poggie RA, Krygier JJ. Clinical Validation of a Structural Porous Tantalum Biomaterial for Adult Reconstruction. J Bone Joint Surg Am. 2004;86-A(Suppl 2):123–9. doi: 10.2106/00004623-200412002-00017. [DOI] [PubMed] [Google Scholar]
- 18.Zardiackas LD, Parsell DE, Dillon LD, Mitchell DW, Nunnery LA, Poggie R. Structure, Metallurgy, and Mechanical Properties of a Porous Tantalum Foam. J Biomed Mater Res. 2001;58(2):180–187. doi: 10.1002/1097-4636(2001)58:2<180::aid-jbm1005>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Shimko DA, Shimko VF, Sander EA, Dickson KF, Nauman EA. Effect of Porosity on the Fluid Flow Characteristics and Mechanical Properties of Tantalum Scaffolds. J Biomed Mater Res B Appl Biomater. 2005;73(2):315–324. doi: 10.1002/jbm.b.30229. [DOI] [PubMed] [Google Scholar]
- 20.Bobyn JD, Stackpool GJ, Hacking SA, Tanzer M, Krygier JJ. Characteristics of Bone Ingrowth and Interface Mechanics of a New Porous Tantalum Biomaterial. J Bone Joint Surg Br. 1999;81(5):907–14. doi: 10.1302/0301-620x.81b5.9283. [DOI] [PubMed] [Google Scholar]
- 21.Levine BR, Sporer S, Poggie RA, Della Valle CJ, Jacobs JJ. Experimental and Clinical Performance of Porous Tantalum in Orthopedic Surgery. Biomaterials. 2006;27(27):4671–81. doi: 10.1016/j.biomaterials.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 22.Schildhauer TA, et al. Bacterial Adherence to Tantalum versus Commonly Used Orthopedic Metallic Implant Materials. J Orthop Trauma. 2006;20(7):476–84. doi: 10.1097/00005131-200608000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Harris WH. Traumatic Arthritis of the Hip After Dislocation and Acetabular Fractures: Treatment by Mold Arthroplasty: an End-result Study Using a New Method of Result Evaluation. J Bone Joint Surg Am. 1969;51:737–55. [PubMed] [Google Scholar]
- 24.Söderman P, Malchau H. Is the Harris Hip Score System Useful to Study the Outcome of Total Hip Replacement? Clin Orthop Relat Res. 2001;384:189–97. doi: 10.1097/00003086-200103000-00022. [DOI] [PubMed] [Google Scholar]
- 25.Soucacos PN, Beris AE, Malizos K, Koropilias A, Zalavras H, Dailiana Z. Treatment of Avascular Necrosis of the Femoral Head with Vascularized Fibular Transplant. Clin Orthop Relat Res. 2001;386:120–30. doi: 10.1097/00003086-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Sotereanos DG, Plakseychuk AY, Rubash HE. Free Vascularized Fibula Grafting for the Treatment of Osteonecrosis of the Femoral Head. Clin Orthop Relat Res. 1997;344:243–56. [PubMed] [Google Scholar]
- 27.Urbaniak JR, Coogan PG, Gunneson EB, Nunley JA. Treatment of Osteonecrosis of the Femoral Head with Free Vascularized Fibular Grafting. A Long-term Follow-up Study of One Hundred and Three Hips. J Bone Joint Surg Am. 1995;77(5):681–94. doi: 10.2106/00004623-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Tsao AK, Roberson JR, Christie MJ. Biomechanical and Clinical Evaluations of a Porous Tantalum Implant for the Treatment of Early-stage Osteonecrosis. J Bone Joint Surg Am. 2005;87 (Suppl 2):22–7. doi: 10.2106/JBJS.E.00490. [DOI] [PubMed] [Google Scholar]
- 29.Veillette CJ, Mehdian H, Schemitsch EH, McKee MD. Survivorship Analysis and Radiographic Outcome Following Tantalum Rod Insertion for Osteonecrosis of the Femoral Head. J Bone Joint Surg Am. 2006;88(Suppl 3):48–55. doi: 10.2106/JBJS.F.00538. In Press. [DOI] [PubMed] [Google Scholar]
- 30.Steinberg ME, Brighton CT, Corces A. Osteonecrosis of the Femoral Head; Results of Core Depression and Grafting with Electrical Stimulation. Clin Orthop. 1989;249:199–208. [PubMed] [Google Scholar]


